A Novel Deep Learning Model as a Donor–Recipient Matching Tool to Predict Survival after Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Selection and Study Population
2.2. Missing Data
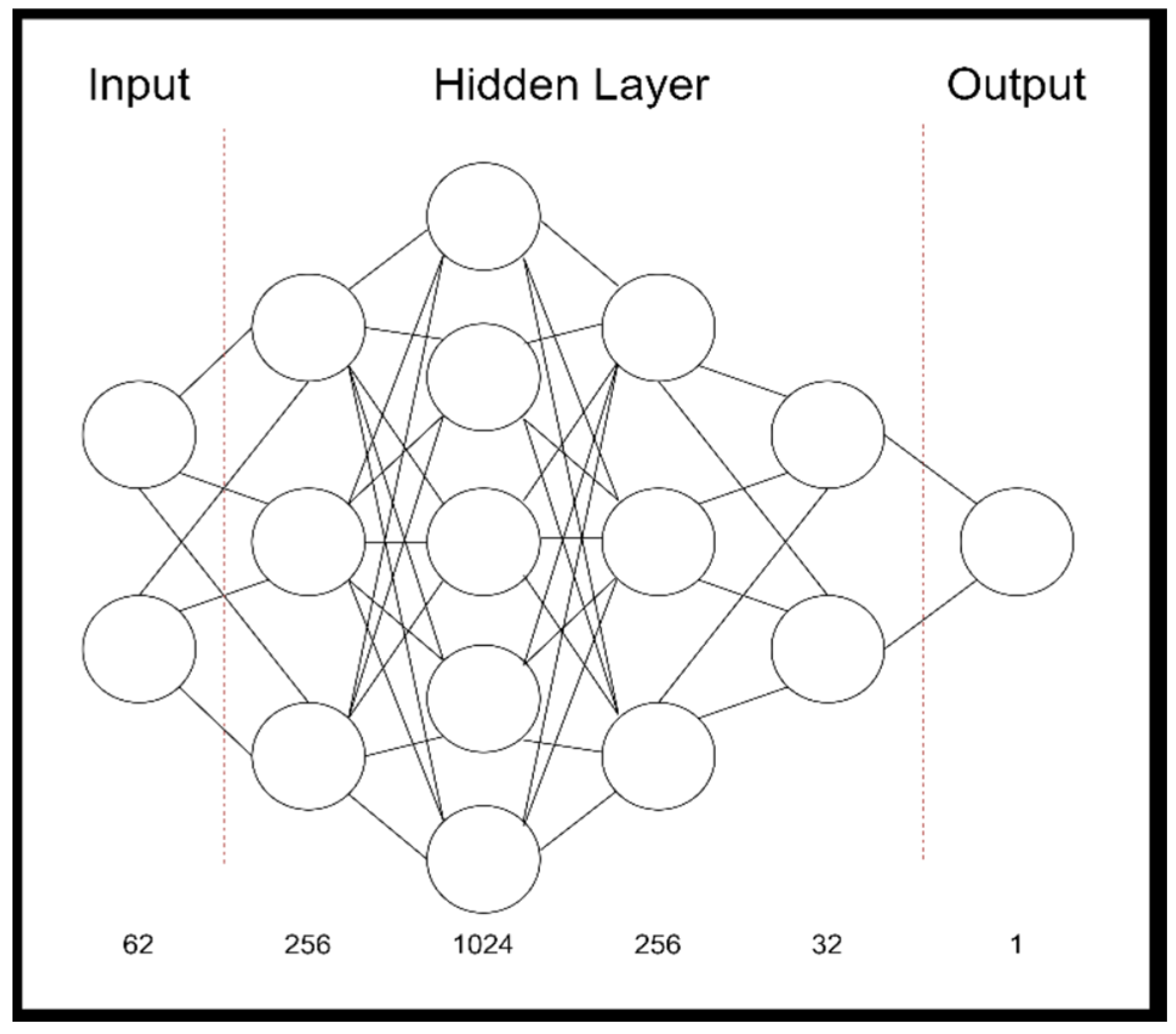
2.3. Model Development
2.4. Code Description
2.5. Outcome Parameter
3. Results
3.1. Demographic Data Characteristics
3.2. Algorithm Performance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.P.; Kao, K.; Ko, C.Y.; Farmer, D.G.; McDiarmid, S.V.; Hong, J.C.; Venick, R.S.; Feist, S.; Goldstein, L.; Saab, S.; et al. Long-term patient outcome and quality of life after liver transplantation: Analysis of 20-year survivors. Ann. Surg. 2010, 252, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Yeh, H. Liver transplantation equity: Supply, demand, and access. Am. J. Transplant. 2017, 17, 2759–2760. [Google Scholar] [CrossRef]
- Kamath, P.S.; Kim, W.R. The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef]
- Jasseron, C.; Francoz, C.; Antoine, C.; Legeai, C.; Durand, F.; Dharancy, S.; Collaborators; Jacquelinet, C.; Azoulay, D.; Duvoux, C.; et al. Impact of the new MELD-based allocation system on waiting list and post-transplant survival—A cohort analysis using the French national CRISTAL database. Transpl. Int. 2019, 32, 1061–1073. [Google Scholar] [CrossRef]
- Schoenberg, M.B. Objective and transparent allocation of postmortal livers for transplantation = Objektive und transparente Allokation von postmortalen Lebern zur Transplantation. Z. Med. Ethik 2022, 68, 109–127. [Google Scholar]
- Flores, A.; Asrani, S.K. The donor risk index: A decade of experience. Liver Transplant. 2017, 23, 1216–1225. [Google Scholar] [CrossRef]
- Rana, A.; Hardy, M.A.; Halazun, K.J.; Woodland, D.C.; Ratner, L.E.; Samstein, B.; Guarrera, J.V.; Brown, R.S., Jr.; Emond, J.C. Survival Outcomes Following Liver Transplantation (SOFT) Score: A Novel Method to Predict Patient Survival Following Liver Transplantation. Am. J. Transplant. 2008, 8, 2537–2546. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Slankamenac, K.; Puhan, M.A.; Schadde, E.; Müllhaupt, B.; Geier, A.; Clavien, P.A. Are There Better Guidelines for Allocation in Liver Transplantation?: A Novel Score Targeting Justice and Utility in the Model for End-Stage Liver Disease Era. Ann. Surg. 2011, 254, 745–754. [Google Scholar] [CrossRef]
- Halldorson, J.B.; Bakthavatsalam, R.; Fix, O.; Reyes, J.D.; Perkins, J.D. D-MELD, a Simple Predictor of Post Liver Transplant Mortality for Optimization of Donor/Recipient Matching. Am. J. Transplant. 2009, 9, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Linecker, M.; Kron, P.; Györi, G.; De Oliveira, M.L.; Müllhaupt, B.; Clavien, P.-A.; Dutkowski, P. Risk Assessment in High- and Low-MELD Liver Transplantation. Am. J. Transplant. 2017, 17, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Light, A.; Alaa, A.; Thurtle, D.; van der Schaar, M.; Gnanapragasam, V.J. Application of a novel machine learning framework for predicting non-metastatic prostate cancer-specific mortality in men using the Surveillance, Epidemiology, and End Results (SEER) database. Lancet Digit. Health 2021, 3, e158–e165. [Google Scholar] [CrossRef]
- Schoenberg, M.B.; Bucher, J.N.; Koch, D.; Börner, N.; Hesse, S.; De Toni, E.N.; Seidensticker, M.; Angele, M.K.; Klein, C.; Bazhin, A.V.; et al. A novel machine learning algorithm to predict disease free survival after resection of hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 434. [Google Scholar] [CrossRef]
- Harris, J.; Purssell, E.; Cornelius, V.; Ream, E.; Jones, A.; Armes, J. Development and internal validation of a predictive risk model for anxiety after completion of treatment for early stage breast cancer. J. Patient-Rep. Outcomes 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Health J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Wingfield, L.R.; Ceresa, C.; Thorogood, S.; Fleuriot, J.; Knight, S. Using Artificial Intelligence for Predicting Survival of Individual Grafts in Liver Transplantation: A Systematic Review. Liver Transplant. 2020, 26, 922–934. [Google Scholar] [CrossRef]
- Yoo, K.D.; Noh, J.; Lee, H.; Kim, D.K.; Lim, C.S.; Kim, Y.H.; Lee, J.P.; Kim, G.; Kim, Y.S. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: A Multicenter Cohort Study. Sci. Rep. 2017, 7, 8904. [Google Scholar] [CrossRef]
- Ayllón, M.D.; Ciria, R.; Cruz-Ramírez, M.; Pérez-Ortiz, M.; Gómez, I.; Valente, R.; O’Grady, J.; de la Mata, M.; Hervás-Martínez, C.; Heaton, N.D.; et al. Validation of artificial neural networks as a methodology for dono’ recipient matching for liver transplantation. Liver Transplant. 2018, 24, 192–203. [Google Scholar] [CrossRef]
- Ershoff, B.D.; Lee, C.K.; Wray, C.L.; Agopian, V.G.; Urban, G.; Baldi, P.; Cannesson, M. Training and Validation of Deep Neural Networks for the Prediction of 90-Day Post-Liver Transplant Mortality Using UNOS Registry Data. Transplant. Proc. 2020, 52, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Regulation, P. Regulation (EU) 2016/679 of the European Parliament and of the Council. Regulation 2016, 679, 2016. [Google Scholar]
- Boecker, J.; Czigany, Z.; Bednarsch, J.; Amygdalos, I.; Meister, F.; Santana, D.A.M.; Liu, W.-J.; Strnad, P.; Neumann, U.P.; Lurje, G. Potential value and limitations of different clinical scoring systems in the assessment of short- and long-term outcome following orthotopic liver transplantation. PLoS ONE 2019, 14, e0214221. [Google Scholar] [CrossRef] [PubMed]
- Elwir, S.; Lake, J. Current Status of Liver Allocation in the United States. Gastroenterol. Hepatol. 2016, 12, 166–170. [Google Scholar]
- Lau, L.; Kankanige, Y.; Rubinstein, B.; Jones, R.; Christophi, C.; Muralidharan, V.; Bailey, J. Machine-Learning Algorithms Predict Graft Failure After Liver Transplantation. Transplantation 2017, 101, e125–e132. [Google Scholar] [CrossRef] [PubMed]
- Childress, J.F. Putting patients first in organ allocation: An ethical analysis of the U.S. debate. Camb. Q. Healthc. Ethics 2001, 10, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Bertsimas, D.; Kung, J.; Trichakis, N.; Wang, Y.; Hirose, R.; Vagefi, P.A. Development and validation of an optimized prediction of mortality for candidates awaiting liver transplantation. Am. J. Transplant. 2019, 19, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M.; Ayloo, S.; Tsung, A.; Jorgensen, D.; Tevar, A.; Rahman, S.H.; Jonassaint, N. Prediction of Perioperative Mortality of Cadaveric Liver Transplant Recipients During Their Evaluations. Transplantation 2019, 103, e297–e307. [Google Scholar] [CrossRef]
| Characteristics | Study Cohort | Training Data | Test Data | Training vs. Test |
|---|---|---|---|---|
| n = 529 | n = 477 | n = 52 | p-Value | |
| Demographics | ||||
| Age at operation in years, mean ± SD | 50.28 ± 12.29 | 50.06 ± 12.46 | 52.31 ± 10.58 | 0.2113 |
| Male/Female | 357/172 | 318/159 | 39/13 | 0.2755 |
| Height (m), mean ± SD | 1.73 ± 0.10 | 1.73 ± 0.10 | 1.73 ± 0.09 | 0.9754 |
| Weight (kg), mean ± SD | 77.57 ± 16.39 | 77.79 ± 16.36 | 75.58 ± 16.66 | 0.3543 |
| BMI, mean ± SD | 25.67 ± 4.59 | 25.74 ± 4.57 | 25.03 ± 4.44 | 0.2903 |
| Liver Disease features | ||||
| Ascites, Y/N | 332/197 | 301/176 | 31/21 | 0.6518 |
| Encephalopathy, Y/N | 216/313 | 194/283 | 22/30 | 0.8822 |
| Dialysis, Y/N | 77/452 | 72/407 | 5/47 | 0.2921 |
| MELD, mean ± SD | 23.79 ± 11.08 | 23.86 ± 11.16 | 23.17 ± 10.50 | 0.6710 |
| Allocation MELD, mean ± SD | 27.75 ± 8.55 | 27.83± 8.66 | 27.15 ± 7.64 | 0.5912 |
| Laboratory Values | ||||
| Na mmol/L, mean ± SD | 135.98 ± 5.42 | 135.98 ± 5.43 | 135.98 ± 5.38 | 0.9983 |
| K mmol/L, mean ± SD | 4.10 ± 0.50 | 4.11 ± 0.49 | 3.95 ± 0.55 | 0.0268 |
| Bilirubin mg/dL, mean ± SD | 12.12 ± 13.56 | 12.02 ± 13.32 | 12.97 ± 15.83 | 0.6296 |
| Albumin g/L, mean ± SD | 3.15 ± 0.67 | 3.15 ± 0.68 | 3.16 ± 0.60 | 0.8627 |
| ALT U/L, mean ± SD | 328.94 ± 876.02 | 306 ± 829.33 | 421.81 ± 1023.03 | 0.0967 |
| AST U/L, mean ± SD | 454.85 ± 1318.16 | 389.63 ± 1125.18 | 684.92 ± 1854.65 | 0.3536 |
| GGT U/L, mean ± SD | 141.45 ± 186.77 | 140.23 ± 186.29 | 144.37 ± 189.98 | 0.8796 |
| AP U/L, mean ± SD | 231.38 ± 252.37 | 225.67 ± 251.54 | 246.48 ± 237.75 | 0.5693 |
| Haemoglobin g/dL, mean ± SD | 10.58 ± 2.50 | 10.60 ± 2.50 | 10.43 ± 2.47 | 0.6348 |
| INR, mean ± SD | 1.76 ± 0.90 | 1.77 ± 0.94 | 1.62 ± 0.51 | 0.2541 |
| Creatinine mg/dL, mean ± SD | 1.66 ± 1.16 | 1.65 ± 1.14 | 1.83 ± 1.30 | 0.2843 |
| CRP mg/dL, mean ± SD | 2.51 ± 3.58 | 2.50 ± 3.64 | 2.60 ± 3.09 | 0.8481 |
| Leukocytes 106/L, mean ± SD | 8.15 ± 6.47 | 8.22 ± 6.66 | 7.50 ± 4.37 | 0.4426 |
| Platelets 106/L, mean ± SD | 100.27 ± 74.17 | 100.49 ± 75.54 | 98.17 ± 60.68 | 0.8305 |
| Characteristic | Study Cohort | Training Data | Test Data | Training vs. Test |
|---|---|---|---|---|
| n = 529 | n = 477 | n = 52 | p-Value | |
| Cold ischemia time (min) ± SD | 630.69 ± 156.61 | 634.28 ± 159.66 | 597.77 ± 121.49 | 0.1104 |
| Full/split liver ± SD | 499/30 | 447/30 | 52/0 | 0.0607 |
| Distance explanation to transplantation (km) ± SD | 312.56 ± 210.99 | 328.52 ± 210.31 | 257.73 ± 208.38 | 0.0215 |
| Duration of stay (days) ± SD | 45.15 ± 39.87 | 44.79 ± 39.64 | 48.42 ± 42.13 | 0.5334 |
| Characteristics | Study Cohort | Training Data | Test Data | Training vs. Test |
|---|---|---|---|---|
| n = 529 | n = 477 | n = 52 | p-Value | |
| Demographics | ||||
| Age at operation in years, mean ± SD | 54.79 ± 16.27 | 54.68 ± 16.21 | 55.71 ± 16.87 | 0.6669 |
| Male/Female | 271/258 | 241/236 | 30/22 | 0.3814 |
| Height (m), mean ± SD | 1.72 ± 0.09 | 1.72 ± 0.09 | 1.73 ± 0.08 | 0.6766 |
| Weight (kg), mean ± SD | 77.81 ± 14.71 | 77.88 ± 14.94 | 77.21 ± 12.48 | 0.7545 |
| Donor reanimation | 136 (25.71%) | 122 (25.58%) | 14 (26.92%) | 0.2429 |
| Donor risk index | 1.98 ± 0.43 | 1.98 ± 0.44 | 1.82 ± 0.37 | 0.0095 |
| Laboratory Values | ||||
| Na mmol/L, mean ± SD | 147.9 ± 8.18 | 147.93 ± 8.10 | 147.60 ± 8.93 | 0.7803 |
| K mmol/L, mean ± SD | 4.2 ± 0.56 | 4.21 ± 0.57 | 4.10 ± 0.50 | 0.1627 |
| Bilirubin mg/dL, mean ± SD | 0.69 ± 0.4 | 0.69 ± 0.4 | 0.66 ± 0.43 | 0.6129 |
| Albumin g/L, mean ± SD | 27.86 ± 6.46 | 27.97 ± 6.44 | 26.82 ± 6.61 | 0.2242 |
| ALT U/L, mean ± SD | 65.72 ± 132.3 | 65.24 ± 137.34 | 59.98 ± 71.44 | 0.7855 |
| AST U/L, mean ± SD | 83.52 ± 135.27 | 82.84 ± 137.30 | 90.46 ± 115.87 | 0.7002 |
| GGT U/L, mean ± SD | 83.12 ± 123.16 | 85.01 ± 128.15 | 65.86 ± 57.50 | 0.2874 |
| AP U/L, mean ± SD | 87.83 ± 55.3 | 86.92 ± 54.14 | 96.20 ± 64.98 | 0.2506 |
| Haemoglobin g/dL, mean ± SD | 10.59 ± 2.3 | 10.58 ± 2.31 | 10.72 ± 2.20 | 0.6897 |
| INR, mean ± SD | 1.24 ± 0.53 | 1.24 ± 0.54 | 1.24 ± 0.43 | 0.9953 |
| Creatinine mg/dL, mean ± SD | 1.14 ± 0.87 | 1.15 ± 0.88 | 1.12 ± 0.73 | 0.8153 |
| CRP mg/dL, mean ± SD | 14.78 ± 10.72 | 14.78 ± 10.51 | 14.86 ± 12.64 | 0.9597 |
| Leukocytes 106/L, mean ± SD | 13.85 ± 5.95 | 13.84 ± 5.56 | 13.91 ± 8.79 | 0.9380 |
| Platelets 106/L, mean ± SD | 191.02 ± 103.2 | 189.69 ± 103.70 | 203.19 ± 98.59 | 0.3708 |
| Metric | Death | Death within 48 h | Death in Hospital | Death within 12 Months | Results |
|---|---|---|---|---|---|
| Accuracy | 99.226 | 94.3396 | 99.3396 | 98.113 | 95.745 |
| Cross-Entropy Loss | 0.0377 | 0.0566 | 0.0566 | 0.0189 | 0.0424 |
| F1 Score | 0.9412 | 0.842 | 0.842 | 0.9697 | 0.8988 |
| AUC Score | 0.9444 | 0.9217 | 0.9217 | 0.9706 | 0.9396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börner, N.; Schoenberg, M.B.; Pöschke, P.; Heiliger, C.; Jacob, S.; Koch, D.; Pöllmann, B.; Drefs, M.; Koliogiannis, D.; Böhm, C.; et al. A Novel Deep Learning Model as a Donor–Recipient Matching Tool to Predict Survival after Liver Transplantation. J. Clin. Med. 2022, 11, 6422. https://doi.org/10.3390/jcm11216422
Börner N, Schoenberg MB, Pöschke P, Heiliger C, Jacob S, Koch D, Pöllmann B, Drefs M, Koliogiannis D, Böhm C, et al. A Novel Deep Learning Model as a Donor–Recipient Matching Tool to Predict Survival after Liver Transplantation. Journal of Clinical Medicine. 2022; 11(21):6422. https://doi.org/10.3390/jcm11216422
Chicago/Turabian StyleBörner, Nikolaus, Markus B. Schoenberg, Philipp Pöschke, Christian Heiliger, Sven Jacob, Dominik Koch, Benedikt Pöllmann, Moritz Drefs, Dionysios Koliogiannis, Christian Böhm, and et al. 2022. "A Novel Deep Learning Model as a Donor–Recipient Matching Tool to Predict Survival after Liver Transplantation" Journal of Clinical Medicine 11, no. 21: 6422. https://doi.org/10.3390/jcm11216422
APA StyleBörner, N., Schoenberg, M. B., Pöschke, P., Heiliger, C., Jacob, S., Koch, D., Pöllmann, B., Drefs, M., Koliogiannis, D., Böhm, C., Karcz, K. W., Werner, J., & Guba, M. (2022). A Novel Deep Learning Model as a Donor–Recipient Matching Tool to Predict Survival after Liver Transplantation. Journal of Clinical Medicine, 11(21), 6422. https://doi.org/10.3390/jcm11216422






