Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Subsection
3.1.1. Characteristics of LTx Patients
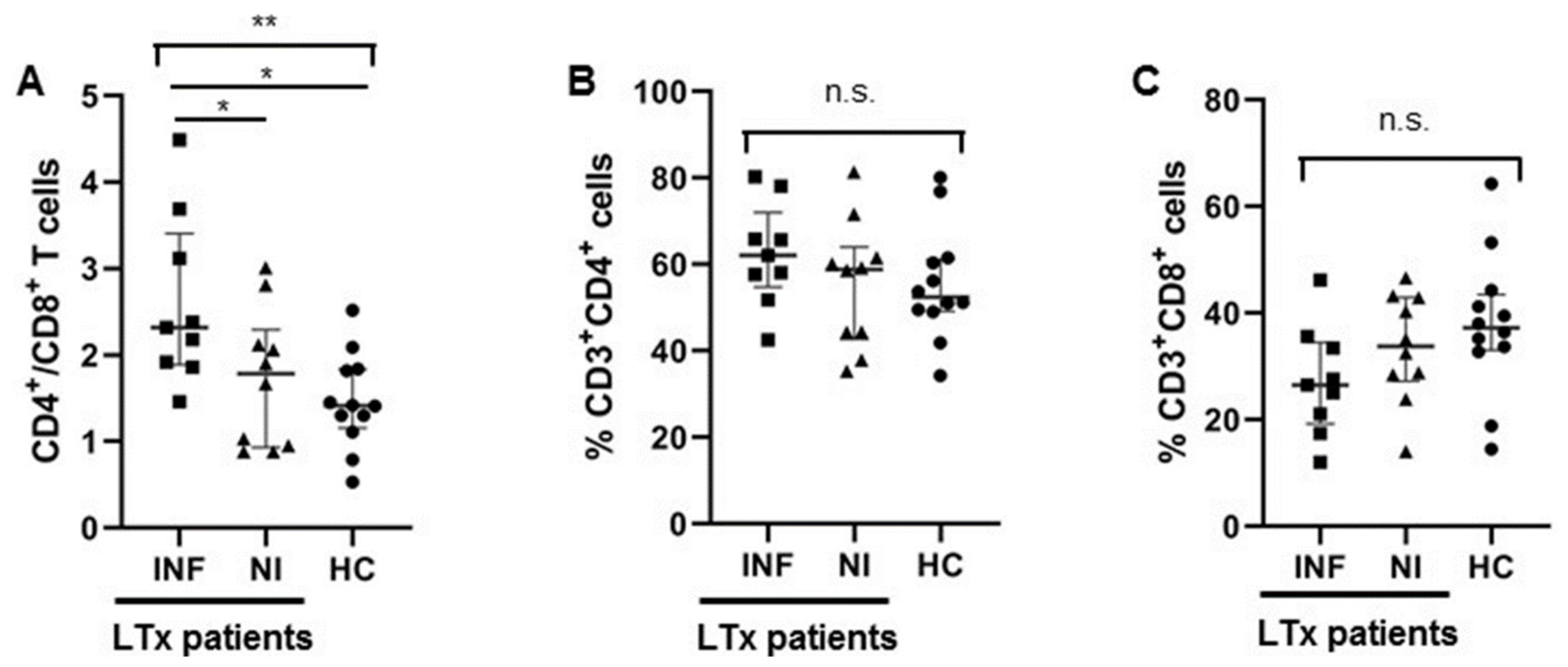
3.1.2. Higher CD4/CD8 Ratio in Transplant Recipients with Infections after LTx
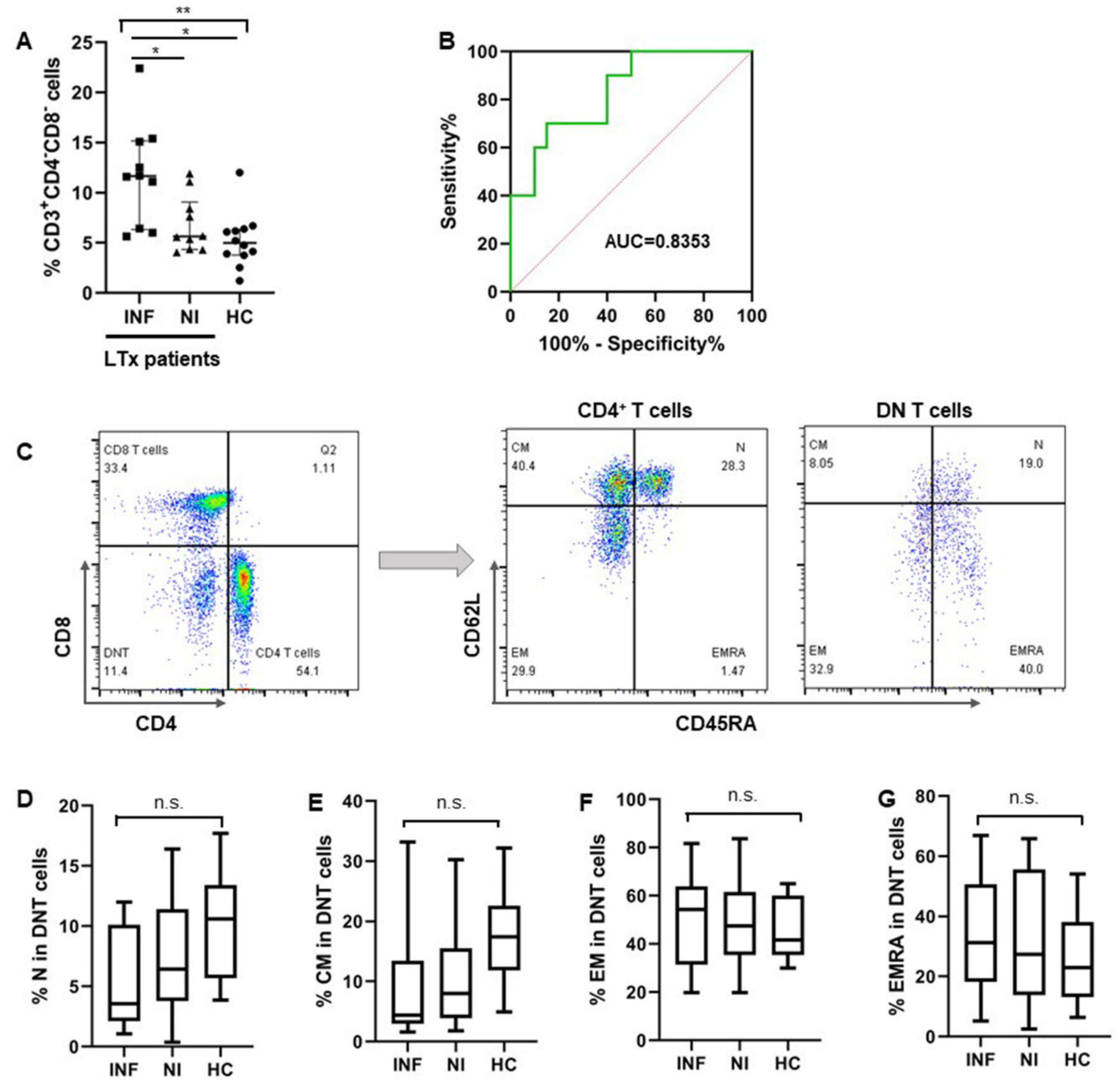
3.1.3. More DNT Cells Were Observed in Pre-LTx Patients Who Developed Post-LTx Infections
3.1.4. CD8+ T Cells Were Exhausted in Pre-LTx Patients Who Developed Post-Transplant Infections
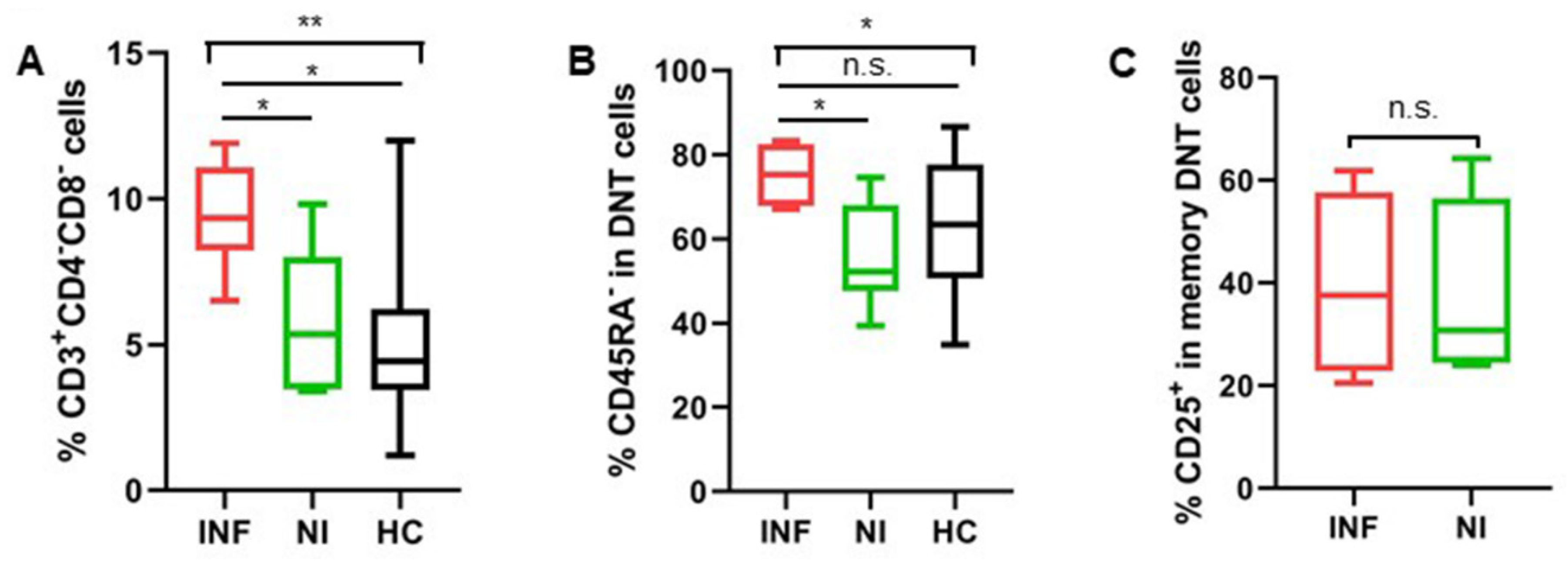
3.1.5. Increased DNT Cells Were Still Observed in Patients after LTx
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwong, A.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; Foutz, J.; Miller, E.; Snyder, J.J.; et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am. J. Transplant. 2020, 20, 193–299. [Google Scholar] [CrossRef] [PubMed]
- Lizaola-Mayo, B.C.; Rodriguez, E.A. Cytomegalovirus infection after liver transplantation. World J. Transplant. 2020, 10, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Scolarici, M.; Jorgenson, M.; Saddler, C.; Smith, J. Fungal Infections in Liver Transplant Recipients. J. Fungi 2021, 7, 524. [Google Scholar] [CrossRef]
- Watt, K.D.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Venugopal, R.; Vij, M.; Rela, M. Post liver transplant recurrent and de novo viral infections. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101689. [Google Scholar] [CrossRef] [PubMed]
- Achita, P.; Dervovic, D.; Ly, D.; Lee, J.B.; Haug, T.; Joe, B.; Hirano, N.; Zhang, L. Infusion of ex-vivo expanded human TCR-αβ+ double-negative regulatory T cells delays onset of xenogeneic graft-versus-host disease. Clin. Exp. Immunol. 2018, 193, 386–399. [Google Scholar] [CrossRef]
- Gagliotti, C.; Morsillo, F.; Moro, M.L.; Masiero, L.; Procaccio, F.; Vespasiano, F.; Pantosti, A.; Monaco, M.; Errico, G.; Ricci, A.; et al. Infections in liver and lung transplant recipients: A national prospective cohort. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Qiao, Y.; Yu, Z.; Huang, Y.; Yang, K.; He, T.; Zhao, J. T-Lymphocyte Subsets Alteration, Infection and Renal Outcome in Advanced Chronic Kidney Disease. Front. Med. 2021, 8, 742419. [Google Scholar] [CrossRef]
- Bortolotti, D.; Gentili, V.; Rotola, A.; Potena, L.; Rizzo, R. Soluble HLA-G pre-transplant levels to identify the risk for development of infection in heart transplant recipients. Hum. Immunol. 2020, 81, 147–150. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Parra, P.; López-Medrano, F.; Ruiz-Merlo, T.; González, E.; Polanco, N.; Origüen, J.; San Juan, R.; Andrés, A.; Aguado, J.M. Serum sCD30: A promising biomarker for predicting the risk of bacterial infection after kidney transplantation. Transpl. Infect. Dis. 2017, 19, e12668. [Google Scholar] [CrossRef]
- Carrasco-Antón, N.; Ibarra-Meneses, A.V.; Carrillo, E.; Fernández-Ruiz, M.; Hernández-Jiménez, P.; Aguado, J.M.; Moreno, J.; López-Medrano, F. An exploratory analysis of C-X-C motif chemokine ligand 10 as a new biomarker of asymptomatic Leishmania infantum infection in solid-organ transplant recipients. J. Infect. 2022, 84, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; Moore, L.W.; Li, X.C.; Mobley, C.M.; Fields, P.A.; Graviss, E.A.; Nguyen, D.T.; Nolte Fong, J.; Saharia, A.; Hobeika, M.J.; et al. Pre-transplant T-cell Clonality: An Observational Study of a Biomarker for Prediction of Sepsis in Liver Transplant Recipients. Ann. Surg. 2021, 274, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.R.; Ghobrial, R.M.; Kannanganat, S.; Minze, L.J.; Graviss, E.A.; Nguyen, D.T.; Perez, K.K.; Li, X.C. Longitudinal assessment of T cell inhibitory receptors in liver transplant recipients and their association with posttransplant infections. Am. J. Transplant. 2018, 18, 351–363. [Google Scholar] [CrossRef]
- Ono, K.; Ide, K.; Tanaka, Y.; Ohira, M.; Tahara, H.; Tanimine, N.; Yamane, H.; Ohdan, H. Molecular mismatch predicts T cell mediated rejection and de novo donor specific antibody formation after living-donor liver transplantation. Liver Transpl. 2021, 27, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Baygeldi, S.; Karakose, O.; Özcelik, K.C.; Pülat, H.; Damar, S.; Eken, H.; Zihni, İ.; Çalta, A.F.; Baç, B. Factors Affecting Morbidity in Solid Organ Injuries. Dis. Mark. 2016, 2016, 6954758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dieterlen, M.T.; John, K.; Bittner, H.B.; Mende, M.; Tarnok, A.; Mohr, F.W.; Barten, M.J. Assessment of Immunological Biomarkers in the First Year after Heart Transplantation. Dis. Mark. 2015, 2015, 678061. [Google Scholar] [CrossRef]
- Lei, H.; Tian, M.; Zhang, X.G.; Meng, L.S.; Zhu, W.H.; Liu, X.M.; Wang, M.Z.; Wang, T.; Chang, P.K.; Chen, H.; et al. Compromised immune status of patients with post-liver transplant biliary complications. Chin. Med. J. 2020, 133, 2622–2624. [Google Scholar] [CrossRef] [PubMed]
- Brandt, D.; Hedrich, C.M. TCRαβ+CD3+)CD4−CD8− (double negative) T cells in autoimmunity. Autoimmun. Rev. 2018, 17, 422–430. [Google Scholar] [CrossRef]
- Tian, D.; Yang, L.; Wang, S.; Zhu, Y.; Shi, W.; Zhang, C.; Jin, H.; Tian, Y.; Xu, H.; Sun, G.; et al. Double negative T cells mediate Lag3-dependent antigen-specific protection in allergic asthma. Nat. Commun. 2019, 10, 4246. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4−CD8− (Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yang, L.; Young, K.J.; DuTemple, B.; Zhang, L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat. Med. 2000, 6, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Ma, Y.; Wang, H.; Arp, J.; Jiang, J.; Huang, X.; He, K.M.; Garcia, B.; Madrenas, J.; Zhong, R. Double-negative T cells, activated by xenoantigen, lyse autologous B and T cells using a perforin/granzyme-dependent, Fas-Fas ligand-independent pathway. J. Immunol. 2006, 177, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Voelkl, S.; Heymann, J.; Przybylski, G.K.; Mondal, K.; Laumer, M.; Kunz-Schughart, L.; Schmidt, C.A.; Andreesen, R.; Mackensen, A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4−CD8− double-negative regulatory T cells. Blood 2005, 105, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, W.; Degauque, N.; Tian, Y.; Mikita, A.; Zheng, X.X. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood 2007, 109, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Maccari, M.E.; Fuchs, S.; Kury, P.; Andrieux, G.; Völkl, S.; Bengsch, B.; Lorenz, M.R.; Heeg, M.; Rohr, J.; Jägle, S.; et al. A distinct CD38+CD45RA+ population of CD4+, CD8+, and double-negative T cells is controlled by FAS. J. Exp. Med. 2021, 218, e20192191. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Li, Z.; Gao, J.; Shi, W.; Zhu, J.; Zhang, D. Ex vivo converted double negative T cells suppress activated B cells. Int. Immunopharmacol. 2014, 20, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; McIntyre, M.S.; Juvet, S.C.; Diao, J.; Li, X.; Vanama, R.B.; Mak, T.W.; Cattral, M.S.; Zhang, L. Regulation of antigen-expressing dendritic cells by double negative regulatory T cells. Eur. J. Immunol. 2011, 41, 2699–2708. [Google Scholar] [CrossRef]
- Su, Y.; Huang, X.; Wang, S.; Min, W.P.; Yin, Z.; Jevnikar, A.M.; Zhang, Z.X. Double negative Treg cells promote nonmyeloablative bone marrow chimerism by inducing T-cell clonal deletion and suppressing NK cell function. Eur. J. Immunol. 2012, 42, 1216–1225. [Google Scholar] [CrossRef]
- Ligocki, A.J.; Niederkorn, J.Y. Advances on Non-CD4+ Foxp3+ T Regulatory Cells: CD8+, Type 1, and Double Negative T Regulatory Cells in Organ Transplantation. Transplantation 2015, 99, 1553–1559. [Google Scholar] [CrossRef]
- Chowdhary, V.R.; Krogman, A.; Tilahun, A.Y.; Alexander, M.P.; David, C.S.; Rajagopalan, G. Concomitant Disruption of CD4 and CD8 Genes Facilitates the Development of Double Negative αβ TCR+ Peripheral T Cells That Respond Robustly to Staphylococcal Superantigen. J. Immunol. 2017, 198, 4413–4424. [Google Scholar] [CrossRef]
- Haug, T.; Aigner, M.; Peuser, M.M.; Strobl, C.D.; Hildner, K.; Mougiakakos, D.; Bruns, H.; Mackensen, A.; Völkl, S. Human Double-Negative Regulatory T-Cells Induce a Metabolic and Functional Switch in Effector T-Cells by Suppressing mTOR Activity. Front. Immunol. 2019, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.N.; Noel, S.; Saxena, A.; Bandapalle, S.; Majithia, R.; Jie, C.; Arend, L.J.; Allaf, M.E.; Rabb, H.; Hamad, A.R. Double-Negative αβ T Cells Are Early Responders to AKI and Are Found in Human Kidney. J. Am. Soc. Nephrol. 2016, 27, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Lv, T.T.; Zhang, C.P.; Wang, T.Q.; Tian, D.; Sun, G.Y.; Wang, Y.; Zhao, X.Y.; Duan, W.J.; Chen, S.; et al. Alteration of liver-infiltrated and peripheral blood double-negative T-cells in primary biliary cholangitis. Liver Int. 2019, 39, 1755–1767. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.; Tian, D.; Wang, S.; Guo, J.; Sun, G.; Jin, H.; Zhang, C.; Shi, W.; Gershwin, M.E.; et al. Transcriptome landscape of double negative T cells by single-cell RNA sequencing. J. Autoimmun. 2021, 121, 102653. [Google Scholar] [CrossRef]
- Lei, H.; Kuchenbecker, L.; Streitz, M.; Sawitzki, B.; Vogt, K.; Landwehr-Kenzel, S.; Millward, J.; Juelke, K.; Babel, N.; Neumann, A.; et al. Human CD45RA− FoxP3hi Memory-Type Regulatory T Cells Show Distinct TCR Repertoires With Conventional T Cells and Play an Important Role in Controlling Early Immune Activation. Am. J. Transplant. 2015, 15, 2625–2635. [Google Scholar] [CrossRef]
- Fukui, S.; Hidaka, M.; Fukui, S.; Morimoto, S.; Hara, T.; Soyama, A.; Adachi, T.; Matsushima, H.; Tanaka, T.; Fuchigami, M.; et al. The Contribution of Serum Complement Component 3 Levels to 90-Day Mortality in Living Donor Liver Transplantation. Front. Immunol. 2021, 12, 652677. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Wu, Y.; Feng, M.; Zhao, X.; Gao, C.; Guo, H.; Luo, J. Double-negative T cells are absolutely elevated in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis. Mol. Immunol. 2021, 132, 250–259. [Google Scholar] [CrossRef]
- Li, H.; Tsokos, G.C. Double-negative T cells in autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 163–172. [Google Scholar] [CrossRef]
- Cong, M.; Liu, T.; Tian, D.; Guo, H.; Wang, P.; Liu, K.; Lin, J.; Tian, Y.; Shi, W.; You, H.; et al. Interleukin-2 Enhances the Regulatory Functions of CD4+T Cell-Derived CD4−CD8− Double Negative T Cells. J. Interferon Cytokine Res. 2016, 36, 499–505. [Google Scholar] [CrossRef]
- Wang, Y.N.; Gao, Z.; Zhang, J.; Song, Y.; Jin, Z.L.; Wang, Z. Exhaustion of CD8+T Lymphocytes Plays a Critical Role in the Pathogenesis of Secondary Hemophagocytic Lymphohistiocytosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 963–968. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Wang, L.; Jia, X.; Lu, T.; Zeng, Y.; Liu, L.; Gao, Y. Follistatin-like 1 deficiency impairs T cell development to promote lung metastasis of triple negative breast cancer. Aging 2021, 13, 7211–7227. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, M.; Noel, S.; Lee, S.A.; Gong, J.; Allaf, M.E.; Pierorazio, P.; Rabb, H.; Hamad, A.R.A. Activation and Proliferation of PD-1+ Kidney Double-Negative T Cells Is Dependent on Nonclassical MHC Proteins and IL-2. J. Am. Soc. Nephrol. 2019, 30, 277–292. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Post-LTx Patients | HCs (n = 12) | p | |
|---|---|---|---|---|
| Infection after LTx (n = 9) | No Infection after LTx (n = 10) | |||
| Age (years), mean ± SD | 50.89 ± 10.89 | 44.90 ± 9.54 | 46.12 ± 10.92 | 0.33 |
| Gender, n | 0.78 | |||
| Female | 2 | 2 | 4 | |
| Male | 7 | 8 | 8 | |
| Primary liver disease, n | 0.94 | |||
| Hepatitis B | 5 | 9 | NA | |
| Hepatitis C | 2 | 1 | NA | |
| HCC | 4 | 5 | NA | |
| MELD scores, median (IQR) | 27.00 (26.00, 28.50) | 27.00 (24.50, 31.50) | NA | 0.97 |
| Cyclosporine A, median (IQR) | 525.0 (371.6, 617.1) | 539.5 (328.7, 713.7) | NA | 0.43 |
| Patient No. | Age (Y) | Gender | MELD Scores Pre LTx | Time of Infections after LTx (D) | Symptoms | Organisms Infected | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 61–65 | M | 27 | 32 | Fever, cough, | Klebsiella | piperacillin-tazobactam |
| 2 | 41–45 | F | 29 | 14 | Leukopenia, nausea, fever | Fungal infection; Staphylococcus | Caspofungin Acetate meropenem |
| 3 | 46–50 | M | 24 | 30 | Nausea, vomiting, abscess | Staphylococcus | Piperacillin-Tazobactam |
| 4 | 46–50 | M | 26 | 62 | Respiratory distress, fevers, cough | Strep pneumoniae | piperacillin-tazobactam |
| 5 | 31–35 | M | 28 | 10 | Fever, abdominal pain, nausea | E.coli sepsis | piperacillin-tazobactam |
| 6 | 61–65 | M | 27 | 92 | Hypopiesia, diarrhea, nausea | Staphylococcus; Fungal infection | Meropenem Caspofungin Acetate |
| 7 | 45–50 | M | 26 | 12, 58 | Fever, vomiting | E.coli UTI in combination with fungal ascites infection | piperacillin-tazobactam Caspofungin Acetate |
| 8 | 61–65 | F | 30 | 30, 122 | Fever, cough, pneumonia | Pseudomonas aeruginosa, influenza B viruses | Cefoperazone Sodium and Sulbactam Sodium, Oseltamivir |
| 9 | 56–60 | M | 27 | 51 | Nausea, vomiting, abscess | Staphylococcus; Fungal infection | Piperacillin-Tazobactam Caspofungin Acetate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Tian, M.; Zhang, X.; Liu, X.; Wang, B.; Wu, R.; Lv, Y. Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections. J. Clin. Med. 2022, 11, 3502. https://doi.org/10.3390/jcm11123502
Lei H, Tian M, Zhang X, Liu X, Wang B, Wu R, Lv Y. Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections. Journal of Clinical Medicine. 2022; 11(12):3502. https://doi.org/10.3390/jcm11123502
Chicago/Turabian StyleLei, Hong, Min Tian, Xiaogang Zhang, Xuemin Liu, Bo Wang, Rongqian Wu, and Yi Lv. 2022. "Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections" Journal of Clinical Medicine 11, no. 12: 3502. https://doi.org/10.3390/jcm11123502
APA StyleLei, H., Tian, M., Zhang, X., Liu, X., Wang, B., Wu, R., & Lv, Y. (2022). Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections. Journal of Clinical Medicine, 11(12), 3502. https://doi.org/10.3390/jcm11123502





