The First Evidence on the Occurrence of Bisphenol Analogues in the Aqueous Humor of Patients Undergoing Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Solvents
2.2. Sample Collection
2.3. Sample Preparation
2.4. LC-MS/MS
2.5. Validation
2.5.1. Calibration Standards and Quality Control Samples
2.5.2. Linearity
2.5.3. Precision and Accuracy
2.5.4. LOD, LOQ
2.5.5. Matrix Effects, Recovery
2.6. Statistical Analysis
3. Results
3.1. UHPLC–MS/MS Analysis
3.2. Validation Protocol
3.3. Concentrations of BPs in AH Samples
3.4. Comparison between the Specific Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caprioli, J. The ciliary epithelia and aqueous humor. In Adler’s Physiology of the Eye: Clinical Application, 9th ed.; Adler, F.H., Hart, W.M., Eds.; Mosby Year Book: St. Louis, MO, USA, 1992. [Google Scholar]
- Gum, G.G.; Gelatt, K.N.; Esson, D.W. Physiology of the eye. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Blackwell Pub.: Ames, IA, USA, 2007. [Google Scholar]
- Biros, D.J. Immunology and the Eye. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Blackwell Pub.: Ames, IA, USA, 2007. [Google Scholar]
- Kim, T.W.; Kang, J.W.; Ahn, J.; Lee, E.K.; Cho, K.C.; Han, B.N.; Hong, N.Y.; Park, J.; Kim, K.P. Proteomic analysis of the aqueous humor in age-related macular degeneration (AMD) patients. J. Proteome Res. 2012, 11, 4034–4043. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Kaur, J.; Sooraj, K.; Goswami, S.; Saxena, R.; Chauhan, V.S.; Sihota, R. Comparative evaluation of the aqueous humor proteome of primary angle closure and primary open angle glaucomas and age-related cataract eyes. Int. Ophthalmol. 2019, 39, 69–104. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Dolar-Szczasny, J.; Rejdak, R.; Majerek, D.; Tatarczak-Michalewska, M.; Proch, J.; Blicharska, E.; Flieger, W.; Baj, J.; Niedzielski, P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 9413. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rong, X.; Lu, Y. Metabolic characterization of human aqueous humor in the cataract progression after pars plana vitrectomy. BMC Ophthalmol. 2018, 18, 63. [Google Scholar] [CrossRef]
- Miric, D.; Kisic, B.; Zoric, L.; Miric, B.M.; Mirkovic, M.; Mitic, R. Influence of cataract maturity on aqueous humor lipid peroxidation markers and antioxidant enzymes. Eye 2014, 28, 72–77. [Google Scholar] [CrossRef]
- Flieger, J.; Święch-Zubilewicz, A.; Śniegocki, T.; Dolar-Szczasny, J.; Pizoń, M. Determination of Tryptophan and Its Major Metabolites in Fluid from the Anterior Chamber of the Eye in Diabetic Patients with Cataract by Liquid Chromotography Mass Spectrometry (LC-MS/MS). Molecules 2018, 23, 3012. [Google Scholar] [CrossRef]
- Chowdhury, U.R.; Madden, B.J.; Charlesworth, M.C.; Fautsch, M.P. Proteome analysis of human aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4921–4931. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Flieger, J.; Kowalska, B.; Majerek, D.; Tatarczak-Michalewska, M.; Zakrocka, I.; Załuska, W.; Rejdak, R. Hemodialysis Effect on the Composition of the Eye Fluid of Cataract Patients. J. Clin. Med. 2021, 10, 5485. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed]
- Makinwa, T.; Uadia, P. A survey of the level of bisphenol A (BPA) in effluents soil leachates, food samples, drinking water and consumer products in south western Nigeria. World Environ. 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Wang, H.; Chang, L.; Aguilar, J.S.; Dong, S.; Hong, Y. Bisphenol-A exposure induced neurotoxicity in glutamatergic neurons derived from human embryonic stem cells. Environ. Int. 2019, 127, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; Welshons, W.V.; Swan, S.H. Bisphenol A data in Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009, 117, 784–789. [Google Scholar] [CrossRef]
- Elsworth, J.D.; Jentsch, J.D.; Groman, S.M.; Roth, R.H.; Redmond, E.D., Jr.; Leranth, C. Low circulating levels of bisphenol-A induce cognitive deficits and loss of asymmetric spine synapses in dorsolateral prefrontal cortex and hippocampus of adult male monkeys. J. Comp. Neurol. 2015, 523, 1248–1257. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Han, H.; Luo, G.; Zhou, B.; Wang, S.; Wang, J. Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of Akt and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology 2016, 341–343, 56–64. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Brock, J.W.; Makino, T.; Nakazawa, H. Measurement of bisphenol A in human serum by gas chromatography/mass spectrometry. Anal. Chim. Acta 2002, 458, 331–336. [Google Scholar] [CrossRef]
- Wan, H.T.; Leung, P.Y.; Zhao, Y.G.; Wei, X.; Wong, M.H.; Wong, C.K.C. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J. Hazard. Mater. 2013, 261, 763–769. [Google Scholar] [CrossRef]
- Kosarac, I.; Kubwabo, C.; Lalonde, K.; Foster, W. A novel method for the quantitative determination of free and conjugated bisphenol A in human maternal and umbilical cord blood serum using a two-step solid phase extraction and gas chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 898, 90–94. [Google Scholar] [CrossRef]
- Kim, A.; Li, C.R.; Jin, C.F.; Lee, K.W.; Lee, S.H.; Shon, K.J.; Park, N.G.; Kim, D.K.; Kang, S.W.; Shim, Y.B.; et al. A sensitive and reliable quantification method for Bisphenol A based on modified competitive ELISA method. Chemosphere 2007, 68, 1204–1209. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Groshart, C.; Okkerman, P.; Pijnenburg, A. Chemical Study on Bisphenol A; RIKZ/1001.027; Rijkswaterstaad, RIKZ: Utrecht, The Netherlands, 2001. [Google Scholar]
- Aristiaawan, Y.; Aryana, N.P.D.; Styarini, D. Analytical Method Development for Bisphenol A in Tuna by Using High Performance Liquid Chromatography-UV. Procedia Chem. 2015, 16, 202–208. [Google Scholar] [CrossRef]
- Kuroda, N.; Kinoshita, Y.; Sun, Y.; Wada, M.; Kishikawa, N.; Nakashima, K.; Makino, T.; Nakazawa, H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003, 30, 1743–1749. [Google Scholar] [CrossRef]
- Inoue, K.; Kato, K.; Yoshimura, Y.; Makino, T.; Nakazawa, H. Determination of bisphenol A in human serum by high-performance liquid chromatography with multi-electrode electrochemical detection. J. Chromatogr. B Biomed. Sci Appl. 2000, 749, 17–23. [Google Scholar] [CrossRef]
- Khedr, A. Optimized extraction method for LC-MS determination of bisphenol A, melamine and di(2-ethylhexyl) phthalate in selected soft drinks, syringes, and milk powder. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 930, 98–103. [Google Scholar] [CrossRef]
- Alabi, A.; Caballero-Casero, N.; Rubio, S. Development and validation of a stable-isotope dilution liquid chromatography–tandem mass spectrometry method for the determination of bisphenols in ready-made meals. J. Chromatogr. A. 2014, 1336, 23–33. [Google Scholar] [CrossRef]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009, 23, 1186–1190. [Google Scholar] [CrossRef]
- Gyllenhammar, I.; Tröger, R.; Glynn, A.; Rosén, J.; Hellenäs, K.E.; Lignell, S. Serum levels of unconjugated bisphenol A are below 0.2ng/ml in Swedish nursing women when contamination is minimized. Environ. Int. 2014, 64, 56–60. [Google Scholar] [CrossRef]
- Servaes, K.; Voorspoels, S.; Lievens, J.; Noten, B.; Allaerts, K.; Van De Weghe, H.; Vanermen, G. Direct analysis of phthalate ester biomarkers in urine without preconcentration: Method validation and monitoring. J. Chromatogr. A. 2013, 1294, 25–32. [Google Scholar] [CrossRef]
- Geens, T.; Bruckers, L.; Covaci, A.; Schoeters, G.; Fierens, T.; Sioen, I.; Vanermen, G.; Baeyens, W.; Morrens, B.; Loots, I.; et al. Determinants of bisphenol A and phthalate metabolites in urine of Flemish adolescents. Environ. Res. 2014, 134, 110–117. [Google Scholar] [CrossRef]
- Provencher, G.; Bérubé, R.; Dumas, P.; Bienvenu, J.F.; Gaudreau, E.; Bélanger, P.; Ayotte, P. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2014, 1348, 97–104. [Google Scholar] [CrossRef]
- Zhou, X.; Kramer, J.P.; Calafat, A.M.; Ye, X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 944, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 831, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Buscher, B.; van de Lagemaat, D.; Gries, W.; Beyer, D.; Markham, D.A.; Budinsky, R.A.; Dimond, S.S.; Nath, R.V.; Snyder, S.A.; Hentges, S.G. Quantitative analysis of unconjugated and total bisphenol A in human urine using solid-phase extraction and UPLC-MS/MS: Method implementation, method qualification and troubleshooting. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1005, 30–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazdinezhad, S.R.; Ballesteros-Gómez, A.; Lunar, L.; Rubio, S. Single-step extraction and cleanup of bisphenol A in soft drinks by hemimicellar magnetic solid phase extraction prior to liquid chromatography/tandem mass spectrometry. Anal. Chim. Acta 2013, 778, 31–37. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Song, C.; You, J. Determination of Bisphenol A and Alkylphenols in Soft Drinks by High-Performance Liquid Chromatography with Fluorescence Detection. Food Anal. Method 2013, 6, 1284–1290. [Google Scholar] [CrossRef]
- Cunha, S.C.; Cunha, C.; Ferreira, A.R.; Fernandes, J.O. Determination of bisphenol A and bisphenol B in canned seafood combining QuEChERS extraction with dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 404, 2453–2463. [Google Scholar] [CrossRef]
- Tuzimski, T.; Szubartowski, S. Application of Solid-Phase Extraction and High-Performance Liquid Chromatography with Fluorescence Detection to Analyze Eleven Bisphenols in Amniotic Fluid Samples Collected during Amniocentesis. Int. J. Environ. Res. Public Health 2022, 19, 2309. [Google Scholar] [CrossRef]
- Philippat, C.; Wolff, M.S.; Calafat, A.M.; Ye, X.; Bausell, R.; Meadows, M.; Stone, J.; Slama, R.; Engel, S.M. Prenatal exposure to environmental phenols: Concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ. Health Perspect. 2013, 121, 1225–1231. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Zhu, H.; Huang, X.; Bai, X.; Kannan, K.; Zhang, T. Concentrations of bisphenol A and its alternatives in paired maternal–fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ. Int. 2020, 136, 105407. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Szubartowski, S. Method Development for Selected Bisphenols Analysis in Sweetened Condensed Milk from a Can and Breast Milk Samples by HPLC–DAD and HPLC-QqQ-MS: Comparison of Sorbents (Z-SEP, Z-SEP Plus, PSA, C18, Chitin and EMR-Lipid) for Clean-Up of QuEChERS Extract. Molecules 2019, 24, 2093. [Google Scholar] [CrossRef] [PubMed]
- Karnam, S.S.; Ghosh, R.C.; Mondal, S.; Mondal, M. Evaluation of subacute bisphenol—A toxicity on male reproductive system. Vet. World 2015, 8, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Y.; Fu, C.; Wang, H.; Huang, P.; Wang, B.; Su, M.; Jiang, F.; Fang, H.; Zhao, Q.; et al. Influence of Bisphenol A on Thyroid Volume and Structure Independent of Iodine in School Children. PLoS ONE 2015, 10, e0141248. [Google Scholar] [CrossRef] [PubMed]
- Teppala, S.; Madhavan, S.; Shankar, A. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int. J. Endocrinol. 2012, 2012, 598180. [Google Scholar] [CrossRef]
- Han, C.; Hong, Y.C. Bisphenol A, Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef]
- USEPA-IRIS; Bisphenol, A. Fuseaction Ľiris.Show Quickview & Substance_nmbrĽ0356. Available online: http://cfpub.epa.gov/ncea/iris/index.cfm? (accessed on 26 October 1988).
- Gyllenhammar, I.; Glynn, A.; Darnerud, P.O.; Lignell, S.; van Delft, R.; Aune, M. 4-Nonylphenol and bisphenol A in Swedish food and exposure in Swedish nursing women. Environ. Int. 2012, 43, 21–28. [Google Scholar] [CrossRef]
- Toxicological and Health Aspects of Bisphenol A, Report of Joint FAO/WHO Expert Meeting 2–5 November 2010 Ottawa, Canada; World Health Organization Press: Geneva, Switzerland, 2011.
- Bae, S.; Hong, Y.C. Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension 2015, 65, 313–319. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Akintola, T.E.; Hammed, M.O.; Amoo, C.O.; Adegoke, A.M.; Ajisafe, L.O. Naringin protects against Bisphenol-A induced oculopathy as implication of cataract in hypertensive rat model. Biomed. Pharmacother. 2020, 126, 110043. [Google Scholar] [CrossRef]
- Xu, G.; Hu, F.; Wang, X.; Zhang, B.; Zhou, Y. Bisphenol A exposure perturbs visual function of adult cats by remodeling the neuronal activity in the primary visual pathway. Arch. Toxicol. 2018, 92, 455–468. [Google Scholar] [CrossRef]
- Iwakura, T.; Iwafuchi, M.; Muraoka, D.; Yokosuka, M.; Shiga, T.; Watanabe, C.; Ohtani-Kaneko, R. In vitro effects of bisphenol A on developing hypothalamic neurons. Toxicology 2010, 272, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vara, H.; Onofri, F.; Benfenati, F.; Sassoè-Pognetto, M.; Giustetto, M. ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc. Natl. Acad. Sci. USA 2009, 106, 9872–9877. [Google Scholar] [CrossRef] [PubMed]
- ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf (accessed on 25 June 2016).
- Pharmaceutical Quality/CMC. Guidance for Industry, Analytical Procedures and Methods Validation for Drugs and Biologics, US Department of Health and Human Services, Food and Drug Administration. Available online: http://www.fda.gov/download/drug/guidancecomplianceregulatoryinformation/.guidances/ucm386366.pdf (accessed on 25 June 2016).
- Chan, C.C.; Lam, H.; Lee, Y.C.; Zhang, X.-M. Analytical Method Validation and Instrument Performance Verification; Wiley-Interscience: New York, NY, USA, 2004. [Google Scholar]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory: A Practical Approach, 1st ed.; Analytical Chemistry Series; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Milton Park/Abingdon, UK, 2009. [Google Scholar]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory: A Practical Approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 31 March 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ggstatsplot approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 13 February 2021).
- Sjoberg, D.D.; Curry, M.; Hannum, M.; Larmarange, J.; Whiting, K.; Zabor, E.C.; Drill, E.; Flynn, J.; Lavery, J.; Lobaugh, S.; et al. Gtsummary: Presentation-Ready Data Summary and Analytic Result Tables. 2021. Available online: https://CRAN.R-project.org/package=gtsummary (accessed on 21 June 2021).
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Neuhäuser, M. Wilcoxon–Mann–Whitney Test. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1656–1658. [Google Scholar] [CrossRef]
- Wilczewska, K.; Namieśnik, J.; Wasik, A. Troubleshooting of the determination of bisphenol A at ultra-trace levels by liquid chromatography and tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 1009–1013. [Google Scholar] [CrossRef]
- Śniegocki, T. Liquid chromatography–mass spectrometry analysis of carvacrol in chicken tissues. J. Vet. Res. 2022, 66, 225–233. [Google Scholar] [CrossRef]
- Arias, J.L.O.; Schneider, A.; Andrade, J.A.B.; Vieira, A.A.; Gehrke, V.R.; Camargo, E.R.; Caldas, S.S.; Primel, E.G. Evaluation of dilute-and-shoot and solid-phase extraction methods for the determination of S-metolachlor and metolachlor-OA in runoff water samples by liquid chromatography tandem mass spectrometry. Anal. Methods 2017, 9, 5777–5783. [Google Scholar] [CrossRef]
- He, X.; Kozak, M. Simultaneous Quantitation of 43 Drugs in Human Urine with a “Dilute-and-Shoot” LC-MS/MS Method; Application Note 576; Thermo Fisher Scientific: San Jose, CA, USA, 2012. [Google Scholar]
- Liu, J.; Li, J.; Wu, Y.; Zhao, Y.; Luo, F.; Li, S.; Yang, L.; Moez, E.K.; Dinu, I.; Martin, J.W. Bisphenol A Metabolites and Bisphenol S in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2017, 51, 2456–2463. [Google Scholar] [CrossRef]
- Owczarek, K.; Kubica, P.; Kudłak, B.; Rutkowska, A.; Konieczna, A.; Rachoń, D.; Namieśnik, J.; Wasik, A. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography-tandem mass spectrometry. Sci. Total. Environ. 2018, 628–629, 1362–1368. [Google Scholar] [CrossRef]
- Tan, D.; Jin, J.; Wang, L.; Zhao, X.; Guo, C.; Sun, X.; Dhanjai; Lu, X.; Chen, J. Ammonium hydroxide enhancing electrospray response and boosting sensitivity of bisphenol A and its analogs. Talanta 2018, 182, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Markham, D.A.; Waechter, J.M.J.; Wimber, M.; Rao, N.; Connolly, P.; Chuang, J.C.; Hentges, S.; Shiotsuka, R.N.; Dimond, S.; Chappelle, A.H. Development of a method for the determination of bisphenol A at trace concentrations in human blood and urine and elucidation of factors influencing method accuracy and sensitivity. J. Anal. Toxicol. 2010, 34, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, W.; Yao, L.; Zhao, N.; Zhang, Y.; Zhao, M.; Jin, H. First report on occurrence of bisphenol A isomers in human serum and whole blood. J. Hazard. Mater. 2022, 424 Pt C, 127549. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Farouk, A.A.; Mogbojuri, O. Metabolic treatment of syndrome linked with Parkinson’s disease and hypothalamus pituitary gonadal hormones by turmeric curcumin in Bisphenol-A induced neuro-testicular dysfunction of wistar rat. Biochem. Biophys. Rep. 2018, 17, 97–107. [Google Scholar] [CrossRef]
- Svitok, P.; Senko, T.; Panakova, Z.; Olexova, L.; Krskova, L.; Okuliarova, M.; Zeman, M. Prenatal exposure to angiotensin II increases blood pressure and decreases salt sensitivity in rats. Clin. Exp. Hypertens. 2017, 39, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Khayat, M.T.; Nayeem, M.A. The role of Adenosine A2A receptor, CYP450s, and PPARs in the regulation of vascular tone. Biomed. Res. Int. 2017, 2017, 1720920. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- Olabiyi, A.A.; Carvalho, F.B.; Bottari, N.B.; Lopes, T.F.; da Costa, P.; Stefanelo, N.; Morsch, V.M.; Akindahunsi, A.A.; Oboh, G.; Schetinger, M.R. Dietary supplementation of tiger nut alters biochemical parameters relevant to erectile function in l-NAME treated rats. Food Res. Int. 2018, 109, 358–367. [Google Scholar] [CrossRef]
- Bozkurt, B.; Okudan, N.; Belviranli, M.; Oflaz, A.B. The evaluation of intraocular pressure fluctuation in glaucoma subjects during submaximal exercise using an ocular telemetry sensor. Indian J. Ophthalmol. 2019, 67, 89–94. [Google Scholar] [CrossRef]
- Kaski, D.; Bronstein, A.M. Ocular Tremor in Parkinson’s Disease: Discussion, Debate, and Controversy. Front. Neurol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Kehinde, A.J. Functional Oil from Black Seed Differentially Inhibits Aldose-reductase and Ectonucleotidase Activities by Up-regulating Cellular Energy in Haloperidol-induced Hepatic Toxicity in Rat Liver. J. Oleo. Sci. 2017, 66, 1051–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kutryb-Zajac, B.; Mateuszuk, L.; Zukowska, P.; Jasztal, A.; Zabielska, M.A.; Toczek, M.; Jablonska, P.; Zakrzewska, A.; Sitek, B.; Rogowski, J.; et al. Increased activity of vascular adenosine deaminase in atherosclerosis and therapeutic potential of its inhibition. Cardiovasc. Res. 2016, 112, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.N.; Sharma, N.; Hallén-Grufman, K.; Jager, E.W.H.; Svennersten, K. The role of ATP signalling in response to mechanical stimulation studied in T24 cells using new microphysiological tools. J. Cell Mol. Med. 2018, 22, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, J.K.; Irondi, A.E.; Ajani, E.O.; Olayemi, T.V. Neuroprotective effect of dietary black seed flour on key enzymes linked with neuronal signaling molecules in rats exposed to mixture of environmental metals. J. Food Biochem. 2018, 42, e12573. [Google Scholar] [CrossRef]
- Velázquez, F.E.; Anastasiou, M.; Carrillo-Salinas, F.J.; Ngwenyama, N.; Salvador, A.M.; Nevers, T.; Alcaide, P. Sialomucin CD43 regulates T helper type 17 cell intercellular adhesion molecule 1 dependent adhesion, apical migration and transendothelial migration. Immunology 2019, 157, 52–69. [Google Scholar] [CrossRef]
- Mangraviti, A.; Raghavan, T.; Volpin, F.; Skuli, N.; Gullotti, D.; Zhou, J.; Asnaghi, L.; Sankey, E.; Liu, A.; Wang, Y.; et al. HIF-1α- Targeting Acriflavine Provides Long Term Survival and Radiological Tumor Response in Brain Cancer Therapy. Sci. Rep. 2017, 7, 14978. [Google Scholar] [CrossRef]
- Jukić, T.; Katusic, D.; Kordić, R.; Cacić, M.; Braunschweig, T.; Thumann, G. Malignes Melanom der Kornea nach stumpfem Trauma [Malignant melanoma of the cornea after blunt trauma]. Ophthalmologe 2009, 106, 625–627. (In German) [Google Scholar] [CrossRef]
- Tolone, A.; Belhadj, S.; Rentsch, A.; Schwede, F.; Paquet-Durand, F. The cGMP Pathway and Inherited Photoreceptor Degeneration: Targets, Compounds, and Biomarkers. Genes 2019, 10, 453. [Google Scholar] [CrossRef]
- Chtourou, Y.; Aouey, B.; Aroui, S.; Kebieche, M.; Fetoui, H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem. Biol. Interact. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Tuscher, J.J.; Luine, V.; Frankfurt, M.; Frick, K.M. Estradiol-Mediated Spine Changes in the Dorsal Hippocampus and Medial Prefrontal Cortex of Ovariectomized Female Mice Depend on ERK and mTOR Activation in the Dorsal Hippocampus. J. Neurosci. 2016, 36, 1483–1489. [Google Scholar] [CrossRef]
- Mangiamele, L.A.; Gomez, J.R.; Curtis, N.J.; Thompson, R.R. GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J. Comp. Neurol. 2017, 525, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.K.; Tremere, L.A.; Burrows, K.; Majewska, A.K.; Pinaud, R. The mouse primary visual cortex is a site of production and sensitivity to estrogens. PLoS ONE 2011, 6, e20400. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.A.; Opanashuk, L.A.; Majewska, A.K. The effects of postnatal exposure to low-dose bisphenol-A on activity-dependent plasticity in the mouse sensory cortex. Front. Neuroanat. 2014, 8, 117. [Google Scholar] [CrossRef] [PubMed]
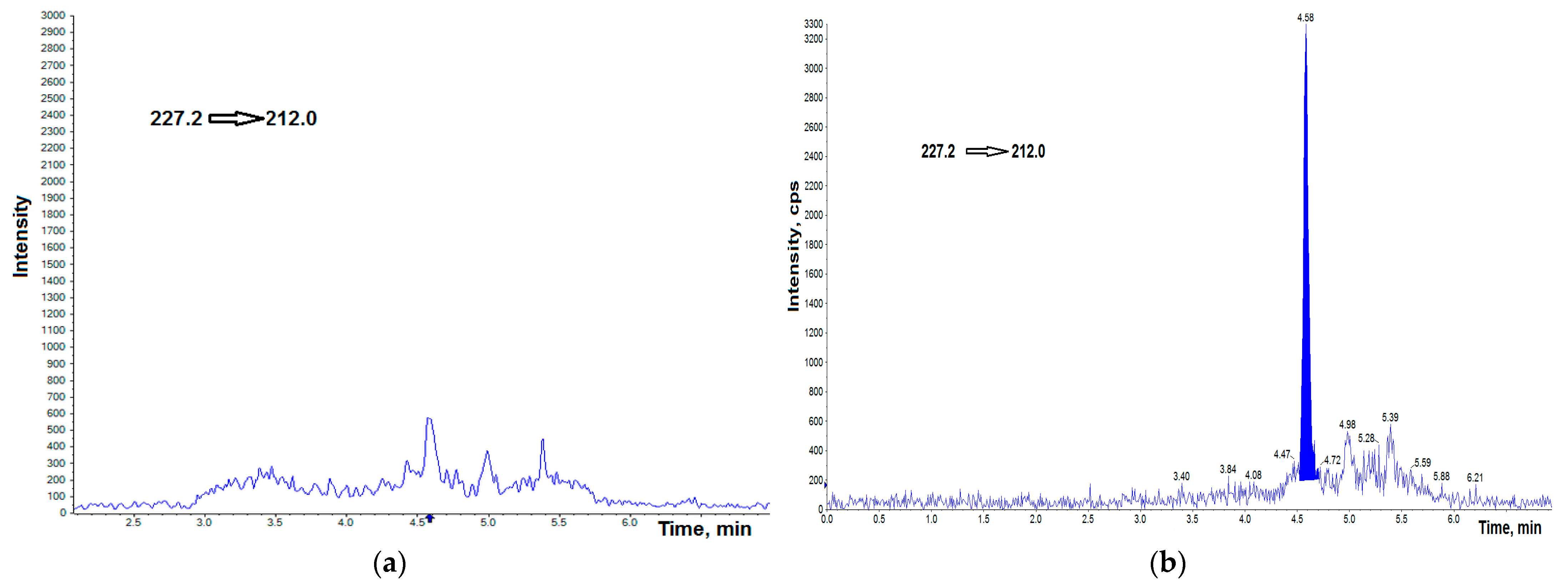
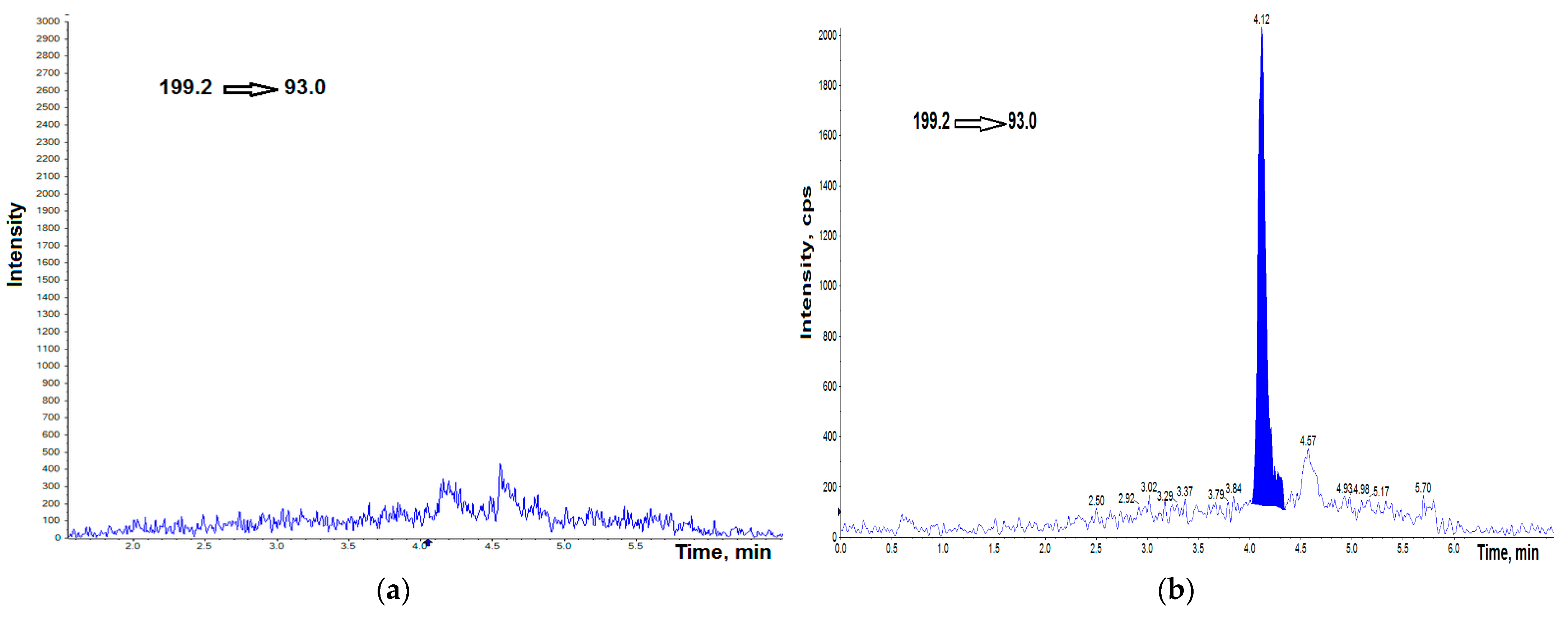

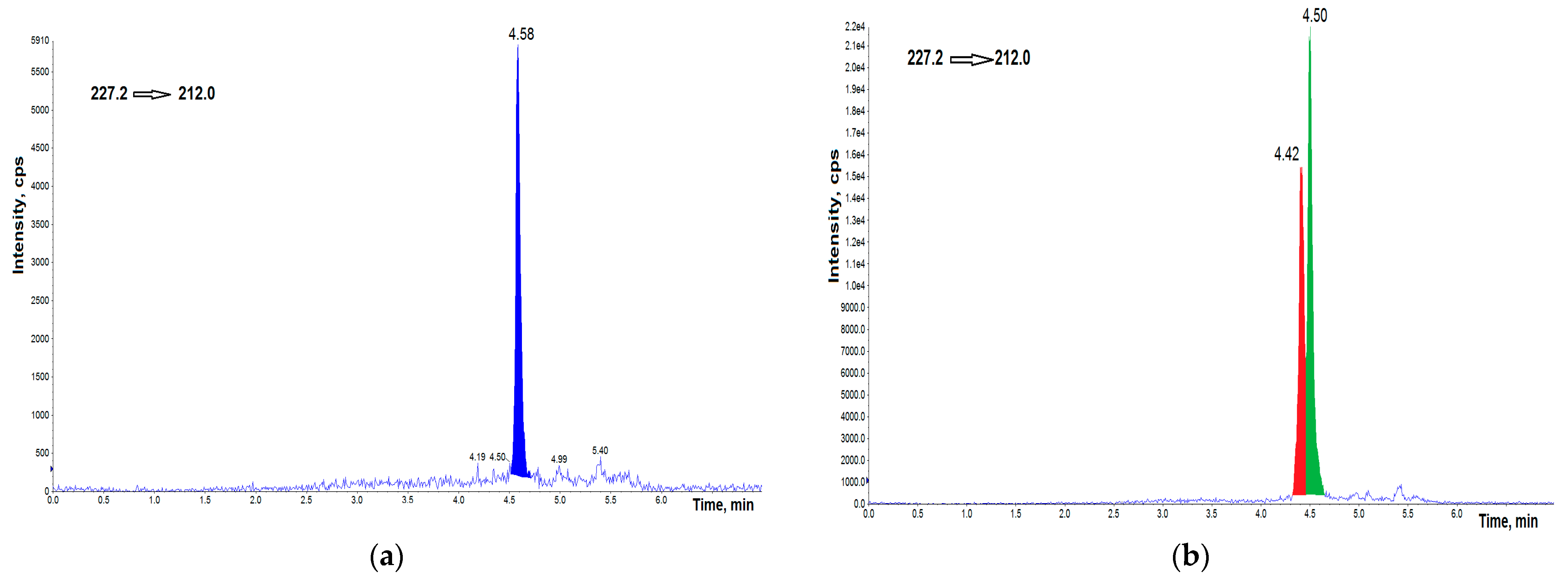
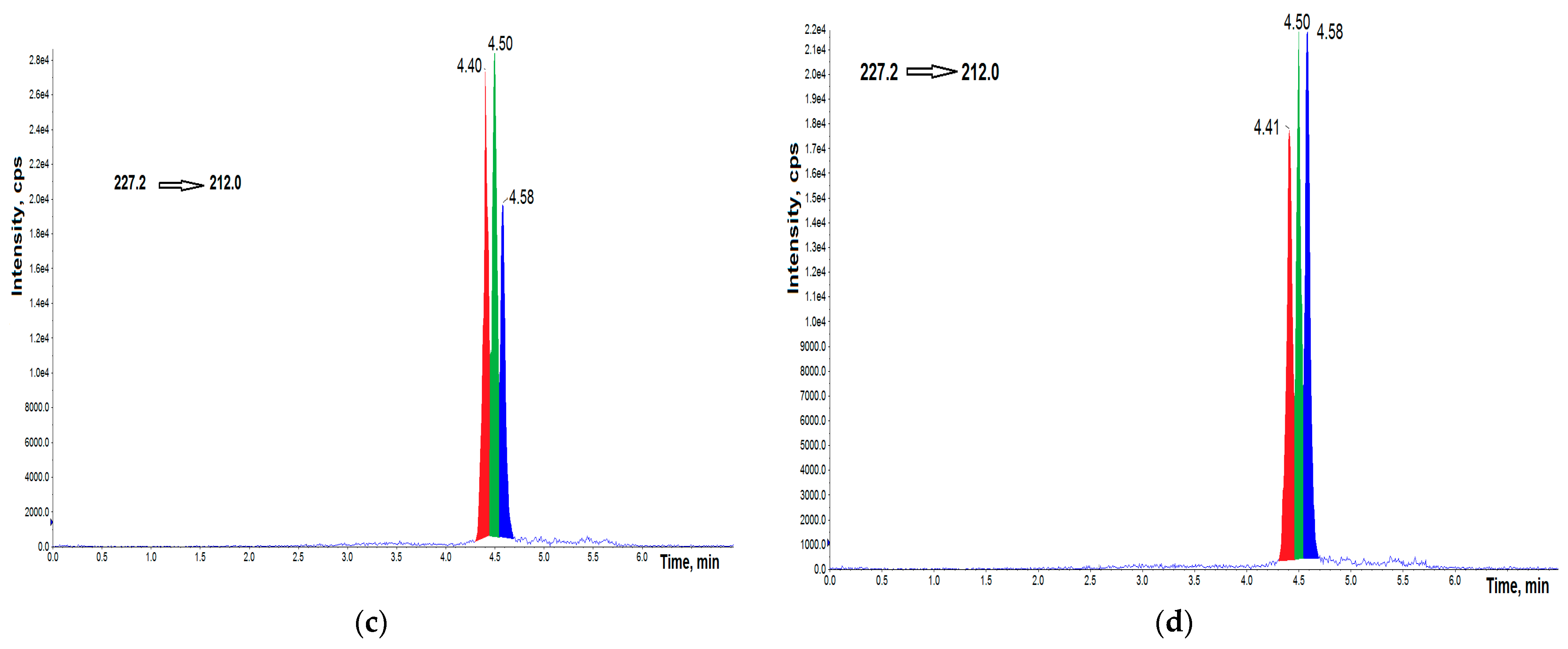
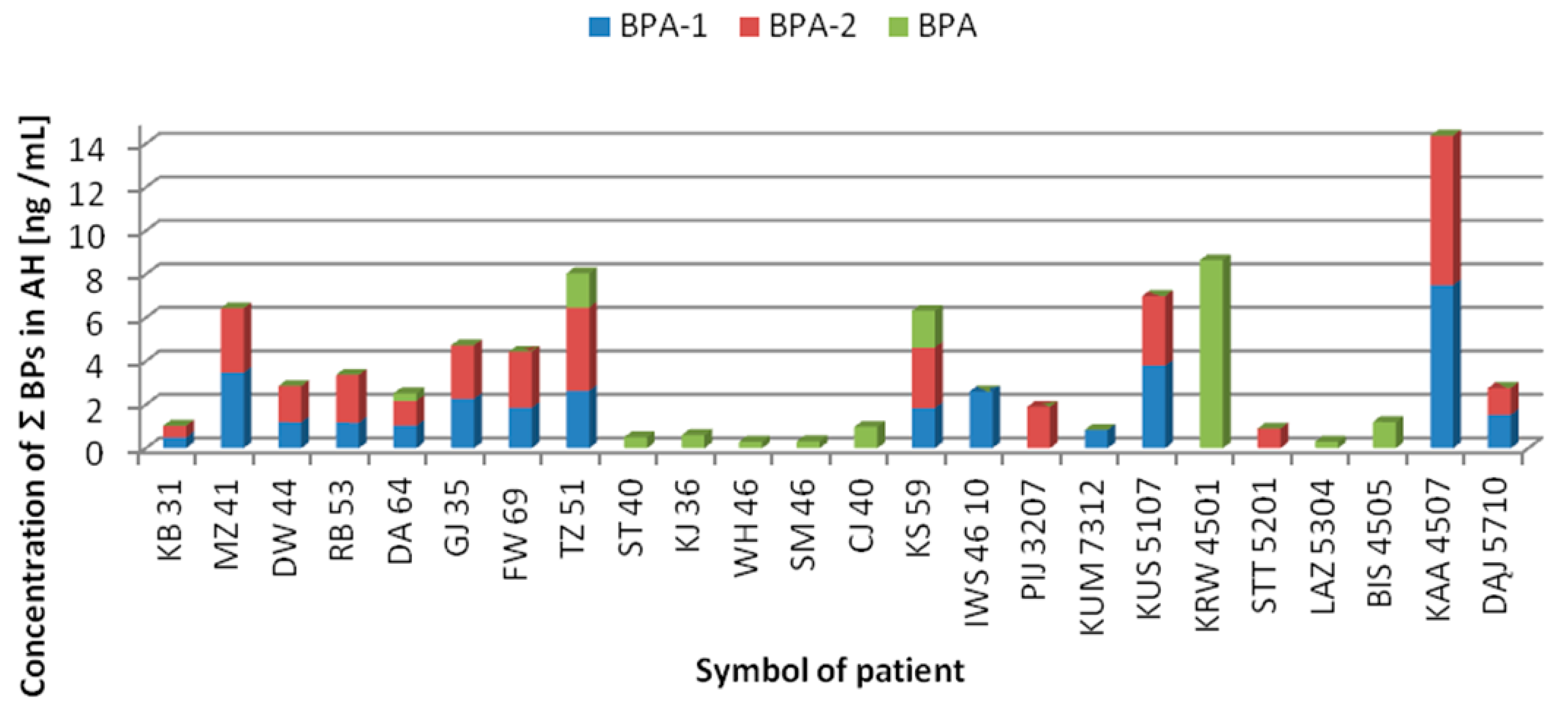

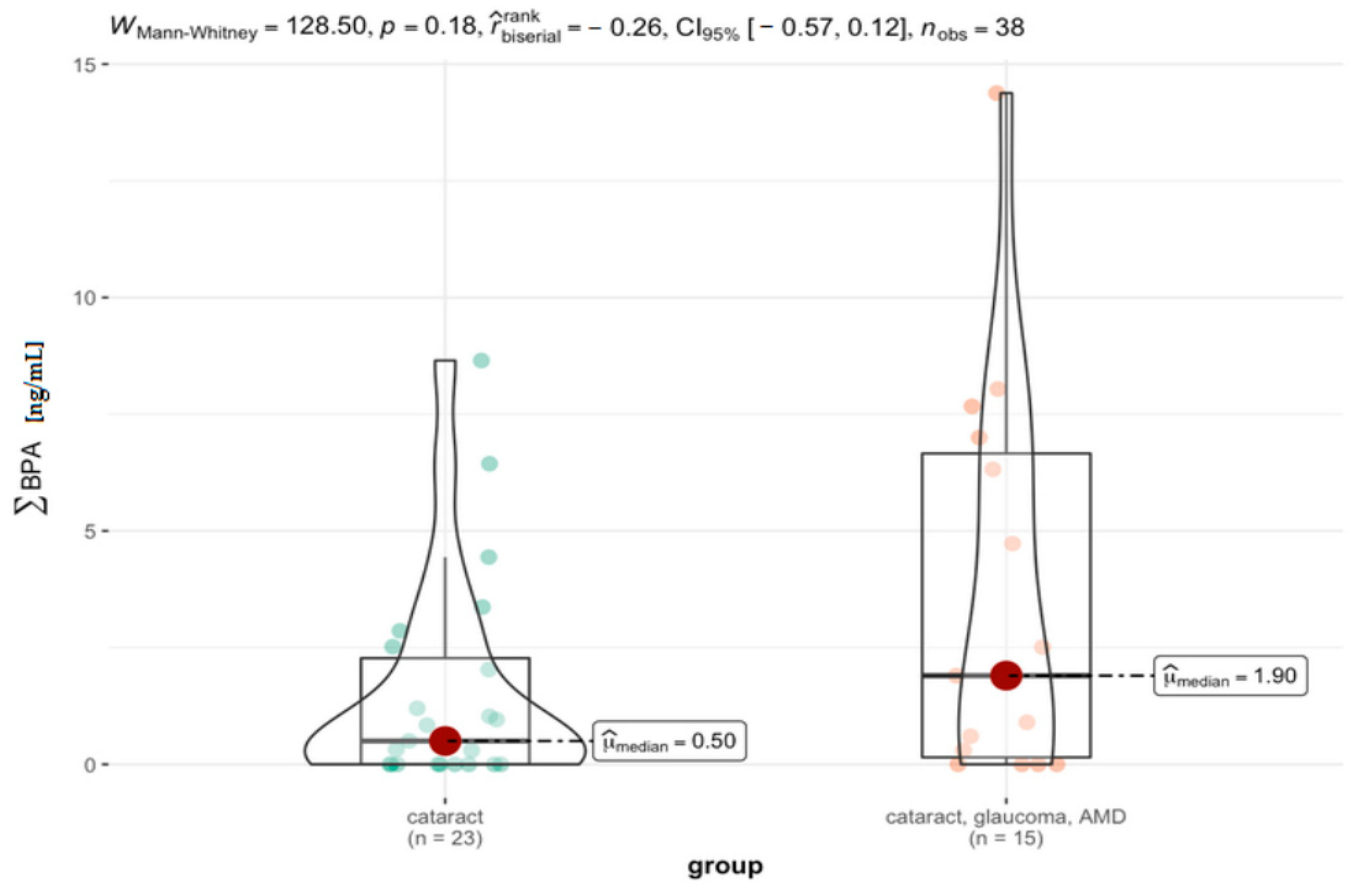
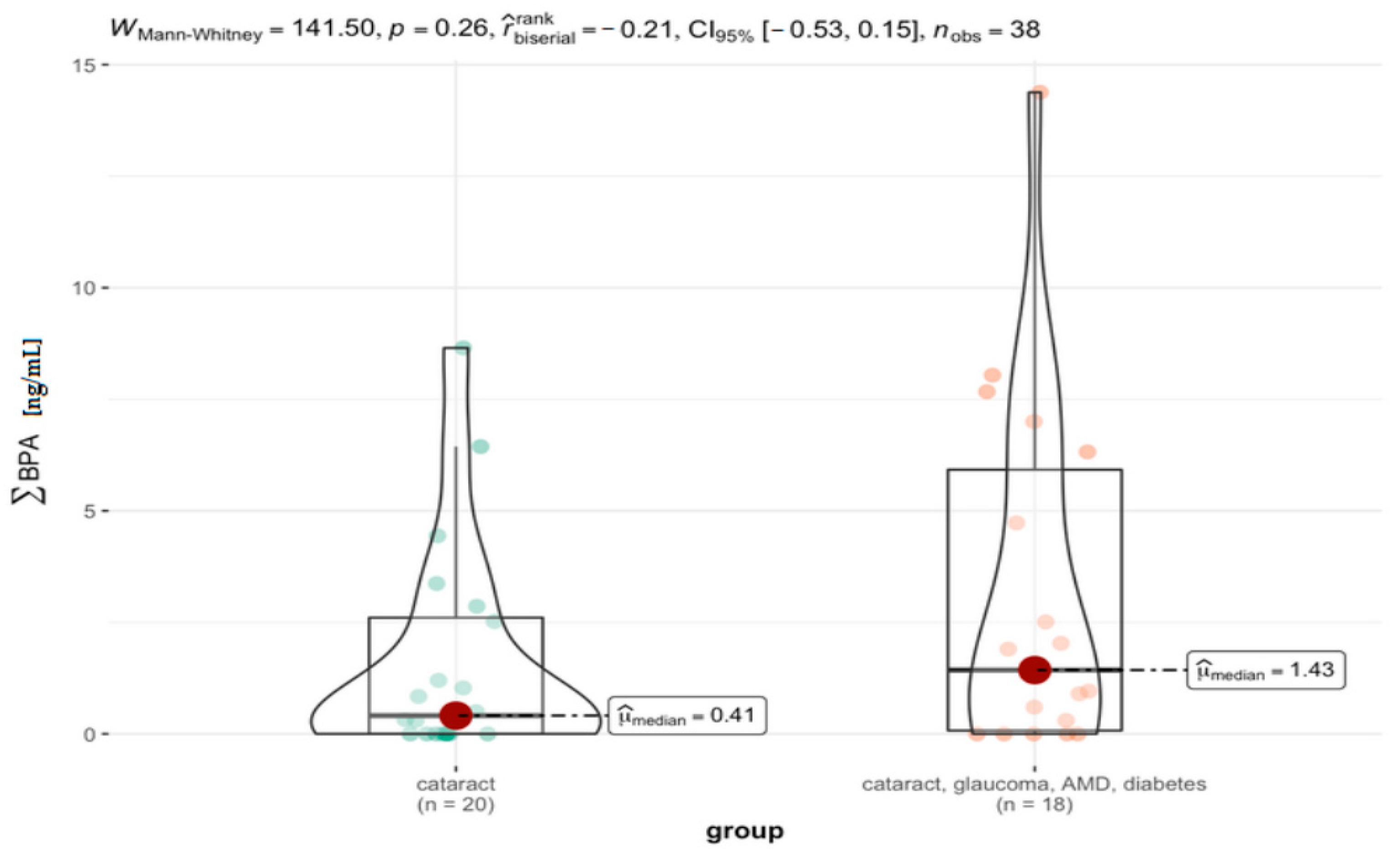
| Group | Gender | n | % | Min–Max Age | Median Age | Mean Age ± SD |
|---|---|---|---|---|---|---|
| cases (n = 44) | Female | 26 | 59.1 | 49–92 | 84.88 | 77.77 ± 7.12 |
| Male | 18 | 40.9 | 53–90 | 75.00 | 73.72 ± 8.22 |
| Compound | Precursor Ion (m/z) | Product Ion (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| Bisphenol A | 227.2 | 212.0 133.0 | −130 −130 | −10 −10 | −24 −32 | −16 −16 |
| Bisphenol F | 199.2 | 93.0 105.0 | −130 −130 | −10 −10 | −28 −28 | −12 −12 |
| Bisphenol A–D16 (IS) | 241.2 | 142.1 | −130 | −10 | −40 | −16 |
| Compounds | LOD (ng mL−1) | LOQ (ng mL−1) | Matrix Effect (%) | Working Range (ng mL−1) | Determination Coefficient (R2) | Calibration Curve |
|---|---|---|---|---|---|---|
| Bisphenol A | 0.13 | 0.248 | 4.0 ± 1.6% | 0.25–20.0 | 0.987 | y = 0.216x + 0.05 |
| Bisphenol F | 0.14 | 0.252 | 3.9 ± 2.5% | 0.25–20.0 | 0.989 | y = 0.291x + 0.01 |
| Concentration (ng mL−1) | Repeatability (RSDr, %) (n = 6) | Within-Lab Reproducibility (RSDwR, %) (n = 18) | Expanded Uncertainty (ng mL−1) | Apparent Recovery (%) |
|---|---|---|---|---|
| Bisphenol A | ||||
| 0.25 | 3.8 ± 4.1 | 4.8 ± 4.2 | - | 103.3 ±3.2 |
| 1.0 | 3.5 ± 3.3 | 4.5 ± 3.9 | 1.0 ± 0.17 | 104.3 ± 2.7 |
| 2.5 | 2.9 ± 2.8 | 3.6 ± 3.4 | - | 102.1 ± 2.5 |
| 10.0 | 3.0 ± 3.1 | 4.0 ± 3.3 | - | 104.3 ± 4.0 |
| 20.0 | 2.9 ± 3.1 | 4.1 ± 3.3 | - | 103.8 ± 3.7 |
| Bisphenol F | ||||
| 0.25 | 3.6 ± 4.5 | 4.6 ± 4.3 | - | 99.9 ± 3.6 |
| 1.0 | 3.6 ± 3.7 | 4.1 ± 3.9 | 1.0 ± 0.22 | 103.2 ± 3.4 |
| 2.5 | 3.1 ± 3.8 | 3.9 ± 3.6 | - | 104.2 ± 3.5 |
| 10.0 | 3.4 ± 3.3 | 4.1 ± 3.6 | - | 103.9 ± 3.4 |
| 20.0 | 3.5 ± 3.1 | 4.2 ± 3.4 | - | 103.6 ± 3.5 |
| Parameter | BPA-1 | BPA-2 | BPA-Free |
|---|---|---|---|
| Mean [ng mL−1] | 0.8809 | 0.9191 | 0.3857 |
| Standard error | 0.2285 | 0.2261 | 0.2021 |
| Median | 0 | 0 | 0 |
| Mode | 0 | 0 | 0 |
| Standard deviation | 1.5157 | 1.4998 | 1.34055 |
| Sample variance | 2.2973 | 2.2494 | 1.7971 |
| Kurtosis | 7.7144 | 4.6902 | 35.4541 |
| Skewness | 2.4863 | 2.02203 | 5.7367 |
| Range | 7.51 | 6.87 | 8.65 |
| Minimum | 0 | 0 | 0 |
| Maximum | 7.51 | 6.87 | 8.65 |
| Sum | 38.76 | 40.44 | 16.97 |
| Count | 44 | 44 | 44 |
| DF (n) | 18 | 18 | 12 |
| DF (%) | 40.91 | 40.91 | 27.27 |
| P25 | 0 | 0 | 0 |
| P50 | 0 | 0 | 0 |
| P75 | 1.185 | 1.458 | 0.3 |
| P90 | 2.638 | 2.906 | 0.866 |
| P99 | 5.915 | 5.597 | 5.657 |
| Gender | Vars | n | Mean | Sd | Median | Trimmed | Mad | Min | Max | Range | Skew | Kurtosis | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| female | 1 | 26 | 1.60 | 3.08 | 0.41 | 0.94 | 0.61 | 0 | 14.38 | 14.38 | 2.88 | 8.72 | 0.60 |
| male | 1 | 18 | 3.03 | 3.16 | 2.20 | 2.87 | 3.27 | 0 | 8.65 | 8.65 | 0.58 | −1.34 | 0.74 |
| Group | Vars | n | Mean | Sd | Median | Trimmed | Mad | Min | Max | Range | Skew | Kurtosis | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cataract | 1 | 23 | 1.54 | 2.30 | 0.50 | 1.07 | 0.74 | 0 | 8.65 | 8.65 | 1.70 | 2.12 | 0.48 |
| cataract, AMD, glaucoma | 1 | 15 | 3.62 | 4.27 | 1.90 | 3.07 | 2.82 | 0 | 14.38 | 14.38 | 1.00 | 0.03 | 1.10 |
| Group | Vars | n | Mean | Sd | Median | Trimmed | Mad | Min | Max | Range | Skew | Kurtosis | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cataract | 1 | 20 | 1.62 | 2.44 | 0.41 | 1.09 | 0.61 | 0 | 8.65 | 8.65 | 1.55 | 1.42 | 0.54 |
| cataract, glaucoma, AMD, diabetes | 1 | 18 | 3.19 | 4.02 | 1.43 | 2.68 | 2.12 | 0 | 14.38 | 14.38 | 1.25 | 0.75 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, J.; Śniegocki, T.; Dolar-Szczasny, J.; Załuska, W.; Rejdak, R. The First Evidence on the Occurrence of Bisphenol Analogues in the Aqueous Humor of Patients Undergoing Cataract Surgery. J. Clin. Med. 2022, 11, 6402. https://doi.org/10.3390/jcm11216402
Flieger J, Śniegocki T, Dolar-Szczasny J, Załuska W, Rejdak R. The First Evidence on the Occurrence of Bisphenol Analogues in the Aqueous Humor of Patients Undergoing Cataract Surgery. Journal of Clinical Medicine. 2022; 11(21):6402. https://doi.org/10.3390/jcm11216402
Chicago/Turabian StyleFlieger, Jolanta, Tomasz Śniegocki, Joanna Dolar-Szczasny, Wojciech Załuska, and Robert Rejdak. 2022. "The First Evidence on the Occurrence of Bisphenol Analogues in the Aqueous Humor of Patients Undergoing Cataract Surgery" Journal of Clinical Medicine 11, no. 21: 6402. https://doi.org/10.3390/jcm11216402
APA StyleFlieger, J., Śniegocki, T., Dolar-Szczasny, J., Załuska, W., & Rejdak, R. (2022). The First Evidence on the Occurrence of Bisphenol Analogues in the Aqueous Humor of Patients Undergoing Cataract Surgery. Journal of Clinical Medicine, 11(21), 6402. https://doi.org/10.3390/jcm11216402







