Incidence and Predictors of Cardiac Implantable Electronic Devices Malfunction with Radiotherapy Treatment
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
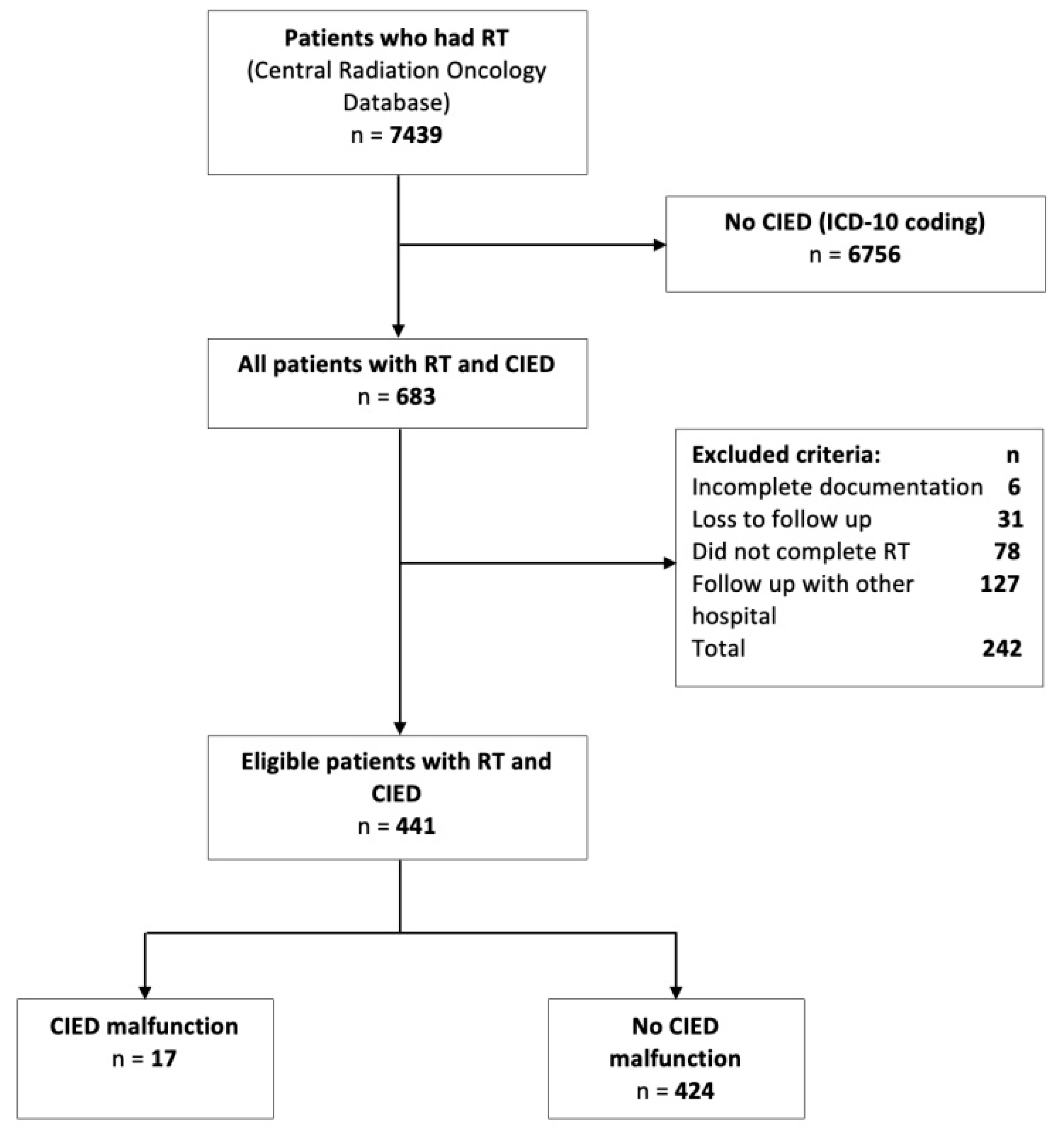
2.2. Participants
2.3. CIED Data
2.4. Radiation Oncology Data
2.5. CIED Malfunction
- (1)
- Electrical reset to backup mode or other minor software error.
- (2)
- Electrical reset or other software error requiring reprogramming of CIED by the manufacturer.
- (3)
- Unexpected decrease in battery life capacity.
- (4)
- Loss of telemetry.
- (5)
- Change in one or several lead parameters eventually resulting in follow-up visits or lead replacement.
- (6)
- Noise oversense without symptomatic pacing inhibition, antitachycardia pacing (ATP), or shock therapy.
- (7)
- Oversense with symptomatic pacing inhibition, ATP, or shock therapy.
2.6. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
3.2. Patients with CIED Malfunction vs. without CIED Malfunction with RT
3.3. CIED Malfunction
3.4. Predictors of CIED Malfunction
4. Discussion
| Summary of Recommendations from the Major CIED Manufacturers Regarding Safe Radiotherapy in CIED Patients | ||||
|---|---|---|---|---|
| Recommendations | Medtronic [25] | St Jude [26] | Boston Scientific [27] | Biotronik [28] |
| Device Checks | ||||
| Before RT course | Not stated | Not stated | Specific to each patient | Yes |
| During RT course | Yes (if exceed recommended safe dose) | Yes (a detailed evaluation once or twice during the RT course in PM-dependent patients) | Specific to each patient | Not stated |
| After RT course | Yes | Yes | Yes, including subsequent close monitoring of the device function | Yes, including a supplementary follow-up shortly after RT |
| Maximal PM dose | 5 Gy | No safe dose | No safe dose (2 Gy used as a reference) | 2 Gy |
| Maximal ICD dose | 1–5 Gy depending on model | No safe dose | No safe dose (2 Gy used as a reference) | 2 Gy |
| Maximal beam energy | ≤10 MV | Not stated | Not stated | ≤10 MV |
| Inactivation of antitachycardia therapies | Yes | Yes | Yes | Yes |
| Lead shielding of device | No (ineffective against neutrons) | Not stated (reduction in device dose is recommended) | All available shielding options | Yes |
| Heart rhythm monitoring during RT | Not stated | Yes | As determined most appropriate by the physician team | Yes |
5. Strengths and Limitations
6. Conclusions
7. Clinical Perspective
- Competency in medical knowledge
- Translational outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CIED | Cardiac implantable cardiac device |
| CMOS | Complementary metal-oxide semiconductor |
| CRT | Cardiac resynchronization therapy |
| CRT–D | Cardiac resynchronization therapy—defibrillator |
| CRT–P | Cardiac resynchronization therapy—pacemaker |
| EP | Electrophysiology |
| Gy | Grays |
| ICD | Implantable cardioverter defibrillator |
| MV | Megavolt |
| PPM | Permanent pacemaker |
| RT | Radiotherapy |
References
- Raatikainen, M.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 report from the European Heart Rhythm Association. Ep Eur. 2017, 19 (Suppl. S2), ii1–ii90. [Google Scholar] [CrossRef]
- Gupta, N.; Kiley, M.L.; Anthony, F.; Young, C.; Brar, S.; Kwaku, K. Multi-Center, Community-Based Cardiac Implantable Electronic Devices Registry: Population, Device Utilization, and Outcomes. J. Am. Heart Assoc. 2016, 5, e002798. [Google Scholar] [CrossRef]
- Stone, K.R.; McPherson, C.A. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit. Care Med. 2004, 32, S155–S165. [Google Scholar] [CrossRef]
- Tondato, F.; Ng, D.W.; Srivathsan, K.; Altemose, G.T.; Halyard, M.Y.; Scott, L.R. Radiotherapy-induced pacemaker and implantable cardioverter defibrillator malfunction. Expert Rev. Med. Devices 2009, 6, 243–249. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Nemec, J. Runaway implantable defibrillator—A rare complication of radiation therapy. Pacing Clin. Electrophysiol. 2007, 30, 716–718. [Google Scholar] [CrossRef]
- Zweng, A.; Schuster, R.; Hawlicek, R.; Weber, H.S. Life-threatening pacemaker dysfunction associated with therapeutic radiation: A case report. Angiology 2009, 60, 509–512. [Google Scholar] [CrossRef]
- Azraai, M.; D’Souza, D.; Lin, Y.-H.; Nadurata, V. Current clinical practice in patients with cardiac implantable electronic devices undergoing radiotherapy: A literature review. EP Europace 2022, 24, 362–374. [Google Scholar] [CrossRef]
- Hilbert, S.; Jahnke, C.; Loebe, S.; Oebel, S.; Weber, A.; Spampinato, R.; Richter, S.; Doering, M.; Bollmann, A.; Sommer, P.; et al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: A device-dependent imaging strategy for improved image quality. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 1051–1061. [Google Scholar] [CrossRef]
- Indik, J.H.; Gimbel, J.R.; Abe, H.; Alkmim-Teixeira, R.; Birgersdotter-Green, U.; Clarke, G.D.; Dickfeld, T.-M.L.; Froelich, J.W.; Grant, J.; Hayes, D.L. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm 2017, 14, e97–e153. [Google Scholar] [CrossRef]
- Miften, M.; Mihailidis, D.; Kry, S.F.; Reft, C.; Esquivel, C.; Farr, J.; Followill, D.; Hurkmans, C.; Liu, A.; Gayou, O. Management of radiotherapy patients with implanted cardiac pacemakers and defibrillators: A Report of the AAPM TG-203. Med. Phys. 2019, 46, e757–e788. [Google Scholar] [CrossRef]
- Zaremba, T.; Jakobsen, A.R.; Søgaard, M.; Thøgersen, A.M.; Johansen, M.B.; Madsen, L.B.; Riahi, S. Risk of device malfunction in cancer patients with implantable cardiac device undergoing radiotherapy: A population-based cohort study. Pacing Clin. Electrophysiol. 2015, 38, 343–356. [Google Scholar] [CrossRef]
- Zagzoog, A.; Wronski, M.; Birnie, D.H.; Yeung, C.; Baranchuk, A.; Healey, J.S.; Golian, M.; Boles, U.; Carrizo, A.G.; Turner, S.; et al. Assessment of Radiation-Induced Malfunction in Cardiac Implantable Electronic Devices. CJC Open 2021, 3, 1438–1443. [Google Scholar] [CrossRef]
- Zaremba, T.; Jakobsen, A.R.; Thøgersen, A.M.; Oddershede, L.; Riahi, S. The effect of radiotherapy beam energy on modern cardiac devices: An in vitro study. Europace 2014, 16, 612–616. [Google Scholar] [CrossRef]
- Marbach, J.; Sontag, M.; Van Dyk, J.; Wolbarst, A. Management of radiation oncology patients with implanted cardiac pacemakers: Report of AAPM Task Group No. 34. Med. Phys. 1994, 21, 85–90. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Bounds, C.; Brown, T.; Gerbi, B.J.; Peltier, J. Cancer-radiotherapy equipment as a cause of soft errors in electronic equipment. IEEE Trans. Device Mater. Reliab. 2005, 5, 449–451. [Google Scholar] [CrossRef]
- Bradley, P.; Normand, E. Single event upsets in implantable cardioverter defibrillators. IEEE Trans. Nucl. Sci. 1998, 45, 2929–2940. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Nitsch, P.L.; Kudchadker, R.J.; Howell, R.M.; Kry, S.F. Out-of-field doses and neutron dose equivalents for electron beams from modern Varian and Elekta linear accelerators. J. Appl. Clin. Med. Phys. 2016, 17, 442–455. [Google Scholar] [CrossRef]
- Hurkmans, C.W.; Scheepers, E.; Springorum, B.G.; Uiterwaal, H. Influence of radiotherapy on the latest generation of implantable cardioverter-defibrillators. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 282–289. [Google Scholar] [CrossRef]
- McCollough, C.H.; Zhang, J.; Primak, A.N.; Clement, W.J.; Buysman, J.R. Effects of CT irradiation on implantable cardiac rhythm management devices. Radiology 2007, 243, 766–774. [Google Scholar] [CrossRef]
- Last, A. Radiotherapy in patients with cardiac pacemakers. Br. J. Radiol. 1998, 71, 4–10. [Google Scholar] [CrossRef]
- Ott, O.J.; Niewald, M.; Weitmann, H.-D.; Jacob, I.; Adamietz, I.A.; Schaefer, U.; Keilholz, L.; Heyd, R.; Muecke, R. DEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther. Und Onkol. 2015, 191, 1–6. [Google Scholar] [CrossRef]
- Zecchin, M.; Morea, G.; Severgnini, M.; Sergi, E.; Baratto Roldan, A.; Bianco, E.; Magnani, S.; De Luca, A.; Zorzin Fantasia, A.; Salvatore, L.; et al. Malfunction of cardiac devices after radiotherapy without direct exposure to ionizing radiation: Mechanisms and experimental data. EP Eur. 2015, 18, 288–293. [Google Scholar] [CrossRef]
- Guidant Corporation Cardiac Rhythm Management Technical Services. Impact of Therapeutic Radiation and Guidant ICD/CRT-D/CRT-P/Pacing Systems; Revision; Guidant Corporation: St. Paul, MN, USA, 2004; pp. 1–6. [Google Scholar]
- Medtronic. Standard Letter; Medtronic: Minneapolis, MN, USA, 2017. [Google Scholar]
- Jude, S. Effects of Therapeutic Radiation on St. Jude Medical Implantable Cardiac Rhythm Devices; Technical Service: Sylmar, CA, USA, 2013. [Google Scholar]
- Scientific, B. Therapeutic Radiation and Implantable Device Systems; Revision 002-1675; Technical Service: Marlborough, MA, USA, 2013. [Google Scholar]
- Biotronik. Radiation Therapy and BIOTRONIK CRM Devices—Pacemakers (IPG), Defibrillators (ICD) and CRT-Devices; Biotronik: Berlin, Germany, 2011. [Google Scholar]
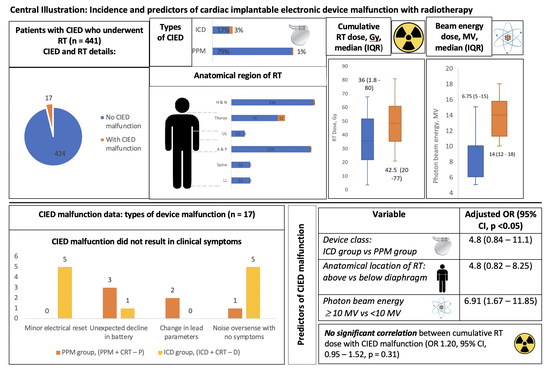
| Category | Characteristics | All n = 441 | CIED Malfunction n = 17 | No CIED Malfunction n = 424 | p Value |
|---|---|---|---|---|---|
| Patient characteristics | Age, years (±SD) | 82 ± 14 | 85 ± 11 | 82 ± 14 | 0.12 |
| Male gender, n (%) | 327 (74) | 14 (82) | 313 (74) | 0.061 | |
| Device type n (%) | SC PPM | 41 (9) | 0 (0) | 41 (9) | <0.001 |
| DC PPM | 303 (69) | 5 (29) | 298 (70) | 0.024 | |
| CRT–P | 9 (2) | 1 (6) | 8 (2) | 0.032 | |
| ICD | 44 (10) | 8 (47) | 36 (8) | 0.047 | |
| CRT–D | 44(10) | 3 (18) | 41 (10) | 0.014 | |
| Age of device, n (IQR) | 5.2 (0.9–11.2) | 8 (4.7–11.2) | 5.1 (0.9–10.9) | 0.072 | |
| Device manufacturer n (%) | Medtronic | 198 (45) | 9 (54) | 189 (45) | 0.016 |
| St Jude | 129 (29) | 4 (21) | 125 (29) | 0.013 | |
| Boston Scientific | 92 (21) | 3 (19) | 89 (21) | 0.021 | |
| Biotronik | 22 (5) | 1 (6) | 21 (5) | 0.045 | |
| Safety measures n (%) | Device dependency | 216 (49) | 10 (59) | 206 (49) | 0.039 |
| Total safety measures | 84 (19) | 0 (0) | 84 (20) | <0.001 | |
| Application of magnet | 49 (11) | 0 (0) | 49 (12) | <0.001 | |
| Device reprogramming | 35 (8) | 0 (0) | 35 (8) | <0.001 | |
| Surgical relocation of CIED | 0 (0) | 0 (0) | 0 (0) | N/A | |
| RT anatomical location n (%) | Head and neck | 137 (31) | 3 (15) | 134 (32) | 0.021 |
| Thorax | 88 (20) | 12 (71) | 76 (18) | 0.014 | |
| Abdomen and pelvis | 132 (30) | 2 (14) | 129 (31) | 0.035 | |
| Spine | 31 (7) | 0 (0) | 31 (7) | <0.01 | |
| Upper limbs | 22 (5) | 0 (0) | 22 (5) | <0.01 | |
| Lower limbs | 31 (7) | 0 (0) | 31 (7) | <0.01 | |
| RT details, n (IQR) | Cumulative dose, Gy | 36 (1.8–80) | 42.5 (20–77) | 36 (1.8–80) | 0.39 |
| Dose per fraction, Gy | 3.75 (1–26) | 12 (10–26) | 3.6 (1–24) | 0.26 | |
| Number of fractions | 13 (1–40) | 6 (2–23) | 12.9 (1–40) | 0.42 | |
| Dose to device, Gy | 0.28 (0–3.3) | 0.29 (0.1–3.3) | 0.26 (0.1–3) | 0.13 | |
| Beam energy, n (%) | Total beam energy used | 423 (96) | 16 (95) | 406 (96) | 0.037 |
| Photon ≥ 10 MV | 185 (44) | 16 (95) | 169 (40) | 0.045 | |
| Photon < 10 MV | 145 (34) | 0 (0) | 145 (34) | <0.01 | |
| Electron | 93 (22) | 0 (0) | 93 (22) | <0.01 | |
| Photon dose in MV, n (IQR) | 7 (5–18) | 14 (12–18) | 6.75 (5–15) | 0.037 | |
| Electron dose in MV, n (IQR) | 9 (3–18) | 0 | 9 (3–18) | <0.01 |
| Types of CIED Malfunction | PPM Group, (%) | ICD Group, (%) | n (%) |
|---|---|---|---|
| Minor electrical reset | 0 (0) | 5 (45) | 5 (29) |
| Major electrical reset | 0 (0) | 0 (0) | 0 (0) |
| Unexpected decline in battery | 3 (50) | 1 (10) | 4 (24) |
| Loss of telemetry | 0 (0) | 0 (0) | 0 (0) |
| Change in lead parameters | 2 (33) | 0 (0) | 2 (12) |
| Noise oversense with no symptoms | 1 (17) | 5 (45) | 6 (35) |
| Noise oversense with inhibition of pacing and/or symptoms | 0 (0) | 0 (0) | 0 (0) |
| ATP treatment | 0 (0) | 0 (0) | 0 (0) |
| Inappropriate shock | 0 (0) | 0 (0) | 0 (0) |
| Total CIED malfunction | 6 (35) | 11 (65) | 17 (100) |
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | p-Value for Adjusted OR |
|---|---|---|---|
| Device class: ICD group vs. PPM group | 4.6 (0.75–10.2) | 4.8 (0.84–11.1) | 0.021 |
| Anatomical location of RT (above vs. below diaphragm) | 5.2 (1.82–15.2) | 4.8 (0.82–8.25) | 0.014 |
| Cumulative tumor dose (10 Gy increment) | 1.20 (0.95–1.52) | 1.13 (0.89–1.44) | 0.31 |
| Fraction dose (1 Gy increment) | 1.0 (0.79–4.7) | 1.1 (0.84–4.98) | 0.43 |
| Photon beam energy ≥10 MV vs. <10 MV | 6.73 (1.58–10.76) | 6.91(1.67–11.85) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azraai, M.; Miura, D.; Lin, Y.-H.; Rodrigues, T.S.; Nadurata, V. Incidence and Predictors of Cardiac Implantable Electronic Devices Malfunction with Radiotherapy Treatment. J. Clin. Med. 2022, 11, 6329. https://doi.org/10.3390/jcm11216329
Azraai M, Miura D, Lin Y-H, Rodrigues TS, Nadurata V. Incidence and Predictors of Cardiac Implantable Electronic Devices Malfunction with Radiotherapy Treatment. Journal of Clinical Medicine. 2022; 11(21):6329. https://doi.org/10.3390/jcm11216329
Chicago/Turabian StyleAzraai, Meor, Daisuke Miura, Yuan-Hong Lin, Thalys Sampaio Rodrigues, and Voltaire Nadurata. 2022. "Incidence and Predictors of Cardiac Implantable Electronic Devices Malfunction with Radiotherapy Treatment" Journal of Clinical Medicine 11, no. 21: 6329. https://doi.org/10.3390/jcm11216329
APA StyleAzraai, M., Miura, D., Lin, Y.-H., Rodrigues, T. S., & Nadurata, V. (2022). Incidence and Predictors of Cardiac Implantable Electronic Devices Malfunction with Radiotherapy Treatment. Journal of Clinical Medicine, 11(21), 6329. https://doi.org/10.3390/jcm11216329