Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare but potentially fatal hyperinflammatory disorder characterized by dysfunctional cytotoxic T and natural killer cells. Liver transplantation is a predisposing factor for HLH. High mortality rates were reported in 40 cases of HLH following liver transplantation in adults and children. Herein, we describe a case of adult HLH triggered by cytomegalovirus (CMV) infection shortly after liver transplantation. The patient was successfully treated with ruxolitinib combined with a modified HLH-2004 treatment strategy. Our case is the first to report the successful use of ruxolitinib with a modified HLH-2004 strategy to treat HLH in a solid organ transplantation recipient.
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome (HPS), is a rare but life-threatening disorder characterized by a high-grade fever, splenomegaly, cytopenia, and hyperferritinemia. It is believed that NK cell dysfunction, activated cytotoxic T-cell activity, and the cytokine storm constitute the underlying mechanism of HLH [1]. HLH can be divided into primary HLH and secondary HLH according to its cause. Primary HLH is considered to be genetic and often occurs in children with an underlying genetic defect. Bone marrow transplant is the only radical treatment for primary HLH [2]. In contrast, secondary HLH can occur at any age and is associated with infection, malignancy, autoimmune disease, transplantation, and drugs [1]. These factors may act as triggers or predisposing factors to the disease, or both. Viral infection is the most frequent trigger, and about 6% of cases are triggered by a cytomegalovirus (CMV) [1]. HLH is a rare disease, but has a very high mortality rate of around 41%, probably due to the nonspecific manifestations and delayed diagnosis [1].
After liver transplantation (LT), patients receive immunosuppression treatment and as a result are susceptible to infections and immune dysregulation. HLH after LT is often overlooked as a fever and cytopenia both of which are common after a liver transplant, and the initial manifestations of HLH are also similar to other complications, such as posttransplant lymphoproliferative disorders (PTLD), graft-versus-host disease (GVHD), autoimmune hemolytic anemia (AIHA), and sepsis [1,3]. To date, 21 pediatric cases (age 18 years) and 19 adult cases of HLH following liver transplantation (LT) have been reported in the literature, with a very high mortality rate of 55% (22/40) (Table 1). The high mortality rate may be attributed to a delayed diagnosis and a debatable treatment. The widely used HLH-2004 treatment protocol, including steroids (dexamethasone), immunosuppressive agents (cyclosporine A), and chemotherapy drugs (etoposide), was first proposed for pediatric patients with primary HLH. However, this protocol may not be appropriate for LT patients as they are already immunosuppressed [4].
Ruxolitinib is an inhibitor of JAK1/2 (the Janus family kinase 1 and 2) and can inhibit the release of cytokines. It is mainly used in the hematological field and also in COVID-19 patients [5]. There are case reports and pilot studies using ruxolitinib as a salvage therapy or first-line treatment for HLH patients showing good efficacy and safety [6,7,8,9,10,11,12,13,14,15]. However, it has never been used for post-transplant patients with HLH. In this study, we present one case of adult CMV-associated HLH following liver transplantation successfully treated by ruxolitinib and a modified HLH-2004 protocol. This report provides an alternative method for the treatment of HLH following LT.

Table 1.
Cases of HLH after liver transplantation.
Table 1.
Cases of HLH after liver transplantation.
| No | Author | Age (Years) | Dx | Donor Type | Time Post LT | IS regimen | Triggers | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chisuwa [16] | 0.75 | Biliary atresia | LRLT | 15 d | Steroid, FK506 | Unx | Steroid, IVIg, G-CSF, stopped IS | Died |
| 2 | Chisuwa [16] | 1 | Biliary atresia | LRLT | 134 d | Steroid, FK506 | Unx | G-CSF, stopped IS | Died |
| 3 | Karasu [17] | 38 | HBV/HDV | LRLT | 131 d | Steroid, FK506 | Unx | IVIg, G-CSF, PE, stopped IS | Alive |
| 4 | Lladó [18] | 63 | AIH | DDLT | 75 d | CsA, basiliximab | Unx | Steroid, IVIg | Died |
| 5 | George [19] | 10 | Unx | Unx | 6 y | FK506 | EBV | Steroid, Stopped IS, etoposide | Alive |
| 6 | Tania [20] | 37 | Unx | LDLT | 11 d | FK506 | Unx | Steroid, IvIg, PE, CHDF | Died |
| 7 | Hardikar [21] | 2.2 | Unx | DDLT | 15 d | Steroid, FK506, Aza | CMV | Stop IS, G-CSF, IVIg | Alive |
| 8 | Akamatsu [22] | 59 | HCV | LDLT | 138 d | FK506 | Aspergillus | Supportive | Died |
| 9 | Akamatsu [22] | 49 | PBC | LDLT | 315 d | Steroid, FK506 | CMV | Steroid, IVIg, PE, FK506 to CsA, Sx | Died |
| 10 | Akamatsu [22] | 48 | HCV | LDLT | 50 d | Steroid, FK506, OKT3 | HCV | G-CSF | Died |
| 11 | Yoshizumi [23] | 63 | HBV | LDLT | 13 d | SFSS | Steroid, change IS, IVIg, G-CSF | Alive | |
| 12 | Dharancy [24] L/K | 49 | Polycystic liver kidney disease | Combined Liver-kidney (CLK) | 19 d | Steroid, CsA, MMF, basiliximab | HHV6 | Supportive | Died |
| 13 | Zhang [25] | 6 | Unx | LRLT | 8 d | Steroid, FK506 | EBV | Steroid, IVIg, G-CSF, stopped IS, etoposide | Alive |
| 14 | Satapathy [26] | 25 | AIH | DDLT | 6 m | Steroid, FK506, MMF | Still’s disease | Steroid, anakinra | Alive |
| 15 | Somyama [27] | 57 | HCV/HCC | LDLT | 32 d | Steroid, FK506 | CMV/HCC | Steroid, IVIg, G-CSF | Died |
| 16 | Somyama [27] | 63 | HBV/HCC | LRLT | 81 d | Steroid, FK506 | Unx | Steroid, IVIg, G-CSF | Died |
| 17 | Rodríguez-Medina [28] | 63 | HCV | Unx | 4 y | Steroid, CsA, Aza | TB | G-CSF | Died |
| 18 | Jha [29] 2015 | 2 | Extrahepatic biliary atresia | LRLT | 9 m | EBV | Reduced IS; rituximab | Alive | |
| 19 | Vijgen [30] | 66 | Alcohol-related liver disease, HCC | DDLT | 1 y | FK506, MMF | HHV8 | Steroid, stopped IS, rituximab | Died |
| 20 | Iseda [31] | 63 | HBV | LDLT | 12 d | Steroid, FK506, MMF | SFSS | Steroid, IVIg, G-CSF, stopped MMF, reduced FK506 | Died |
| 21 | Cohen [32] | 31 | Drug-induced liver disease | DDLT | 4 y | FK506 | HHV8 | Steroid, IVIg, rituximab, reduced FK506 | Died |
| 22 | Jarchin [33] | 18 | Neonatal hepatitis | LRLT | 17 y | FK506 | Unx | Steroid, etoposide, stopped FK506 | Died |
| 23 | Valenzuela [34] | 48 | AIH, PBC | DDLT | 13 d | Steroid, FK506 | EBV | Steroid, etoposide, IS switched to CsA | Died |
| 24 | Yamada [35] | 2 | Biliary atresia | Liver inteatinal transplantation | 254 d | Steroid, FK506, everolimus | EBV | Steroid, etoposide, reduced IS, THP-COP | Died |
| 25 | Chesner [36] | 58 | AIH | Unx | 16 y | Steroid, FK506, Aza | EBV | supportive | Died |
| 26 | Arikan [37] | 1 | Caroli disease | Unx | 6 m | Steroid, FK506, sirolimus, MMF | CMV | Steroid, IVIg, rituximab | Died |
| 27 | Arikan [37] | 2 | Biliary atresia | 8 m | Steroid, FK506, sirolimus | EBV | Steroid, IVIg, VP16, rituximab | Alive | |
| 28 | Arikan [37] | 1.5 | Unx cholestasis | 7 m | Steroid, FK506 | HHV8 | Steroid, IVIg, VP16 | Alive | |
| 29 | Arikan [37] | 9 | PFIC | 4 m | Steroid, FK506, sirolimus | Acinetobacter | IVIg | Died | |
| 30 | Arikan [37] | 0.5 | Biliary atresia | 10 m | FK506 | EBV | IVIg, VP16, rituximab | Alive | |
| 31 | Arikan [37] | 0.5 | Unx cholestasis | 7 m | Steroid, FK506 | Klebsiella | Steroid, IVIg, VP16 | Alive | |
| 32 | Arikan [37] | 0.67 | Biliary atresia | 9 m | Steroid, FK506 | EBV | Steroid, IVIg, VP16, rituximab | Alive | |
| 33 | Arikan [37] | 0.67 | Unx | 5 m | Steroid, FK506, MMF | CMV, EBV | Steroid, IVIg, rituximab | Died | |
| 34 | Arikan [37] | 0.75 | Unx cholestasis | 6 m | Steroid, FK506 | EBV | Steroid, IVIg, rituximab | Alive | |
| 35 | Arikan [37] | 2.1 | PFIC | 10 m | FK506 | EBV | IVIg, VP16, rituximab | Alive | |
| 36 | Arikan [37] | 0.4 | Unx cholestasis | 8 m | FK506 | EBV | Steroid, IVIg, rituximab | Alive | |
| 37 | Arikan [37] | 1 | Bile salt synthesis defect | 10 m | FK506, sirolimus | EBV | IVIg, rituximab | Alive | |
| 38 | Gandotra [38] | 47 | HBV/HCV/HCC | LDLT | 3 y | Steroid | TB | Steroid, IVIg | Alive |
| 39 | Nakajima [39] | 2 | EBV-LPD | LRLT | 10 d | EBV | Steroid, etoposide, HCT | Alive | |
| 40 | Ferreira [40] | 52 | Cryptogenic cirrhosis | DDLT | 2 m | Steroid, FK506, MMF | TB | Steroid, etoposide, stopped FK506 | Died |
AIH: autoimmune hepatitis, Aza: azathioprine, CMV: cytomegalovirus, CsA: cyclosporin A, d: days, DDLT: deceased donor liver transplantation, EBV: Epstein-Barr virus, EBV-G-CSF: granulocyte colony-stimulating factor, LPD: EBV associated T and NK cell lymphoproliferative disease, FK506: tacrolimus, HBV: hepatitis B virus, HCV: hepatitis C virus, HDV: hepatitis D virus, HCC: hepatocellular carcinoma, HCT: hematopoietic cell transplantation, HHV: human herpes virus, IVIg: intravenous immunoglobin, IS: immunosuppression, LDLT: living donor liver transplantation, LRLT: living related donor liver transplantation, m: months, MMF: mycophenolate mofetil, PBC: primary biliary cholangitis, PE: plasma exchange, PFIC: progressive familial intrahepatic cholestasis, SFSS: small-for-size syndrome, Sx: splenectomy, TB: tuberculosis, Unx: unknown, VP-16: etoposide, y: years.
2. Case Report
A 53-year-old male presented with fulminant hepatic failure, probable drug-induced liver injury and alcohol-induced hepatitis. Serologic tests for hepatitis A-D, Epstein-Barr virus (EBV), and tuberculosis were all negative. The autoimmune workup was normal. The CMV DNA was detected by PCR in peripheral blood with 4.64copies/mL, with a positive cytomegalovirus (CMV) IgG and a negative IgM.
On admission, the patient’s vitals were within normal ranges and his temperature was 36.5 °C. Initial examination showed normal hematological findings and an abnormal liver function and coagulopathy: total bilirubin 544.6 , direct bilirubin 386.8 , alanine aminotransferase (ALT) 537 U/L, aspartate aminotransferase (AST) 373 U/L, PT 24.1 s, INR 2.3, and fibrinogen 1.15 g/L.
On day 10 of admission, the patient underwent a deceased donor liver transplantation. The donor was a 50-year-old male with a height of 165 cm and weight of 55 kg (BMI 20.2 kg/m2), diagnosed with brain death induced by intracranial hemorrhage. The donor was CMV-seronegative and had an identical blood type (type B) to the patient. The graft weight was 1515 g. The liver transplant took 6 h with 35 min of anhepatic time. During the surgery, 500 mg methylprednisolone was used for immunosuppression induction. The patient received a standard immunosuppression treatment with methylprednisolone tapering (tapering from 240 mg to 20 mg within one week), mycophenolate mofetil (720 mg/day), tacrolimus (4 mg/day), and basiliximab (20 mg on POD 0 and 4) after the surgery.
The post-transplant outcome is shown in Figure 1. On postoperative day (POD) 3, the patient developed a high-grade fever (above 38.5 °C), thrombocytopenia and anemia: WBC 4.97/L (neutrophil 4.36/L), hemoglobin 73 g/L, and platelets 43/L. Experimental anti-infection treatment was initiated with vancomycin and meropenem to treat the fever. Granulocyte colony-stimulating factor (G-CSF, 75g/day) was administered three times on POD 5, 9, and 17 to prevent white blood cell (WBC) count decline, but only temporary improvement was observed. A blood culture was immediately performed and repeated twice, revealing no bacterial infections. Next Generation Sequencing screening, bacterial culture of the intubation and drainage liquid, G test, GM test, TORCH test, respiratory virus test, and a chest X-ray were also all negative. On POD 8, the CMV DNA in peripheral blood increased to 4.64 copies/mL, and ganciclovir (400 mg/day) was initiated to treat the infection. Considering the normal hepatobiliary enzymes, bilirubin levels and normal daily ultrasonogram, immunosuppression therapy (tacrolimus, mycophenolate mofetil and methylprednisolone) was temporarily stopped in order to promote an immune response to overcome the infection.
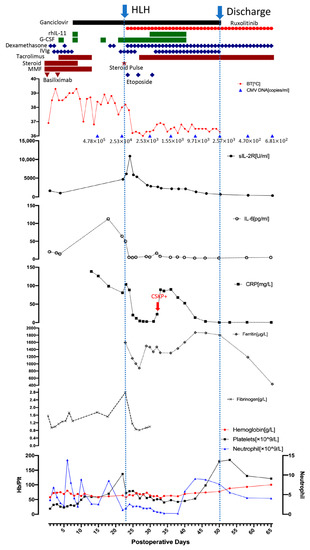
Figure 1.
The posttransplant outcome of the patient. (BT: body temperature, CRP: C-reactive protein, CSKP: carbapenem-sensitive klebsiella pneumoniae, G-CSF: granulocyte colony-stimulating factor, Hb: hemoglobin, IL-6: interleukin-6, IVIg: intravenous immunoglobulin, MMF: mycophenolate mofetil, Plt: platelet, rhIL-11: recombinant human interleukin-11, sIL-2R: soluble interleukin-2 receptor).
Despite the use of ganciclovir, the patient’s inflammatory factors, fever and pancytopenia showed no improvement. On POD 22, the WBC count declined to 1.21/L (neutrophil 1.09/L), and the patient exhibited a persistent high-grade fever. He was then tested for HCMV drug-resistant mutation, but the result was negative. Therefore, hemophagocytic lymphohistiocytosis (HLH) was strongly suspected even without a relevant family history. Bone marrow aspiration was performed on POD 23 and revealed hemophagocytosis (Figure 2). Laboratory findings: hemoglobin 58 g/L, platelets 72/L, WBC 1.65 × 109/L (neutrophils 1.54/L), ferritin 1592.5 ng/mL, fasting triglyceride 1.06 mmol/L, fibrinogen 2.78 g/L, sIL-2R 6150 U/mL, and low NK cell activity (2.1%). The patient met seven out of the eight HLH-2004 diagnostic criteria [4] and was diagnosed with HLH following LT. According to the HLH-2004 protocol, a combination of intravenous immunoglobulin (25 g/day), G-CSF (150 g/d), steroids (methylprednisolone pulse 80 mg), and etoposide (100 mg/d) was immediately administered [4]. The JAK1/2 inhibitor ruxolitinib is a novel treatment for HLH and was started the next day after diagnosis in combination with dexamethasone. The dose of ruxolitinib was maintained at 5 mg/day until discharge, and dexamethasone was tapered from 20 mg/day to 5 mg/day over three weeks and maintained at 5 mg/day until discharge (POD 28 15 mg/day, POD 34 10 mg/day, POD38 7.5 mg/day, and POD 44 5 mg/day). The patient’s fever improved rapidly following treatment initiation. On POD 25, the patient’s temperature returned to normal. The inflammatory factors IL-6 and C-reactive protein declined rapidly after the treatment. Ferritin and sIL-2R are two reliable prognostic factors of HLH; sIL-2R showed a significant decline while ferritin showed a gradual decrease after treatment initiation [41]. The WBC, hemoglobin and platelet levels showed improvement after two weeks of HLH and supportive treatment. GSF and recombinant human interleukin-11 were given to improve the platelet level, and a therapeutic plasma exchange was performed to clear the excessive cytokines. On POD29, tacrolimus was resumed due to a slight increase in liver enzymes. On POD33, the patient tested positive for carbapenem-sensitive klebsiella pneumoniae (CSKP). Minocycline and meropenem were used. Dexamethasone was reduced to 10 mg/day the next day and then to 7.5 mg/day on POD38, which led to a significant improvement in neutropenia.
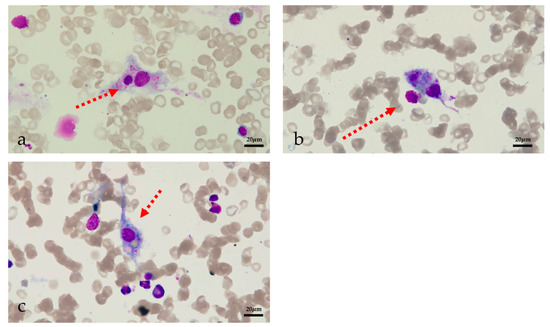
Figure 2.
Image of bone marrow aspiration (a) phagocytic neutrophils (b) phagocytic platelets (c) erythrophagocytosis.
With a stable temperature and lab findings, the patient was discharged on POD 50. His maintenance treatment included ruxolitinib (5 mg/day), dexamethasone (4.5 mg/day), tacrolimus (1 mg/day), ganciclovir, and entecavir. Regular follow-ups were performed after discharge. Dexamethasone was reduced by one tablet (0.75 mg) per week forone1 month, which resulted in a rapid decline in WBC count, which was successfully treated by increasing ruxolitinib to 10 mg/day. On POD 71 (three weeks after discharge), the peripheral blood CMV DNA was undetectable, and ganciclovir was stopped. Three months after discharge, dexamethasone (2.25 mg/day) was changed to prednisone (5 mg/day), and ruxolitinib was stopped. The patient’s temperature remained stable and the patient is doing well to date (12 months).
3. Discussion
HLH is hard to diagnose due to its non-specific symptoms. The patient’s CMV DNA was 4.64 copies/mL before transplant. After liver transplantation, with the use of immunosuppression agents, the CMV DNA increased to 4.64 copies/mL on POD8. The initial symptoms were thought to be caused by CMV reactivation, and differential diagnoses such as HLH were only considered after failure of the initial treatment. Failure to treat CMV with ganciclovir in a timely manner was indeed one of the possible causes of secondary HLH in our case. Basiliximab, plus a delayed and low-dose immunosuppressive regimen of tacrolimus were intended to reduce further CMV flareups after the transplant. Bone marrow aspiration was performed to confirm the diagnosis, which is not a mandatory diagnostic criterion, but provides valuable information for differential diagnosis.
The HLH-2004 treatment protocol includes a combination of chemotherapy (etoposide), immunosuppressive therapy (cyclosporin A, CsA), glucocorticoid, and bone marrow transplantation, which has a 5-year survival rate of about 61% [4]. CsA is proven to be beneficial in pediatric and autoimmune cases and is recommended in the HLH-2004 protocol, while tacrolimus’s efficacy has not been proved [1]. Hence, some clinicians choose to change the immunosuppression to CsA. It has been reported that tacrolimus has a stronger inhibitory effect on CD8 T cells and has shown efficacy in the treatment of HLH [42,43]. Thus, the need for switching tacrolimus to CsA is controversial (Table 1).
However, the HLH-2004 treatment protocol is a conflicting strategy for patients with HLH after transplantation, as these patients are already in an immunosuppressive state, which could also be a predisposing factor for HLH. Clinicians argued that CsA was ineffective in preventing HLH progression after solid organ transplantation [44]. The myelotoxicity, hepatotoxicity, and nephrotoxicity of etoposide may also have resulted in complications. Therefore, the immunosuppressants were stopped, and etoposide was given only three times during the early stages. Most importantly, the novel drug ruxolitinib was added to the treatment strategy.
Ruxolitinib is an oral JAK1/2 inhibitor and can inhibit the production of interferon- (IFN-) [6]. It has been approved to treat myelofibrosis, polycythemia, and steroid-refractory acute graft-versus-host disease (SR-aGVHD) by the US Food and Drug administration [45]. Considering the complexity of the cytokine storm in HLH, the pathogenesis is likely mediated by a network of cytokines rather than a single one. However, it is generally believed that the pathway involving STAT1 (signal transducer and activator of transcription 1), JAK1, JAK2, and IFN- is the common pathway of HLH [46,47,48]. Ruxolitinib has been reported to be effective in treating 61 secondary HLH patients, including 19 adults and 42 children. In nine cases, ruxolitinib was used as the first-line treatment, while in other cases it was used as a salvage therapy. However, ruxolitinib has never been reported to be used in HLH after solid organ transplantation.
In 2019, Ahmed et al. conducted a small pilot study that included seven patients, which had shown that ruxolitinib is an active and well-tolerated treatment for secondary HLH in adult patients and can be managed through an outpatient clinic [6]. Two of the patients’ conditions were triggered by an autoimmune disorder, and three were idiopathic [6]. Among the seven patients, three of them achieved a complete response, and the others achieved a partial response with improved cytopenia within the first week [6]. The use of ruxolitinib allowed the reduction or discontinuation of corticosteroids [6]. All the patients were alive with a median follow-up of 490 days (range: 190-1075 days) [6]. However, one patient was diagnosed with primary HLH and suffered a relapse, while another patient experienced a drug-related adverse event (neuropathic foot pain) and stopped the treatment [6]. The only serious side effect of ruxolitinib was grade 4 febrile neutropenia without any drug-related death [6]. Currently, a multi-center phase 2 clinical trial is investigating the efficacy of ruxolitinib (NCT04551131). In addition, participants are being recruited for a phase 4 clinical trial focusing on malignancy-related HLH and the effect of ruxolitinib (NCT04999878).
Wang et al. conducted a clinical trial, which included 34 children with refractory/relapsed HLH [7]. Ruxolitinib was given as a salvage therapy after the HLH-94 regimen failure. Twenty five children responded to the therapy and five of them had complete remission. Leukopenia and thrombocytopenia were common side effects, but all the patients could tolerate them. At the end of the study, 15 patients died, and the median survival time was 22 weeks. The clinicians argued that ruxolitinib could alleviate the hyperinflammatory state but was not sufficiently potent. They suggested combining ruxolitinib with other treatment strategies to achieve better outcomes. A large-scale clinical trial is being conducted to validate the effectiveness of the DEP-ruxolitinib regimen (NCT03533790)
In 2019, Meng performed a single-center retrospective analysis evaluating 12 patients who developed SR-aGVHD after allogeneic hematopoietic stem cell transplantation (allo-HSCT), complicated by Epstein-Barr virus-associated HLH [8]. Among the 12 patients, seven achieved a complete response, three achieved a partial response, and two exhibited treatment failure. The side effects only included neutropenia (grade 3 to 4) and thrombocytopenia (grade 3 to 4). Ruxolitinib was apparently effective for patients with EBV-HLH combined with SR-aGVHD.
In 2020, Wei conducted a retrospective analysis of pediatric cases with refractory or recurrent HLH and found that ruxolitinib was effective as a salvage therapy [9]. Among the nine analyzed cases, five cases of HLH were secondary to EBV, one was secondary to an autoimmune disorder, two were familial HLH, and one was idiopathic. Before the use of ruxolitinib, all of them were treated according to the HLH-94 treatment strategy but did not show improvement. After receiving a continuous 28-day course of ruxolitinib, three of them achieved partial remission, five improved and one died.
Furthermore, six case reports argued that ruxolitinib is beneficial to refractory HLH both for children and adults. Slostad reported a case of histoplasmosis-related HLH treated with ruxolitinib as a first-line treatment, which achieved a rapid improvement [10]. Zandvakili presented a case of autoimmune disease-related HLH, also using ruxolitinib as a first-line therapy with a good outcome even after the patient developed sepsis [11]. Goldsmith reported two HLH cases, one autoimmune disorder-associated and the other EBV-related [12]. Ruxolitinib was used as a salvage therapy and led to a rapid symptom improvement. Zhao reported a pediatric case of EBV-associated HLH successfully treated with ruxolitinib as a bridge to allogeneic stem cell transplantation [13]. Brogile reported a pediatric case of idiopathic HLH receiving ruxolitinib after several failed treatments, including the biological treatment anakinra. The patient’s fever subsided within 24 h of initiating ruxolitinib, and recovery was achieved without other treatments [14]. Ono successfully used ruxolitinib in a pediatric patient who developed HLH after hematopoietic cell transplantation [15].
The most commonly reported adverse effects of ruxolitinib were hematologic adverse reactions including thrombocytopenia and anemia [49,50]. Dizziness, headache, and infections are common nonhematologic adverse effects [45,49]. Hepatotoxicity is rare and only moderate liver toxicity was reported [51,52]. For patients with hepatic impairment, the dosage of ruxolitinib should be modified according to platelet counts [45]. Both tacrolimus and ruxolitinib are mainly metabolized through cytochrome P450 (CYP) 3A4 enzyme, but no drug interaction between them has been reported [53,54]. For ruxolitinib, dose reduction is only needed when a potent CYP3A4 inhibitor is concomitantly used [53]. However, tacrolimus has a narrow therapeutic index and is more likely to be influenced by other drugs that also metabolize through CYP3A4 [54]. So close monitoring of tacrolimus whole blood concentrations is recommended. Based on this information, we decided to lower the dose of ruxolitinib to 5 mg/day, in comparison to 20 mg twice daily for myelofibrosis patients [45]. The platelet count and liver function were closely monitored, and tacrolimus trough concentration was tested every day.
Our patient developed a high-grade fever shortly after the operation and only CMV infection was detected. However, no symptom improvement was observed following the initiation of ganciclovir. Furthermore, steroids and immunosuppression drugs, as the two major components of HLH-2004 treatment, had already been given for around two weeks. Hence, we considered that the patient was unresponsive to the HLH-2004 treatment protocol. In addition, a pilot study reported ruxolitinib as a first-line treatment for adult secondary HLH [6], and case reports described the use of ruxolitinib as a first-line therapy to treat Hematopoietic Cell Transplantation-associated HLH [10,11,15]. Moreover, some clinicians argued that ruxolitinib may exert a synergistic effect with HLH-2004 treatment [55]. Ruxolitinib was added to our treatment strategy and the dosage and frequency of etoposide were significantly reduced (100 mg three times instead of 150 mg ten times). Dexamethasone was tapered much more rapidly and was maintained at a low level (within three weeks instead of eight weeks according to HLH-2004 strategy).
Our case is the first to report the use of ruxolitinib as an initial therapy instead of a salvage therapy in HLH patients after solid organ transplantation. The patient’s fever subsided within 24 h, with a rapid improvement in ferritin, and sIL-2R levels. However, ruxolitinib treatment resulted in a slow improvement in cytopenia, possibly due to the use of ganciclovir and tacrolimus or the adverse effect of ruxolitinib (neutropenia and thrombocytopenia). The neutropenia and thrombocytopenia spontaneously improved after a reduction in the dexamethasone dosage. The patient is now doing fine with a normal liver function and minimal immunosuppressants almost one year after liver transplantation.
In summary, we present an adult case of secondary HLH associated with CMV infection following a liver transplant successfully treated with a modified HLH-2004 treatment protocol, which included ruxolitinib. The rapid improvement and good prognosis indicate that ruxolitinib may be useful as an initial therapy for patients with HLH after solid organ transplantation.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; A Khamashta, M.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Jordan, M.B.; Filipovich, A.H. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: A journey of a thousand miles begins with a single (big) step. Bone Marrow Transpl. 2008, 42, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ohdan, H.; Tashiro, H.; Itamoto, T.; Tanaka, Y.; Mizunuma, K.; Tokita, D.; Onoe, T.; Ito, R.; Asahara, T. Differential diagnosis between graft-versus-host disease and hemophagocytic syndrome after living-related liver transplantation by mixed lymphocyte reaction assay. J. Investig. Surg. 2004, 17, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Di Trolio, R.; Kozlakidis, Z.; Busto, G.; Ingenito, C.; Buonerba, L.; Ferrara, C.; Libroia, A.; Ragone, G.; Ioio, C.D.; et al. COVID 19 therapies and anti-cancer drugs: A systematic review of recent literature. Crit. Rev. Oncol. 2020, 152, 102991. [Google Scholar] [CrossRef]
- Ahmed, A.; A Merrill, S.; Alsawah, F.; Bockenstedt, P.; Campagnaro, E.; Devata, S.; Gitlin, S.D.; Kaminski, M.; Cusick, A.; Phillips, T.; et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: An open-label, single-centre, pilot trial. Lancet Haematol. 2019, 6, e630–e637. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wu, L.; Wang, X.; Jin, Z.; Gao, Z.; Wang, Z. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica 2019, 105, e210–e212. [Google Scholar] [CrossRef]
- Meng, G.; Wang, J.; Wang, X.; Wang, Y.; Wang, Z. Ruxolitinib treatment for SR-aGVHD in patients with EBV-HLH undergoing allo-HSCT. Ann. Hematol. 2020, 99, 343–349. [Google Scholar] [CrossRef]
- Wei, A.; Ma, H.; Li, Z.; Zhang, L.; Zhang, Q.; Wang, D.; Lian, H.; Zhang, R.; Wang, T. Short-term effectiveness of ruxolitinib in the treatment of recurrent or refractory hemophagocytic lymphohistiocytosis in children. Int. J. Hematol. 2020, 112, 568–576, Epub 20200714. [Google Scholar] [CrossRef]
- Slostad, J.; Hoversten, P.; Haddox, C.L.; Cisak, K.; Paludo, J.; Tefferi, A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: A single patient experience. Am. J. Hematol. 2017, 93, E47–E49. [Google Scholar] [CrossRef]
- Zandvakili, I.; Conboy, C.B.; Ayed, A.O.; Cathcart-Rake, E.J.; Tefferi, A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: A second experience. Am. J. Hematol. 2018, 93, E123–E125. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Rehman, S.S.U.; Shirai, C.L.; Vij, K.; DiPersio, J.F. Resolution of secondary hemophagocytic lymphohistiocytosis after treatment with the JAK1/2 inhibitor ruxolitinib. Blood Adv. 2019, 3, 4131–4135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, J.; Li, X.; Wang, J.; Sun, J.; Zhou, J.; Huang, H. Salvage therapy with dose-escalating ruxolitinib as a bridge to allogeneic stem cell transplantation for refractory hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2020, 55, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Broglie, L.; Pommert, L.; Rao, S.; Thakar, M.; Phelan, R.; Margolis, D.; Talano, J.-A. Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosis. Blood Adv. 2017, 1, 1533–1536. [Google Scholar] [CrossRef]
- Ono, R.; Ashiarai, M.; Hirabayashi, S.; Mizuki, K.; Hosoya, Y.; Yoshihara, H.; Ohtake, J.; Mori, S.; Manabe, A.; Hasegawa, D. Ruxolitinib for hematopoietic cell transplantation-associated hemophagocytic lymphohistiocytosis. Int. J. Hematol. 2021, 113, 297–301. [Google Scholar] [CrossRef]
- Chisuwa, H.; Hashikura, Y.; Nakazawa, Y.; Kamijo, T.; Nakazawa, K.; Nakayama, J.; Oh-Ishi, T.; Ikegami, T.; Terada, M.; Kawasaki, S. Fatal hemophagocytic syndrome after living-related liver transplantation: A report of two cases. Transplantation 2001, 72, 1843–1846. [Google Scholar] [CrossRef]
- Karasu, Z.; Kilic, M.; Cagirgan, S.; Lebe, E.; Yilmaz, F.; Demirbas, T.; Tokat, Y. Hemophagocytic syndrome after living-related liver transplantation. Transplant. Proc. 2003, 35, 1482–1484. [Google Scholar] [CrossRef]
- Lladó, L.; Figueras, J.; Comí, S.; Torras, J.; Serrano, T.; Castellote, J.; Motomura, T.; Mano, Y.; Itoh, S.; Harada, N.; et al. Haemophagocytic syndrome after liver transplantation in adults. Transpl. Int. 2004, 17, 221–223. [Google Scholar] [CrossRef]
- George, T.I.; Jeng, M.; Berquist, W.; Cherry, A.M.; Link, M.P.; Arber, D.A. Epstein-Barr virus-associated peripheral T-cell lymphoma and hemophagocytic syndrome arising after liver transplantation: Case report and review of the literature. Pediatr. Blood Cancer 2005, 44, 270–276. [Google Scholar] [CrossRef]
- Taniai, N.; Akimaru, K.; Kawano, Y.; Mizuguchi, Y.; Shimizu, T.; Takahashi, T.; Mamada, Y.; Yoshida, H.; Tajiri, T. Hemophagocytic syndrome after living-donor liver transplantation for fulminant liver failure: A case report. Hepatogastroenterology 2005, 52, 923–926. [Google Scholar]
- Hardikar, W.; Pang, K.; Al-Hebbi, H.; Curtis, N.; Couper, R. Successful treatment of cytomegalovirus-associated haemophagocytic syndrome following paediatric orthotopic liver transplantation. J Paediatr Child Health. 2006, 42, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Tamura, S.; Matsui, Y.; Hasegawa, K.; Imamura, H.; Kokudo, N.; Makuuchi, M. Hemophagocytic syndrome after adult-to-adult living donor liver transplantation. Transplant. Proc. 2006, 38, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Taketomi, A.; Kayashima, H.; Harada, N.; Uchiyama, H.; Yamashita, Y.-I.; Ikegami, T.; Soejima, Y.; Nishizaki, T.; Shimada, M.; et al. Successful treatment for a patient with hemophagocytic syndrome after a small-for-size graft liver transplantation. Hepatogastroenterology 2008, 55, 359–362. [Google Scholar] [PubMed]
- Dharancy, S.; Crombe, V.; Copin, M.; Boleslawski, E.; Bocket, L.; Declerck, N.; Canva, V.; Dewilde, A.; Mathurin, P.; Pruvot, F. Fatal hemophagocytic syndrome related to human herpesvirus-6 reinfection following liver transplantation: A case report. Transplant. Proc. 2008, 40, 3791–3793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, C.; Yan, L.; Pu, C.; Li, Y.; Kang, Q.; Ren, Z.; Jin, X. Successful treatment of Epstein-Barr Virus-associated haemophagocytic syndrome arising after living donor liver transplantation. Hepatol. Res. 2009, 39, 421–426. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Fiel, M.I.; Martin, J.D.R.; Aloman, C.; Schiano, T.D. Hemophagocytic syndrome occurring in an adult liver transplant recipient having Still’s disease. Hepatol. Int. 2010, 5, 597–602. [Google Scholar] [CrossRef][Green Version]
- Soyama, A.; Eguchi, S.; Takatsuki, M.; Hidaka, M.; Tomonaga, T.; Yamanouchi, K.; Miyazaki, K.; Inokuma, T.; Tajima, Y.; Kanematsu, T. Hemophagocytic syndrome after liver transplantation: Report of two cases. Surg. Today 2011, 41, 1524–1530. [Google Scholar] [CrossRef]
- Rodríguez-Medina, B.; Blanes, M.; Vinaixa, C.; Aguilera, V.; Rubín, A.; Prieto, M.; Berenguer, M. Haemophagocytic syndrome in a liver transplant patient during treatment with Telaprevir. Ann. Hepatol. 2013, 12, 974–978. [Google Scholar] [CrossRef]
- Jha, B.; Mohan, N.; Gajendra, S.; Sachdev, R.; Goel, S.; Sahni, T.; Raina, V.; Soin, A. Prompt diagnosis and management of Epstein-Barr virus-associated post-transplant lymphoproliferative disorder and hemophagocytosis: A dreaded complication in a post-liver transplant child. Pediatr. Transplant. 2015, 19, E177–E180. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, S.; Wyss, C.; Meylan, P.R.; Bisig, B.; Letovanec, I.; Manuel, O.; Pascual, M.; de Leval, L. Fatal Outcome of Multiple Clinical Presentations of Human Herpesvirus 8-related Disease after Solid Organ Transplantation. Transplantation 2016, 100, 134–140. [Google Scholar] [CrossRef]
- Iseda, N.; Yoshizumi, T.; Toshima, T.; Morinaga, A.; Tomiyama, T.; Takahashi, J.; Motomura, T.; Mano, Y.; Itoh, S.; Harada, N.; et al. Hemophagocytic syndrome after living donor liver transplantation: A case report with a review of the literature. Surg. Case Rep. 2018, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M.; Langer, A.L.; Sima, H.; Chang, C.; Troy, K.; Taimur, S. Hemophagocytic Lymphohistiocytosis Due to Primary HHV-8 Infection in a Liver Transplant Recipient. Transplant. Direct 2018, 4, e411. [Google Scholar] [CrossRef] [PubMed]
- Jarchin, L.; Chu, J.; Januska, M.; Merola, P.; Arnon, R. Autoimmune hemolytic anemia: An unusual presentation of hemophagocytic lymphohistiocytosis in a pediatric post-liver transplant patient. Pediatr. Transplant. 2018, 22, e13281. [Google Scholar] [CrossRef]
- Valenzuela, E.F.; García, A.C.; García-Pajares, F.; Tejada, J.T.; Muñoz, R.N.; Martín, C.M.; Delgado, L.S.; Martín, C.A.; Álvarez, C.A.; Fernández-Fontecha, E.; et al. Hemophagocytic Syndrome as Uncommon Cause of Severe Pancytopenia after Liver Transplantation. Transplant. Proc. 2020, 52, 1500–1502. [Google Scholar] [CrossRef]
- Yamada, M.; Sakamoto, S.; Sakamoto, K.; Uchida, H.; Shimizu, S.; Osumi, T.; Kato, M.; Shoji, K.; Arai, K.; Miyazaki, O.; et al. Fatal Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis with virus-infected T cells after pediatric multivisceral transplantation: A proof-of-concept case report. Pediatr. Transplant. 2021, 25, e13961. [Google Scholar] [CrossRef] [PubMed]
- Chesner, J.; Schiano, T.D.; Fiel, M.I.; Crismale, J.F. Hemophagocytic lymphohistiocytosis occurring after liver transplantation: A case series and review of the literature. Clin. Transplant. 2021, 35, e14392. [Google Scholar] [CrossRef]
- Arikan, C.; Erbey, F.; Ozdogan, E.; Akyildiz, M. Posttransplant Hemophagocytic Lymphohistiocytosis in Pediatric Liver Transplant Recipients. Liver Transpl. 2021, 27, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Gandotra, A.; Mehtani, R.; Premkumar, M.; Duseja, A.; De, A.; Mallik, N.; Durgadevi, S.; Das, A.; Kalra, N. Invasive Pulmonary Aspergillosis and Tuberculosis Complicated by Hemophagocytic Lymphohistiocytosis—Sequelae of COVID-19 in a Liver Transplant Recipient. J. Clin. Exp. Hepatol. 2022, 12, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hiejima, E.; Nihira, H.; Kato, K.; Honda, Y.; Izawa, K.; Kawabata, N.; Kato, I.; Ogawa, E.; Sonoda, M.; et al. Case Report: A Case of Epstein-Barr Virus-Associated Acute Liver Failure Requiring Hematopoietic Cell Transplantation after Emergent Liver Transplantation. Front. Immunol. 2022, 13, 825806. [Google Scholar] [CrossRef]
- Ferreira, G.D.S.A.; Moreira, M.L.; Watanabe, A.L.C.; Trevizoli, N.C.; Murta, M.C.B.; Figueira, A.V.F.; Caja, G.O.N.; Ferreira, C.A.; Jorge, F.M.F.; Couto, C.D.F. Hemophagocytic Lymphohistiocytosis Secondary to Tuberculosis after Liver Transplantation: A Case Report. Transplant. Proc. 2022, 54, 1384–1387. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Sokol, L. Hereditary and acquired hemophagocytic lymphohistiocytosis. Cancer Control 2014, 21, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; McKeage, K.; Keam, S.J.; Plosker, G.L. Tacrolimus: A further update of its use in the management of organ transplantation. Drugs 2003, 63, 1247–1297. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, S.; Yasuda, S.; Hashimoto, T.; Oku, K.; Kataoka, H.; Horita, T.; Atsumi, T.; Koike, T. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: Analysis of 30 cases. Rheumatology 2008, 47, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Karras, A.; Thervet, E.; Legendre, C. Groupe Cooperatif de transplantation d’Ile de F. Hemophagocytic syndrome in renal transplant recipients: Report of 17 cases and review of literature. Transplantation 2004, 77, 238–243. [Google Scholar] [CrossRef]
- Jakafi. Highlights of Prescribing Information. US Food and Drug Administration. 2020. Available online: https://www.jakafi.com/pdf/prescribing-information.pdf (accessed on 23 October 2022).
- Jordan, M.B.; Hildeman, D.; Kappler, J.; Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004, 104, 735–743. [Google Scholar] [CrossRef]
- Schmid, J.P.; Ho, C.-H.; Chrétien, F.; Lefebvre, J.M.; Pivert, G.; Kosco-Vilbois, M.; Ferlin, W.; Geissmann, F.; Fischer, A.; Basile, G.D.S. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol. Med. 2009, 1, 112–124. [Google Scholar] [CrossRef]
- Das, R.; Guan, P.; Sprague, L.; Verbist, K.; Tedrick, P.; An, Q.A.; Cheng, C.; Kurachi, M.; Levine, R.; Wherry, E.J.; et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 2016, 127, 1666–1675. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Deyà-Martínez, A.; Rivière, J.G.; Roxo-Junior, P.; Ramakers, J.; Bloomfield, M.; Hernandez, P.G.; Lobo, P.B.; Abu Jamra, S.R.; Esteve-Sole, A.; Kanderova, V.; et al. Impact of JAK Inhibitors in Pediatric Patients with STAT1 Gain of Function (GOF) Mutations-10 Children and Review of the Literature. J. Clin. Immunol. 2022, 42, 1071–1082. [Google Scholar] [CrossRef]
- Redondo, S.; Esquirol, A.; Novelli, S.; Caballero, A.C.; Garrido, A.; Oñate, G.; López, J.; Moreno, C.; Saavedra, S.-D.; Granell, M.; et al. Efficacy and Safety of Ruxolitinib in Steroid-Refractory/Dependent Chronic Graft-versus-Host Disease: Real-World Data and Challenges. Transplant. Cell. Ther. 2021, 28, 43.e1–43.e5. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.G.; Chen, X.; Emm, T.; Scherle, P.A.; McGee, R.F.; Lo, Y.; Landman, R.R.; McKeever, E.G.; Punwani, N.G.; Williams, W.V.; et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J. Clin. Pharmacol. 2012, 52, 809–818. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T. Drug interactions with tacrolimus. Drug Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, J.; Liang, X.; Mao, X.; Wang, Z.; Huang, W. Low dose ruxolitinib plus HLH-94 protocol: A potential choice for secondary HLH. Semin. Hematol. 2020, 57, 26–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).