Abstract
This retrospective study aims to investigate the factors associated with the occurrence of ADEs using nationally representative claims data. All patients with at least one claim with diagnosis codes denoting potential ADE between 1 July 2015 and 31 December 2015 were included. Potential ADE was defined as ADE identified in the claims data, because it was not verified. The index date was defined as the date of the first claim with potential ADEs. Demographic data were collected at the index date, while data on comorbidities and number of medications used were collected six months before the index date. Multivariate logistic regression was used to explore the association between potential ADEs and several factors, including sex, age group, insurance type, comorbidities, and number of prescribed medications. Patients with potential ADEs were older, had more chronic diseases, and used more medications than those without potential ADEs. In the multivariate analysis, occurrence of potential ADEs was associated with age (≥65 years, odds ratio [OR] 1.15, 95% confidence interval [CI] 1.08–1.21), Medical Aid program (OR 1.37, 95% CI 1.27–1.47), Charlson Comorbidity Index scores (≥5, OR 2.87, 95% CI 2.56–3.20), and use of six or more medications (6–10 medications, OR 1.89, 95% CI 1.79–1.99). Age, Medical Aid program, comorbidities, and number of medications were associated with occurrence of potential ADEs.
1. Introduction
With increasing drug use, adverse drug events (ADEs) have become a critical public health concern. In previous studies, approximately 5–10% of patients had ADEs, which increased the risk of death, and the costs associated with ADEs were substantial [1,2,3,4,5,6,7,8]. Furthermore, nearly one third of ADEs are considered preventable and are more likely to be serious and life-threatening events [5]. A study conducted in the late 1990s in two tertiary hospitals in the USA reported that annual costs attributable to preventable ADEs were approximately $2.8 million [5,6,7]. In addition, a recent systematic review of 18 observational studies conducted in the USA and Europe found that the estimated costs of preventable ADEs reached €9000 [9].
One potentially effective strategy for preventing ADEs is identifying those patients at risk of ADEs. Despite concerns that ADEs contribute to significant clinical and economic problems, little is known about the factors associated with ADEs among Asian populations, including the South Korean population. According to a number of studies conducted in Western countries, important independent risk factors for ADEs include sex, age, comorbidities, polypharmacy, health service utilization, and inappropriate drug use [10,11,12]. However, risk factors for ADE may differ according to the characteristics of the study population, including culture, economic status, and patterns of drug use. For instance, a study conducted in 81 hospitals in Italy reported that female gender was a risk factor for ADR-related hospital admissions [13]. However, a prospective observational study conducted in two hospitals in Spain found that sex was not associated with ADEs [14]. Moreover, previous studies determined that risk factors related to the occurrence of ADEs were restricted to hospitals representing a local population or specific age groups [11,15,16]. Previous studies in South Korea that explored risk factors associated with ADEs were often limited to genetic factors for specific drugs [17,18]. Thus, these results are difficult to generalize in clinical practice. The proportion of older adults in South Korea is increasing rapidly, and, similarly to other countries in Asia, South Korea has a cultural preference for consuming health supplements such as herbal medicines [19,20]. Therefore, the risk factors associated with ADEs in South Korea may differ from those reported in previous studies conducted in Western countries. Understanding the factors associated with ADEs will aid in prioritizing policy activities for ADE prevention.
Therefore, this retrospective study was conducted using population-based data to investigate the associated factors for ADEs in South Korea.
2. Materials and Methods
2.1. Data Source
We used the National Patient Sample database from the Health Insurance Review and Assessment Service (HIRA-NPS) from 1 January 2015 to 31 December 2015. The HIRA-NPS database is a secondary data source of sex- and age-stratified random samples (approximately 1,400,000 persons) from health insurance claims data covering approximately 50 million South Korean enrollees. Based on a validation study, the HIRA-NPS database provided the national representativeness of all enrollees [21,22]. In South Korea, there are two government-run mandatory national health security systems: The National Health Insurance (NHI) program is a wage-based, contributory insurance program that covers 96% of the population; the Medical Aid (MA) program is a government-subsidized public health assistance program for low-income households and individuals who are unable to pay for health care [23]. The HIRA-NPS database provides claims records for all types of healthcare services, including outpatient care, inpatient care, emergency department visits, diagnoses, prescribed medications, and sociodemographic information of enrollees, including sex, age, and type of health insurance [21]. The International Classification of Diseases, Tenth Revision (ICD-10) was recorded as diagnoses in the database. Individuals could not be identified because data are anonymized [24].
2.2. Study Subjects
Patients were identified as having a potential ADE if they had at least one claim record of ADE diagnosis codes during a six-month intake period from 1 July to 31 December 2015. Figure 1 shows the study scheme used in this study. We defined the index date for patients with potential ADE as the date of the first ADE claim during the intake period. For patients without potential ADE, we defined the index date as the date of the first claim record during the intake period. We also obtained baseline characteristics of the study population, such as comorbidities and number of prescribed medications, six months from the pre-index period before the index date. To identify patients with new cases of potential ADE, individuals were excluded if they had at least one claim record of ADE within the six months before the index date.
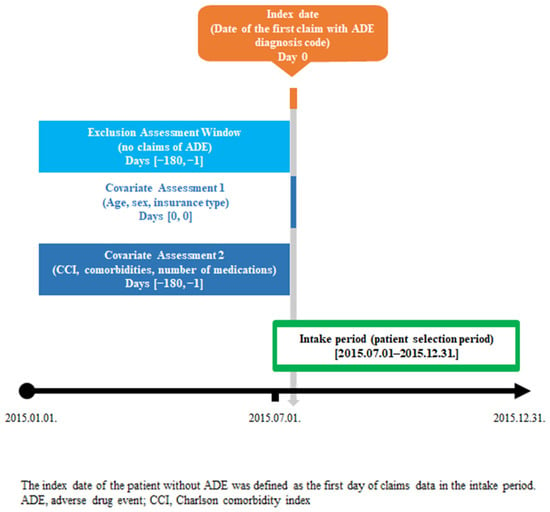
Figure 1.
Study scheme.
2.3. Identification of Potential ADEs
In the selection of patients with ADE, we defined an ADE as “harm caused by a drug or the inappropriate use of a drug” [25]. Compared to adverse drug reactions, defined as “harmful and unintended consequences occurring due to appropriate use of a drug,” ADE consists of a broad spectrum of events or reactions [26]. Thus, we included adverse effects of therapeutic use, poisoning, failure of medical care, and medication errors.
To identify ADEs in the claims data, we used ICD-10 codes that met one of the following criteria based on the previous study: (1) code description includes the phrase “caused by a drug” or “caused by a drug or other substance”; (2) code description includes the phrase “poisoning by a drug” or “poisoning by a drug or other substance”; (3) code description includes the phrase “caused by vaccine”; and (4) code description does not refer to a drug but implies as “ADE very likely” [27]. Finally, we included 586 ICD-10 codes that identified ADEs (Supplementary Table S1). However, we did not verify whether identified ADEs in the claims data were true ADEs in the patients’ original health records. Therefore, we defined it as potential ADEs.
2.4. Statistical Analysis
To compare the baseline characteristics of patients with and without potential ADE, we used the Student t-test for continuous variables and chi-square test or Fisher’s exact test for categorical variables. A p-value < 0.05 indicated a difference in the characteristics between the two groups.
To identify the factors associated with the occurrence of potential ADEs, we performed univariate logistic analysis to explore the potentially important variables to be used in the subsequent multivariate logistic regression. We included variables and categorized them as follows: sex (male or female), age group (<20, 20–44, 45–64, and ≥65 years), insurance type (NHI or MA), Charlson Comorbidity Index score (0, 1, 2, 3, 4, and ≥5), comorbidities, and number of medications (<6, 6–10, 11–20, and ≥21). We obtained odds ratios (ORs) and 95% confidence intervals (CIs) after adjusting for sex, age group, insurance type, Charlson Comorbidity Index score, comorbidities, and number of prescribed medications within six months from the index date. The Charlson Comorbidity Index score is a standard measure of disease burden, and a high index score indicates poor health conditions [28]. According to Quan’s coding algorithms, we calculated the Charlson Comorbidity Index score by analyzing all diagnosis codes within six months from the index date [29]. Comorbidities included in the Charlson Comorbidity Index were measured within six months from the index date. To measure the number of prescribed medications within six months, we collected the Korean national drug codes denoting the general name code of drugs and counted the number of unique active drug ingredients per patient within six months, whether different drugs were prescribed on the same day or on different days. The code consists of nine digits, including six numbers and three English letters. The active ingredient of a medication can be identified by the first four digits of the Korean national drug codes. Accordingly, we defined different medications by identifying distinct active ingredients. All variables used in the present study were identified in previous studies as factors influencing ADEs [12,30,31].
All analyses were performed using the SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
During the intake period, from 1 July 2015 to 31 December 2015, the study population was 1,326,638. Of these, 15,713 (1.18%) individuals were identified as new patients with potential ADEs who met the eligibility criteria. The numbers of patients with potential ADEs who had outpatient, inpatient, and emergency department visits were 12,612 (80.26%), 2093 (13.32%), and 1008 (6.42%), respectively. There were significant differences in most baseline characteristics among patients with and without potential ADEs (Table 1).

Table 1.
Baseline characteristics of the study population.
Compared to patients without potential ADEs, patients with potential ADEs were older and more frequently enrolled in the MA program. Furthermore, the average Charlson Comorbidity Index scores and number of prescribed medications within 6 months in patients with potential ADEs were significantly higher than in those without potential ADEs.
To investigate independent associations with potential ADEs, we conducted a multivariate logistic regression analysis followed by univariate logistic regression analysis (Table 2). We found that independent factors associated with potential ADEs were age ≥ 20 years (20–44 years, OR 1.24, 95% CI 1.18–1.30; 45–64 years, OR 1.17, 95% CI 1.12–1.23; ≥65 years, OR 1.09, 95% CI 1.03–1.16) and MA enrollees (OR 1.35, 95% CI 1.25–1.45). Female sex showed a relationship with potential ADEs in the univariate analysis, but this correlation was not observed after controlling for age, insurance type, Charlson Comorbidity Index score, and number of prescribed medications.

Table 2.
Unadjusted and adjusted odds ratio of potential adverse drug events for characteristics of the study population.
Particularly, comorbidities and number of medications had considerable association with the occurrence of potential ADEs. Moreover, there were increasing patterns of ORs for potential ADEs with higher Charlson Comorbidity Index scores and number of medications. Chronic diseases significantly associated with the occurrence of potential ADEs were connective tissue/rheumatic disease (OR 1.22, 95% CI 1.11–1.34), peptic ulcer disease (OR 1.08, 95% CI 1.02–1.13), mild liver disease (OR 1.30, 95% CI 1.24–1.37), moderate-to-severe liver disease (OR 1.56, 95% CI 1.18–2.06), diabetes without complications (OR 1.12, 95% CI 1.06–1.17), cancer (OR 1.15, 95% CI 1.05–1.25), and metastatic carcinoma (OR 1.43, 95% CI 1.17–1.76).
4. Discussion
This retrospective study investigated the association of patient characteristics, disease, and medication with the occurrence of potential ADEs using nationally representative claims data. Multivariate logistic regression analysis revealed that age, MA program, comorbidities, and number of medications were associated with potential ADEs after controlling for baseline characteristics of the study population.
We defined the study outcomes as ADEs to comprehensively capture the harm related to drug use. Adverse drug reaction (ADR), often used as another research outcome of adverse effects associated with medication, involves harmful and unintended consequences that occur at doses normally used in humans for prophylaxis, diagnosis, or treatment of disease [26]. However, ADR does not include clinically significant issues, such as poisoning and medication errors, because it involves appropriate drug use only. For example, hearing loss due to overdose of a potential drug would not be considered an ADR but an ADE. Moreover, the term ADE is preferable because the diagnosis code cannot differentiate the cause of adverse effects in the claims data [32].
Our results showed that sex was not associated with potential adverse effects. This finding is consistent with previous results [11,33]. However, female gender was frequently reported as an independent factor for ADEs [13,16,34,35,36]. A prospective multicenter study in Europe reported that women had a higher risk of ADE occurrence than men, as reflected in the significant OR of 1.60 (95% CI 1.31–1.94) [36]. In a study conducted in the UK, including individuals aged ≥65 years, female gender was associated with a higher occurrence of ADEs [16]. Potential reasons for ADE risk in women are differences in physical (body fat and organ function) and physiological features (pregnancy and menopause), as well as differences in pharmacodynamics (effects of drugs) and pharmacokinetics (absorption, distribution, metabolism, and excretion) [37,38,39,40]. However, other studies reported that male gender was an independent factor for ADEs [15,41].
Several studies have already found an apparent increase in ADE risk with age [11,16,33,42,43]. A possible explanation for the effect of age on the occurrence of ADE is that the physiological changes associated with advancing age may render individuals vulnerable to adverse impacts [44]. However, there have been inconsistent findings regarding the effect of age on the occurrence of ADEs [11,45,46]. For instance, a multicenter survey that included internal medicine and geriatric wards reported that age was not a significant predictor of ADEs [45]. Conversely, a study conducted in two Dutch hospitals reported that patients aged ≥80 years had lower risk of occurrence of ADEs than those aged ≤60 years [46]. Interestingly, in our study, the risk of potential ADEs showed a tendency to decrease with increasing age after adjusting for the characteristics of the study patients. Further studies are required to explore the causes of the different effects of age on the occurrence of ADEs.
Patients enrolled in the MA program showed a higher OR for the occurrence of potential ADEs than those enrolled in the NHI program. A 2014 study in South Korea reported that polypharmacy was associated with MA enrollees after controlling for sex, age, and chronic diseases [47]. They also expected to use excessive healthcare utilization because of having a low perception of efficient use of healthcare resources [48]. Although we did not consider healthcare utilization, such as history of hospitalization, outpatient visits, and emergency department visits in this study, service and drug utilization patterns might be a potential reason for the effects on the occurrence of ADEs.
The important finding of this study is that comorbidities and number of medications showed a significant association with the occurrence of potential ADEs. Patients with chronic comorbidities have an increased likelihood of polypharmacy [49]. Several previous studies frequently reported that polypharmacy was an important independent predictor for ADEs [11,13,16]. For example, according to a study in New England, USA, Medicare enrollees using eight or more medications showed a 2.9 odds of ADEs compared to those with one or fewer medications [16]. Furthermore, concomitant drug use was considerably associated with an increased risk of serious adverse effects [50]. A potential reason for the impact of polypharmacy on ADEs might be that using several medications may increase the probability of drug–drug interactions and inappropriate drug use [51]. Furthermore, in this study, patients with moderate-to-severe liver disease and metastatic cancer were highly associated with the occurrence of potential ADEs compared to other chronic conditions. Impaired liver function may influence the pharmacokinetics and pharmacodynamics of drugs, as most drugs are predominantly metabolized in the liver. For instance, lower hepatic blood flow may affect drug clearance, and hypoalbuminemia can cause increased concentrations of highly protein-bound drugs. Patients with metastatic cancer receive chemotherapy to delay disease progression and improve their quality of life. However, cancer chemotherapy also causes various severe adverse effects, such as neutropenia, vomiting, diarrhea, and weakness. Furthermore, these patients are frequently exposed to drug–drug interactions because they receive a high number of medications and are significantly susceptible to adverse effects due to their intensive medical treatment [52,53,54]. Therefore, physicians and pharmacists should be more aware of potential drug–drug interactions and pay attention to ADEs in fragile patients.
A better understanding of individual factors associated with ADE is crucial for improving patient safety. In the present study, older adults, MA program enrollees, and patients with polypharmacy or high comorbid conditions were at greater risk of potential ADEs. Our findings highlight the importance of health policy activities in prioritizing ADE prevention for vulnerable patients.
To the best of our knowledge, this is the first study to explore factors associated with the occurrence of potential ADEs using a representative database that appropriately reflects the entire South Korean population. The HIRA-NPS data contain valuable healthcare resource information, including sociodemographic characteristics, diagnoses, and drug utilization. Therefore, claims data have the advantage of comprehensively investigating the association between individual-level factors, such as disease and medication, and occurrence of ADEs. The International Classification of Diseases, eleventh Revision (ICD-11) was adopted in 2019 and implemented worldwide. Therefore, to identify ADEs using the ICD-11 codes, it is necessary to perform direct mapping from ICD-10 to ICD-11 and determine whether the individually matched ICD-11 codes indicate an ADE. Furthermore, the ICD-11 codes provide more health information; therefore, we expect that more ADEs can be detected using the ICD-11 codes in the claims data. We suggest that further studies should be conducted on the epidemiology of ADEs using the ICD-11 codes.
However, this study has several limitations. Firstly, as with other previous studies using administrative databases, we could not capture all ADEs, including abnormal laboratory values, because limited clinical information was available in the claims data. Furthermore, there is a limitation in detecting patients with ADEs owing to the restricted diagnosis codes indicating ADEs. Several approaches to identify ADE have been developed, including spontaneous reporting, chart review, computerized monitoring systems, and administrative data [55]. Spontaneous reporting is the most widely used method, but under-reporting is a problem that limits its effectiveness. Chart review is the gold standard, but it is time- and cost-intensive. Computerized monitoring systems, compared to chart reviews, are more efficient in identifying ADEs, but it is challenging to develop algorithms with high-specificity signals. Administrative data have emerged as an alternative for the identification of ADEs. Compared to other methods, it is relatively inexpensive and readily available. However, no specific methodology has been applied for ADE detection. Based on previous studies, we believe that identifying ADEs using diagnosis codes associated with ADE can be an alternative method for pharmacovigilance [56,57,58]. Administrative data can be used to identify ADEs at the population level [57]. The second limitation is that we were not able to identify whether individuals were using over-the-counter drugs, dietary supplements, or herbal medicines that were not covered by health coverage. Moreover, with our definition of the number of medications, we included both drugs used regularly for chronic disease and those used in the short-term for acute disease. Hence, the effects of the number of medications on the occurrence of ADEs may differ from actual estimates. Thirdly, as the claims data were collected for accounting purposes, the effect of coding quality on identifying ADEs is uncertain. Therefore, some ADEs in the claims data may not have been true ADEs, and the majority of mild ADEs may have been missed because they were incompletely documented. Therefore, careful interpretation should be required to understand the risk factors of ADEs in our results. In addition, the reliability and validity of the claims data may limit the generalization of our results. As suggested by previous studies, validation of codes related to ADEs is necessary to enhance sensitivity and specificity of ADEs detection using ICD codes [27,59]. In addition, validation needs to be performed in various healthcare settings (outpatient, inpatient, and emergency department) on the different severities of ADEs (mild, moderate, and severe) to understand the risk factors of ADEs in the general population. Fourthly, the data source used in our study was not recent. Therefore, the factors associated with ADE may differ from the current estimates. However, several diagnosis codes that are classified as sensitive information are not provided in the HIRA-NPS databases established after 2015. Therefore, we used the 2015 database, which is the most recent database in which diagnosis codes could be fully identified. Lastly, other potential factors, such as lifestyle (alcohol consumption, smoking status), compliance with therapy, healthcare service utilization, and genetic features, were not considered in the present study. Therefore, unmeasured cofounders may have been present in our results. Nevertheless, we included frequently reported factors such as patient-, disease-, and medication-related characteristics [12], and most of our findings were consistent with those of previous studies.
5. Conclusions
This study evaluates the sociodemographics, comorbidities, and number of medications associated with potential ADEs in South Korea using population-based data. Our study demonstrates that increased age, MA enrollment, high number of comorbidities, and high number of medications used are all independent factors of occurrence of potential ADEs. This finding indicates that, to prevent and reduce ADEs, healthcare professionals and policymakers should keep in mind those who have a higher risk of developing ADEs, particularly those with a high disease burden.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216248/s1, Table S1: List of the International Classification of Diseases, Tenth Revision (ICD-10) codes associated with adverse drug events.
Author Contributions
Conceptualization, E.C., S.K. and H.S.S.; methodology, E.C., S.K. and H.S.S.; software, E.C. and S.K.; validation, E.C. and S.K.; formal analysis, E.C. and S.K.; investigation, E.C., S.K. and H.S.S.; resources, E.C. and H.S.S.; data curation, E.C. and S.K.; writing—original draft preparation, E.C., S.K. and H.S.S.; writing—review and editing, S.K. and H.S.S.; visualization, E.C., S.K. and H.S.S.; supervision, H.S.S.; project administration, H.S.S.; funding acquisition, H.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (21153MFDS601) from Ministry of Food and Drug Safety in 2022. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1F1A1069526).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Pusan National University (PNU IRB/2020_152_HR; 16 November 2020).
Informed Consent Statement
Patient consent was waived due to the retrospective study design and anonymity of the HIRA database.
Data Availability Statement
Not applicable.
Acknowledgments
We used Health Insurance Review and Assessment Service-National Patient Sample 2015 (HIRA-NPS-2015-0199); however, we declare that the results do not reflect the positions of either the Health Insurance Review and Assessment Service or the Ministry of Health and Welfare in Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laatikainen, O.; Miettunen, J.; Sneck, S.; Lehtiniemi, H.; Tenhunen, O.; Turpeinen, M. The prevalence of medication-related adverse events in inpatients—A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 1539–1549. [Google Scholar] [CrossRef]
- Kang, M.-G.; Lee, J.-Y.; Woo, S.-I.; Kim, K.-S.; Jung, J.-W.; Lim, T.H.; Yoon, H.J.; Kim, C.W.; Yoon, H.-R.; Park, H.-K. Adverse drug events leading to emergency department visits: A multicenter observational study in Korea. PLoS ONE 2022, 17, e0272743. [Google Scholar] [CrossRef]
- Gyllensten, H.; Hakkarainen, K.M.; Hägg, S.; Carlsten, A.; Petzold, M.; Rehnberg, C.; Jönsson, A.K. Economic impact of adverse drug events–a retrospective population-based cohort study of 4970 adults. PLoS ONE 2014, 9, e92061. [Google Scholar] [CrossRef]
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of adverse drug reactions in Europe: A review of recent observational studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef]
- Bates, D.W.; Cullen, D.J.; Laird, N.; Petersen, L.A.; Small, S.D.; Servi, D.; Laffel, G.; Sweitzer, B.J.; Shea, B.F.; Hallisey, R. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA 1995, 274, 29–34. [Google Scholar] [CrossRef]
- Bates, D.W.; Spell, N.; Cullen, D.J.; Burdick, E.; Laird, N.; Petersen, L.A.; Small, S.D.; Sweitzer, B.J.; Leape, L.L. The costs of adverse drug events in hospitalized patients. JAMA 1997, 277, 307–311. [Google Scholar] [CrossRef]
- Classen, D.C.; Pestotnik, S.L.; Evans, R.S.; Lloyd, J.F.; Burke, J.P. Adverse drug events in hospitalized patients: Excess length of stay, extra costs, and attributable mortality. JAMA 1997, 277, 301–306. [Google Scholar] [CrossRef]
- Leendertse, A.J.; Visser, D.; Egberts, A.C.; van den Bemt, P.M. The relationship between study characteristics and the prevalence of medication-related hospitalizations. Drug Saf. 2010, 33, 233–244. [Google Scholar] [CrossRef]
- Formica, D.; Sultana, J.; Cutroneo, P.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert Opin. Drug Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef]
- Passarelli, M.C.G.; Jacob-Filho, W.; Figueras, A. Adverse drug reactions in an elderly hospitalised population. Drugs Aging 2005, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.N.; Wong, I.C.; Cranswick, N.; Tomlin, S.; Rascher, W.; Neubert, A. Risk factors associated with adverse drug reactions in hospitalised children: International multicentre study. Eur. J. Clin. Pharmacol. 2012, 68, 801–810. [Google Scholar] [CrossRef]
- Zhou, L.; Rupa, A.P. Categorization and association analysis of risk factors for adverse drug events. Eur. J. Clin. Pharmacol. 2018, 74, 389–404. [Google Scholar] [CrossRef]
- Onder, G.; Pedone, C.; Landi, F.; Cesari, M.; Della Vedova, C.; Bernabei, R.; Gambassi, G. Adverse drug reactions as cause of hospital admissions: Results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J. Am. Geriatr. Soc. 2002, 50, 1962–1968. [Google Scholar] [CrossRef]
- Sánchez Muñoz-Torrero, J.F.; Barquilla, P.; Velasco, R.; Fernández Capitan, M.d.C.; Pacheco, N.; Vicente, L.; Chicón, J.L.; Trejo, S.; Zamorano, J.; Lorenzo Hernandez, A. Adverse drug reactions in internal medicine units and associated risk factors. Eur. J. Clin. Pharmacol. 2010, 66, 1257–1264. [Google Scholar] [CrossRef]
- Minkowitz, H.S.; Gruschkus, S.K.; Shah, M.; Raju, A. Adverse drug events among patients receiving postsurgical opioids in a large health system: Risk factors and outcomes. Am. J. Health Syst. Pharm. 2014, 71, 1556–1565. [Google Scholar] [CrossRef]
- Field, T.S.; Gurwitz, J.H.; Harrold, L.R.; Rothschild, J.; DeBellis, K.R.; Seger, A.C.; Auger, J.C.; Garber, L.A.; Cadoret, C.; Fish, L.S. Risk factors for adverse drug events among older adults in the ambulatory setting. J. Am. Geriatr. Soc. 2004, 52, 1349–1354. [Google Scholar] [CrossRef]
- Yi, J.H.; Cho, Y.-J.; Kim, W.-J.; Lee, M.G.; Lee, J.H. Genetic variations of ABCC2 gene associated with adverse drug reactions to valproic acid in Korean epileptic patients. Genom. Inform. 2013, 11, 254. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jee, Y.-K.; Lee, J.-H.; Lee, B.-H.; Kim, Y.-S.; Park, J.-S.; Kim, S.-H. ABCC2 haplotype is associated with antituberculosis drug-induced maculopapular eruption. Allergy Asthma Immunol. Res. 2012, 4, 362–366. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, E.; Jung, H.-W.; Jang, I.-Y. Geriatrics fact sheet in Korea 2021. Ann. Geriatr. Med. Res. 2021, 25, 65. [Google Scholar] [CrossRef]
- Park, J.; Yi, E.; Yi, J. The Provision and Utilization of Traditional Korean Medicine in South Korea: Implications on Integration of Traditional Medicine in a Developed Country. Proc. Healthc. 2021, 9, 1379. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.-A.; Kim, S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Kim, L.; Sakong, J.; Kim, Y.; Kim, S.; Kim, S.; Tchoe, B.; Jeong, H.; Lee, T. Developing the inpatient sample for the National Health Insurance claims data. Health Policy Manag. 2013, 23, 152–161. [Google Scholar] [CrossRef]
- Song, Y.J. The South Korean health care system. JMAJ 2009, 52, 206–209. [Google Scholar]
- Kim, J.-A.; Yoon, S.; Kim, L.-Y.; Kim, D.-S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef]
- Nebeker, J.R.; Barach, P.; Samore, M.H. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004, 140, 795–801. [Google Scholar] [CrossRef]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Hohl, C.M.; Karpov, A.; Reddekopp, L.; Stausberg, J. ICD-10 codes used to identify adverse drug events in administrative data: A systematic review. J. Am. Med. Inform. Assoc. 2014, 21, 547–557. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Bourgeois, F.T.; Shannon, M.W.; Valim, C.; Mandl, K.D. Adverse drug events in the outpatient setting: An 11-year national analysis. Pharmacoepidemiol. Drug Saf. 2010, 19, 901–910. [Google Scholar] [CrossRef]
- Zhang, M.; Holman, C.D.A.J.; Price, S.D.; Sanfilippo, F.M.; Preen, D.B.; Bulsara, M.K. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: Retrospective cohort study. BMJ 2009, 338, a2752. [Google Scholar] [CrossRef]
- Stausberg, J.; Hasford, J. Identification of adverse drug events: The use of ICD-10 coded diagnoses in routine hospital data. Dtsch. Arztebl. Int. 2010, 107, 23. [Google Scholar]
- Friedman, B.W.; Cisewski, D.H.; Holden, L.; Bijur, P.E.; Gallagher, E.J. Age but not sex is associated with efficacy and adverse events following administration of intravenous migraine medication: An analysis of a clinical trial database. Headache J. Head Face Pain 2015, 55, 1342–1355. [Google Scholar] [CrossRef]
- Lee, J.S.; Yang, J.; Stockl, K.M.; Lew, H.; Solow, B.K. Evaluation of eligibility criteria used to identify patients for medication therapy management services: A retrospective cohort study in a Medicare Advantage Part D population. J. Manag. Care Spec. Pharm. 2016, 22, 22–30. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Liang, L.; Lian, Z.; Liu, J.; Luo, X.; Chen, W.; Li, X.; Liang, C.; Zhang, S. The incidence, classification, and management of acute adverse reactions to the low-osmolar iodinated contrast media Isovue and Ultravist in contrast-enhanced computed tomography scanning. Medicine 2016, 95, e3170. [Google Scholar] [CrossRef]
- Zopf, Y.; Rabe, C.; Neubert, A.; Gassmann, K.; Rascher, W.; Hahn, E.; Brune, K.; Dormann, H. Women encounter ADRs more often than do men. Eur. J. Clin. Pharmacol. 2008, 64, 999–1004. [Google Scholar] [CrossRef]
- Tanaka, E. Gender-related differences in pharmacokinetics and their clinical significance. J. Clin. Pharm. Ther. 1999, 24, 339–346. [Google Scholar] [CrossRef]
- Kashuba, A.D.; Nafziger, A.N. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin. Pharmacokinet. 1998, 34, 203–218. [Google Scholar] [CrossRef]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Shiozawa, T.; Tadokoro, J.-i.; Fujiki, T.; Fujino, K.; Kakihata, K.; Masatani, S.; Morita, S.; Gemma, A.; Boku, N. Risk factors for severe adverse effects and treatment-related deaths in Japanese patients treated with irinotecan-based chemotherapy: A postmarketing survey. Jpn. J. Clin. Oncol. 2013, 43, 483–491. [Google Scholar] [CrossRef]
- Pedrós, C.; Quintana, B.; Rebolledo, M.; Porta, N.; Vallano, A.; Arnau, J.M. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur. J. Clin. Pharmacol. 2014, 70, 361–367. [Google Scholar] [CrossRef]
- Morimoto, T.; Gandhi, T.K.; Fiskio, J.M.; Seger, A.C.; So, J.W.; Cook, E.F.; Fukui, T.; Bates, D.W. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J. Eval. Clin. Pract. 2004, 10, 499–509. [Google Scholar] [CrossRef]
- Corsonello, A.; Pedone, C.; Incalzi, R.A. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Carbonin, P.; Bernabei, R.; Sgadari, A. Is age an independent risk factor of adverse drug reactions in hospitalized medical patients? J. Am. Geriatr. Soc. 1991, 39, 1093–1099. [Google Scholar] [CrossRef]
- Van den Bemt, P.; Egberts, A.; Lenderink, A.; Verzijl, J.; Simons, K.; Van der Pol, W.; Leufkens, H. Risk factors for the development of adverse drug events in hospitalized patients. Pharm. World Sci. 2000, 22, 62–66. [Google Scholar] [CrossRef]
- Kim, H.-A.; Shin, J.-Y.; Kim, M.-H.; Park, B.-J. Prevalence and predictors of polypharmacy among Korean elderly. PLoS ONE 2014, 9, e98043. [Google Scholar] [CrossRef]
- Suh, H.S.; Kang, H.-Y.; Kim, J.; Shin, E. Effect of health insurance type on health care utilization in patients with hypertension: A national health insurance database study in Korea. BMC Health Serv. Res. 2014, 14, 570. [Google Scholar] [CrossRef]
- Clague, F.; Mercer, S.W.; McLean, G.; Reynish, E.; Guthrie, B. Comorbidity and polypharmacy in people with dementia: Insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017, 46, 33–39. [Google Scholar] [CrossRef]
- Macedo, A.F.; Alves, C.; Craveiro, N.; Marques, F.B. Multiple drug exposure as a risk factor for the seriousness of adverse drug reactions. J. Nurs. Manag. 2011, 19, 395–399. [Google Scholar] [CrossRef]
- Steinman, M.A.; Seth Landefeld, C.; Rosenthal, G.E.; Berthenthal, D.; Sen, S.; Kaboli, P.J. Polypharmacy and prescribing quality in older people. J. Am. Geriatr. Soc. 2006, 54, 1516–1523. [Google Scholar] [CrossRef]
- Wahlang, J.B.; Laishram, P.D.; Brahma, D.K.; Sarkar, C.; Lahon, J.; Nongkynrih, B.S. Adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital. Ther. Adv. Drug Saf. 2017, 8, 61–66. [Google Scholar] [CrossRef]
- Van Leeuwen, R.; Brundel, D.; Neef, C.; van Gelder, T.; Mathijssen, R.; Burger, D.; Jansman, F. Prevalence of potential drug–drug interactions in cancer patients treated with oral anticancer drugs. Br. J. Cancer 2013, 108, 1071–1078. [Google Scholar] [CrossRef]
- Franz, C.C.; Egger, S.; Born, C.; Rätz Bravo, A.E.; Krähenbühl, S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur. J. Clin. Pharmacol. 2012, 68, 179–188. [Google Scholar] [CrossRef]
- Morimoto, T.; Gandhi, T.K.; Seger, A.C.; Hsieh, T.C.; Bates, D.W. Adverse drug events and medication errors: Detection and classification methods. Qual. Saf. Health Care 2004, 13, 306–314. [Google Scholar] [CrossRef]
- Kuklik, N.; Stausberg, J.; Jockel, K.H. Adverse drug events in German hospital routine data: A validation of International Classification of Diseases, 10th revision (ICD-10) diagnostic codes. PLoS ONE 2017, 12, e0187510. [Google Scholar] [CrossRef]
- Miguel, A.; Azevedo, L.F.; Lopes, F.; Freitas, A.; Pereira, A.C. Methodologies for the detection of adverse drug reactions: Comparison of hospital databases, chart review and spontaneous reporting. Pharmacoepidemiol. Drug Saf. 2013, 22, 98–102. [Google Scholar] [CrossRef]
- Hohl, C.M.; Kuramoto, L.; Yu, E.; Rogula, B.; Stausberg, J.; Sobolev, B. Evaluating adverse drug event reporting in administrative data from emergency departments: A validation study. BMC Health Serv. Res. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Ock, M.; Kim, H.J.; Jeon, B.; Kim, Y.-J.; Ryu, H.M.; Lee, M.-S. Identifying adverse events using international classification of diseases, tenth revision Y codes in Korea: A cross-sectional study. J. Prev. Med. Public Health 2018, 51, 15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).