Purely Off-Clamp Partial Nephrectomy: Robotic Approach Better than Open Using a Pentafecta Outcome with Propensity Score Matching
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population, Data Assessment and Surgical Technique
2.2. Measurements and Outcomes
2.3. Propensity Score-Matching Analysis
2.4. Statistical Methods
3. Results
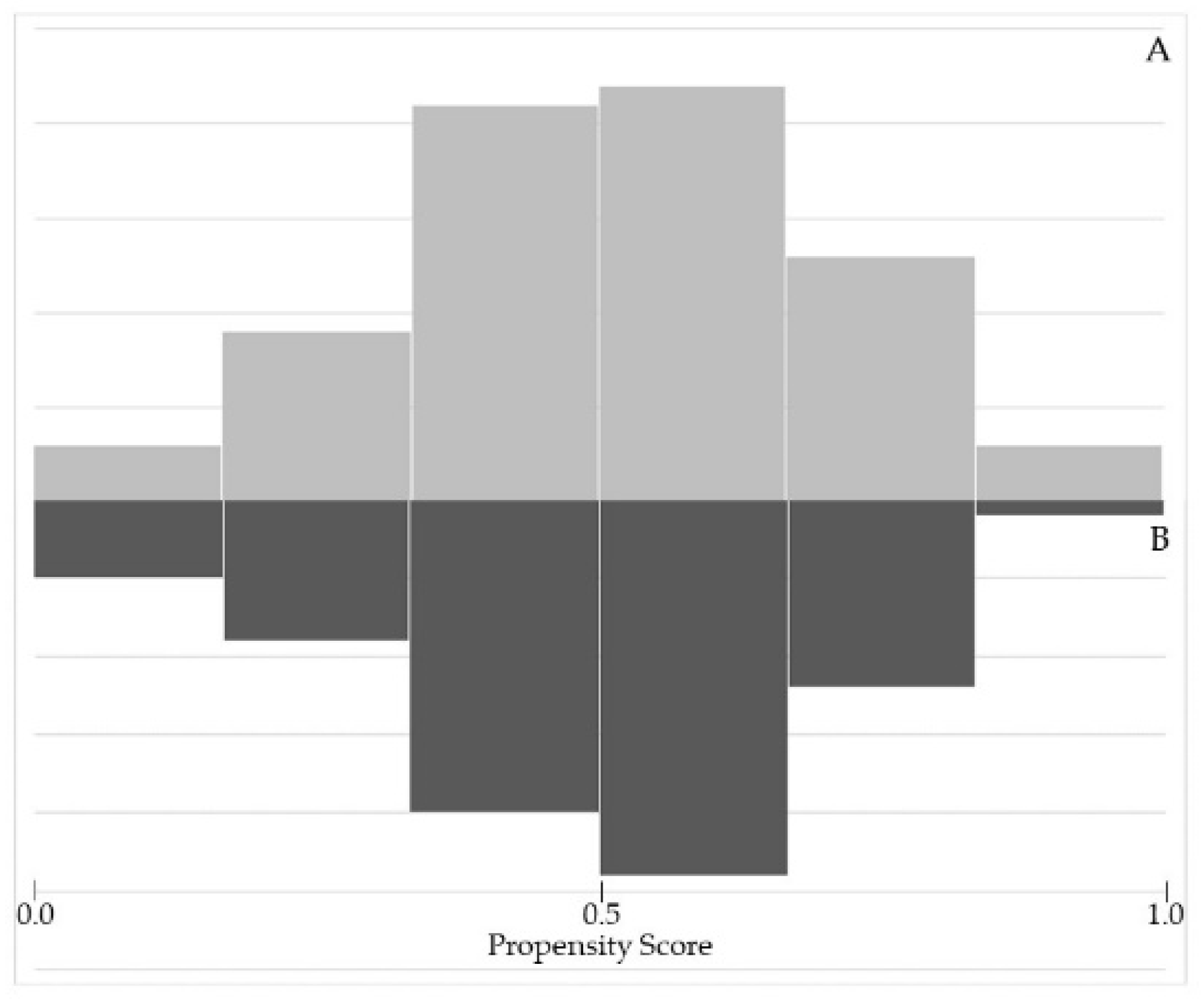
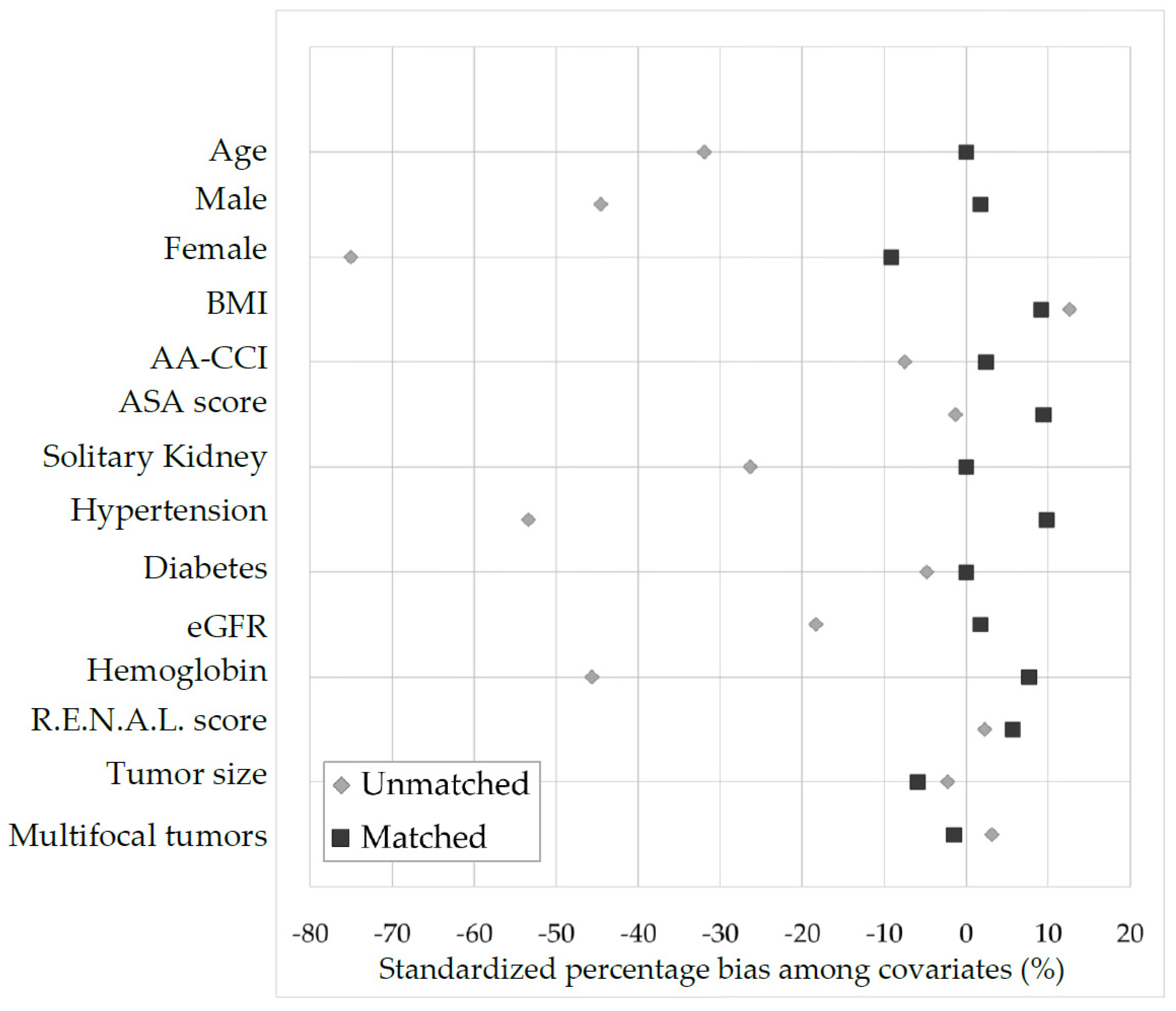
3.1. Matching Procedure
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. Sensitivity Analysis and Diagnostics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G.; Xia, Q.; Shang, Z.; Yu, X.; Wang, M.; Jin, X. Partial nephrectomy vs. radical nephrectomy for renal tumors: A meta-analysis of renal function and cardiovascular outcomes. Urol. Oncol. 2016, 34, 533.e11–533.e19. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Minervini, A.; Sandri, M.; Bertini, R.; Bertolo, R.; Carini, M.; Furlan, M.; Larcher, A.; Mantica, G.; Mari, A.; et al. Below Safety Limits, Every Unit of Glomerular Filtration Rate Counts: Assessing the Relationship between Renal Function and Cancer-specific Mortality in Renal Cell Carcinoma. Eur. Urol. 2018, 74, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.C.; Autorino, R.; Porpiglia, F. Ischemia time and beyond: The concept of global renal damage. Minerva Urol. Nefrol. 2018, 70, 447–449. [Google Scholar] [CrossRef]
- Ficarra, V.; Crestani, A.; Bertolo, R.; Antonelli, A.; Longo, N.; Minervini, A.; Novara, G.; Simeone, C.; Carini, M.; Mirone, V.; et al. Tumor contact surface area as a predictor of postoperative complications and renal function in patients undergoing partial nephrectomy for renal tumors. BJU Int. 2019, 123, 639–645. [Google Scholar] [CrossRef]
- Minervini, A.; Carini, M.; Uzzo, R.G.; Campi, R.; Smaldone, M.C.; Kutikov, A. Standardized reporting of resection technique during nephron-sparing surgery: The surface-intermediate-base margin score. Eur. Urol. 2014, 66, 803–805. [Google Scholar] [CrossRef]
- Bertolo, R.; Campi, R.; Klatte, T.; Kriegmair, M.C.; Mir, M.C.; Ouzaid, I.; Salagierski, M.; Bhayani, S.; Gill, I.; Kaouk, J.; et al. Suture techniques during laparoscopic and robot-assisted partial nephrectomy: A systematic review and quantitative synthesis of peri-operative outcomes. BJU Int. 2019, 123, 923–946. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef]
- Smith, G.L.; Kenney, P.A.; Lee, Y.; Libertino, J.A. Non-clamped partial nephrectomy: Techniques and surgical outcomes. BJU Int. 2011, 107, 1054–1058. [Google Scholar] [CrossRef]
- Bermudez, H.; Guillonneau, B.; Gupta, R.; Rosa, J.A.; Cathelineau, X.; Fromont, G.; Vallancien, G. Initial experience in laparoscopic partial nephrectomy for renal tumor with clamping of renal vessels. J. Endourol. 2003, 17, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Laven, B.A.; Orvieto, M.A.; Chuang, M.S.; Ritch, C.R.; Murray, P.; Harland, R.C.; Inman, S.R.; Brendler, C.B.; Shalhav, A.L. Renal tolerance to prolonged warm ischemia time in a laparoscopic versus open surgery porcine model. J. Urol. 2004, 172, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Babineau, D.C.; Poggio, E.D.; Weight, C.J.; Larson, B.T.; Gill, I.S.; Novick, A.C. Factors predicting renal functional outcome after partial nephrectomy. J. Urol. 2008, 180, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; Van Poppel, H.; Hakenberg, O.W.; Stief, C.; Gill, I.; Guazzoni, G.; Montorsi, F.; Russo, P.; Stockle, M. Assessing the impact of ischaemia time during partial nephrectomy. Eur. Urol. 2009, 56, 625–634. [Google Scholar] [CrossRef]
- Ghani, K.R.; Sukumar, S.; Sammon, J.D.; Rogers, C.G.; Trinh, Q.D.; Menon, M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. J. Urol. 2014, 191, 907–912. [Google Scholar] [CrossRef]
- Ficarra, V.; Minervini, A.; Antonelli, A.; Bhayani, S.; Guazzoni, G.; Longo, N.; Martorana, G.; Morgia, G.; Mottrie, A.; Porter, J.; et al. A multicentre matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int. 2014, 113, 936–941. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Hong, S.K.; Lee, S.E.; Byun, S.S. Open versus robot-assisted partial nephrectomy: Effect on clinical outcome. J. Endourol. 2011, 25, 1181–1185. [Google Scholar] [CrossRef]
- Grivas, N.; Kalampokis, N.; Larcher, A.; Tyritzis, S.; Rha, K.H.; Ficarra, V.; Buffi, N.; Ploumidis, A.; Autorino, R.; Porpiglia, F.; et al. Robot-assisted versus open partial nephrectomy: Comparison of outcomes. A systematic review. Minerva Urol. Nefrol. 2019, 71, 113–120. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Artibani, W.; Delahunt, B.; Ficarra, V.; Knuechel, R.; Montorsi, F.; Patard, J.J.; Stief, C.G.; Sulser, T.; Wild, P.J. Reassessing the current UICC/AJCC TNM staging for renal cell carcinoma. Eur. Urol. 2009, 56, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Wusek, J.K.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612, Correction in Ann. Intern. Med. 2011, 155, 408. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in partial nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef]
- Buffi, N.; Lista, G.; Larcher, A.; Lunghezzani, G.; Ficarra, V.; Cestari, A.; Lazzeri, M.; Guazzoni, G. Margin, ischemia, and complications (MIC) score in partial nephrectomy: A new system for evaluating achievement of optimal outcomes in nephron-sparing surgery. Eur. Urol. 2012, 62, 617–618. [Google Scholar] [CrossRef]
- Zargar, H.; Allaf, M.E.; Bhayani, S.; Stifelman, M.; Rogers, C.; Ball, M.W.; Larson, J.; Marshall, S.; Kumar, R.; Kaouk, J.H. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: A multi-institutional study. BJU Int. 2015, 116, 407–414. [Google Scholar] [CrossRef]
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottrie, A.; et al. Surgical quality, cancer control and functional preservation: Introducing a novel trifecta for robot-assisted partial nephrectomy. Minerva Urol. Nefrol. 2020, 72, 82–90. [Google Scholar] [CrossRef]
- DeCoster, J.; Gallucci, M.; Iselin, A.-M.R. Best Practices for Using Median Splits, Artificial Categorization, and their Continuous Alternatives. J. Exp. Psychopathol. 2011, 2, 197–209. [Google Scholar] [CrossRef]
- Simone, G.; Capitanio, U.; Tuderti, G.; Presicce, F.; Leonardo, C.; Ferriero, M.; Misuraca, L.; Costantini, M.; Larcher, A.; Minisola, F.; et al. On-clamp versus off-clamp partial nephrectomy: Propensity score-matched comparison of long-term functional outcomes. Int. J. Urol. 2019, 26, 985–991. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Qu, L.; Ye, H.; Liu, B.; Yang, Q.; Sheng, J.; Xiao, L.; Lv, C.; Yang, B. A propensity-score matched comparison of perioperative and early renal functional outcomes of robotic versus open partial nephrectomy. PLoS ONE 2014, 9, e94195. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Grootendorst, P.; Anderson, G.M. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat. Med. 2007, 26, 734–753. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R. Observational Studies, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-1-4757-3692-2. [Google Scholar]
- Sacco, E.; Gandi, C.; Marino, F.; Totaro, A.; Di Gianfrancesco, L.; Palermo, G.; Pierconti, F.; Racioppi, M.; Bassi, P.F. Artificial urinary sphincter significantly better than fixed sling for moderate post-prostatectomy strees urinary incontinence: A propensity score-matched study. BJU Int. 2021, 127, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Liu, B.; Cai, C.; Ye, H.; Lv, C.; Yang, Q.; Sheng, J.; Song, S.; Qu, L.; et al. Robotic versus open partial nephrectomy: A systematic review and meta-analysis. PLoS ONE 2014, 9, e94878. [Google Scholar] [CrossRef]
- Leow, J.J.; Heah, N.H.; Chang, S.L.; Chong, Y.L.; Png, K.S. Outcomes of Robotic versus Laparoscopic Partial Nephrectomy: An Updated Meta-Analysis of 4919 Patients. J. Urol. 2016, 196, 1371–1377. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Anal. 2007, 15, 199–236. [Google Scholar] [CrossRef]
- Normand, S.T.; Landrum, M.B.; Guadagnoli, E.; Ayanian, J.Z.; Ryan, T.J.; Cleary, P.D.; McNeil, B.J. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: A matched analysis using propensity scores. J. Clin. Epidemiol. 2001, 54, 387–398. [Google Scholar] [CrossRef]
- Arnastauskaite, J.; Ruzgas, T.; Brazenas, M. An Exhaustive Power Comparison of Normality Tests. Mathematics 2021, 9, 788. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Brito, M.R.; Quiroz, A.J. On the goodness-of-fit procedure for normality based on the empirical characteristic function for ranked set sampling data. Metrika 2013, 76, 161–177. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J. Natl. Cancer. Inst. 2017, 109, djw323. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, X.; Xu, T.; Guzzo, T.J. Systematic Review and Meta-Analysis of Comparative Studies Reporting Perioperative Outcomes of Robot-Assisted Partial Nephrectomy Versus Open Partial Nephrectomy. J. Endourol. 2017, 31, 893–909. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Blute, M.I.; Ficarra, V.; Gill, I.S.; Kutikov, A.; Porpiglia, F.; Rogers, C.; Touijer, K.A.; Van Poppel, H.; Thompson, R.H. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur. Urol. 2015, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Campbell, S.C.; Blute, M.L. Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur. Urol. 2010, 58, 331–336. [Google Scholar] [CrossRef]
- Rogers, C.G.; Patard, J.J. Open to debate. The motion: Robotic partial nephrectomy is better than open partial nephrectomy. Eur. Urol. 2009, 56, 568–570. [Google Scholar] [CrossRef]
- Han, K.S.; Song, G.H.; You, D.; Song, C.; Jeong, I.G.; Hong, J.H.; Ahn, H.; Kim, C.S.; Hong, B. Comparison of Hand-Assisted Laparoscopic vs Robot-Assisted Laparoscopic vs Open Partial Nephrectomy in Patients with T1 Renal Masses. J. Endourol. 2017, 31, 374–379. [Google Scholar] [CrossRef]
- Minervini, A.; Vittori, G.; Antonelli, A.; Celia, A.; Crivellaro, S.; Dente, D.; Di Santo, V.; Frea, B.; Gacci, M.; Gritti, A.; et al. Open versus robotic-assisted partial nephrectomy: A multicenter comparison study of perioperative results and complications. World J. Urol. 2014, 32, 287–293, Correction in World J. Urol. 2014, 32, 295. [Google Scholar] [CrossRef]
- Porpiglia, F.; Mari, A.; Bertolo, R.; Antonelli, A.; Bianchi, G.; Fidanza, F.; Fiori, C.; Furlan, M.; Morgia, G.; Novara, G. Partial Nephrectomy in Clinical T1b Renal Tumors: Multicenter Comparative Study of Open, Laparoscopic and Robot-assisted Approach (the RECORd Project). Urology 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Miyake, H.; Hinata, N.; Imai, S.; Furukawa, J.; Tanaka, K.; Fujisawa, M. Partial nephrectomy for hilar tumors: Comparison of conventional open and robot-assisted approaches. Int. J. Clin. Oncol. 2015, 20, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, J.; Ma, X.; Du, Q.; Gong, H.; Zhang, X. Robotic and open partial nephrectomy for complex renal tumors: A matched-pair comparison with a long-term follow-up. World J. Urol. 2017, 35, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Laydner, H.; Isac, W.; Autorino, R.; Kassab, A.; Yakoubi, R.; Hillyer, S.; Khalifeh, A.; Campbell, S.C.; Fergany, A.; Simmons, M.; et al. Single institutional cost analysis of 325 robotic, laparoscopic, and open partial nephrectomies. Urology 2013, 81, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.J.; Byun, S.; Hong, S.K.; Jeong, C.W.; Lee, S.E. Comparison of robotic and open partial nephrectomy: Single-surgeon matched cohort study. Can. Urol. Assoc. J. 2014, 8, E471–E475. [Google Scholar] [CrossRef]
- Pignot, G.; Méjean, A.; Bernhard, J.C.; Bigot, P.; Timsit, M.O.; Ferriere, J.M.; Zerbib, M.; Villers, A.; Mouracade, P.; Lang, H.; et al. The use of partial nephrectomy: Results from a contemporary national prospective multicenter study. World J. Urol. 2015, 33, 33–40. [Google Scholar] [CrossRef]

| Mean ± SD or n (%) | Unmatched Population | Matched Population | ||||||
|---|---|---|---|---|---|---|---|---|
| RAPN | OPN | SMD | p Value | RAPN | OPN | SMD | p Value | |
| Matched Variables | (n = 87) | (n = 253) | (n = 71) | (n = 71) | ||||
| Age, years | 61.34 ± 12.43 | 60.93 ± 13.92 | 0.0311 | 0.87 | 60.81 ± 12.99 | 61.01 ± 14.31 | −0.0146 | 0.600 |
| Gender, n (%) | 0.29 | 0.189 | ||||||
| Male | 52 (59.77) | 154 (60.86) | −0.0224 | 41 (57.74) | 43 (60.56) | −0.059 | ||
| Female | 35 (40.22) | 99 (39.13) | 0.0225 | 30 (42.25) | 28 (39.43) | 0.0569 | ||
| BMI, kg/m2 | 25.90 ± 4.05 | 28.20 ± 5.86 | −0.4566 | 0.001 | 26.24 ± 4.06 | 25.90 ± 4.74 | 0.077 | 0.472 |
| AA-CCI | 4.51 ± 1.55 | 4.82 ± 1.82 | −0.1833 | 0.12 | 4.58 ± 1.64 | 4.55 ± 1.76 | 0.0176 | 0.916 |
| ASA score | 2.07 ± 0.33 | 2.09 ± 0.48 | −0.0485 | 0.63 | 2.07 ± 1.35 | 2.07 ± 0.52 | 0,00 | 0.951 |
| Solitary kidney, n (%) | 1 (1.14) | 15 (5.92) | −0.5341 | 0.05 | 1 (1.40) | 0 | 0.0987 | 0.477 |
| Hypertension, n (%) | 42 (48.27) | 156 (61.66) | −0.2633 | 0.03 | 36 (50.70) | 36 (50.70) | 0,00 | 0.932 |
| Diabetes, n (%) | 14 (16.09) | 42 (16.60) | −0.0135 | 0.05 | 13 (18.30) | 10 (14.08) | 0.0941 | 0.466 |
| eGFR, mL/min/1.73 m2 | 82.94 ± 20.95 | 87.79 ± 88.71 | −0.0752 | 0.69 | 83.10 ± 22.13 | 82.52 ± 25.29 | 0.0244 | 0.794 |
| Hemoglobin, g/dL | 13.86 ± 1.32 | 13.67 ± 1.67 | 0.1262 | 0.20 | 13.75 ± 1.41 | 13.60 ± 1.84 | 0.0915 | 0.401 |
| R.E.N.A.L. score | 4.47 ± 0.75 | 5.68 ± 1.33 | −0.7502 | <0.0001 | 4.57 ± 0.79 | 4.65 ± 0.95 | −0.0915 | 0.873 |
| Tumor size, cm | 2.70 ± 1.91 | 3.59 ± 2.08 | −0.4457 | <0.0001 | 2.90 ± 2.05 | 2.87 ± 1.31 | 0.0174 | 0.597 |
| Multifocal tumors, n (%) | 1 (1.14) | 19 (7.50) | −0.3194 | 0.01 | 1 (1.40) | 1 (1.40) | 0,00 | 0.476 |
| Unmatched Variables | ||||||||
| R.E.N.A.L. complexity, n (%) | 0.003 | 1.000 | ||||||
| Low (4–6) | 85 (97.70) | 212 (83.79) | 0.4944 | 67 (94.35) | 66 (92.95) | 0.0574 | ||
| Moderate (7–9) | 2 (2.29) | 38 (15.01) | −0.4337 | 4 (5.65) | 5 (7.04) | −0.0574 | ||
| High (10–12) | 0 | 3 (1.18) | −0.1545 | 0 | 0 | |||
| Tumor laterality, n (%) | 0.118 | 0.1569 | ||||||
| Right | 38 (43.67) | 137 (54.15) | −0.2108 | 32 (45.07) | 33 (46.47) | −0.0281 | ||
| Left | 49 (56.32) | 116 (45.84) | 0.2108 | 39 (54.92) | 38 (53.52) | 0.0281 | ||
| ECOG score | 1.20 ± 0.40 | 1.28 ± 0.52 | −0.1724 | 0.929 | 1.22 ± 0.41 | 1.23 ± 0.46 | −0.0229 | 0.929 |
| Mean ± SD or n (%) | Matched Population | ||
|---|---|---|---|
| Surgical, Pathological and Functional Outcomes | RAPN | OPN | p Value |
| (n = 71) | (n = 71) | ||
| Operative time, min | 149.8 ± 41.1 | 173.9 ± 51.8 | 0.003 |
| EBL, mL | 182.1 ± 198.9 | 329.3 ± 305.6 | 0.001 |
| Tranfusions, n (%) | 5 (7.0) | 4 (5.6) | 1.00 |
| LHS, days | 5.8 ± 2.3 | 6.9 ± 3.9 | 0.02 |
| LHS ≤ 5, n (%) | 41 (57.7) | 17 (23.9) | 0.0001 |
| Complications, n (%) | |||
| Intra-operative | 1 (1.4) | 5 (7.0) | 0.21 |
| Post-operative | 11 (15.5) | 12 (16.9) | 1.00 |
| C-D 1–2 | 8 (11.3) | 10 (14.1) | 0.8 |
| C-D 3–4 | 3 (4.2) | 2 (2.8) | 1.00 |
| PSM, n (%) | 2 (2.8) | 6 (8.4) | 0.27 |
| Tumour histology, n (%) | |||
| Malignant | 48 (67.6) | 47 (66.2) | 1.00 |
| Clear cell RCC | 32 (45.1) | 29 (40.8) | 0.73 |
| Papillary RCC | 13 (18.3) | 11 (15.5) | 0.82 |
| Chromophobe RCC | 2 (2.8) | 7 (9.9) | 0.17 |
| Others | 1 (1.4) | 0 (0) | 1.00 |
| Benign | 23 (32.4) | 24 (33.8) | 1.00 |
| Angiomyolipoma | 8 (11.3) | 7 (9.9) | 1.00 |
| Oncytoma | 13 (18.3) | 14 (19.7) | 1.00 |
| Others | 2 (2.8) | 3 (4.2) | 1.00 |
| Pathological stage, n (%) | |||
| T1a | 65 (91.55) | 59 (83.09) | 0.14 |
| T1b | 4 (5.63) | 9 (12.68) | 0.16 |
| T2a | 0 (0) | 0 (0) | 1.00 |
| T2b | 1 (1.41) | 2 (2.81) | 0.97 |
| T3 | 1 (1.41) | 1 (1.41) | 1.00 |
| Fuhrman grade, n (%) | |||
| Low (1–2) | 40 (56.3) | 36 (50.7) | 0.12 |
| High (3–4) | 3 (4.2) | 4(5.6) | 1.00 |
| Not specified | 5 (7.0) | 7 (9.9) | 0.76 |
| Last eGFR, mL/min per 1.73 m2 | 83.3 ± 24.7 | 78.2 ± 30.4 | 0.29 |
| Change in eGFR | −0.06 ± 0.3 | −0.08 ± 0.3 | 0.58 |
| CKD upstaging, n (%) | 15 (21.1) | 18 (25.3) | 0.69 |
| Pentafecta outcome, n (%) | 40 (56.3) | 15 (21.1) | <0.0001 |
| Unmatched Population | Matched Population | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 340) | (n = 142) | |||||||
| Univariate Analisys | Multivariate Analysis | Univariate Analisys | Multivariate Analysis | |||||
| Predictors of Pentafecta Outcome | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 0.97 (0.95–1.00) | 0.05 | 0.96 (0.94–0.99) | 0.04 | 0.97 (0.94–0.99) | 0.04 | 0.96 (0.93–1-00) | 0.06 |
| Gender: F vs. M (ref.) | 1.12 (0.58–2.15) | 0.72 | / | / | 1.5 (0.70–3.28) | 0.28 | / | / |
| BMI | 0.89 (0.83–0.96) | 0.003 | 0.94 (0.86–1.02) | 0.14 | 0.90 (0.82–0.99) | 0.04 | 0.89 (0.80–0.99) | 0.04 |
| AA-CCI | 0.82 (0.67–1.00) | 0.05 | 0.91 (0.72–1.16) | 0.47 | 0.80 (0.63–1.01) | 0.05 | 0.89 (0.68–1.17) | 0.42 |
| ASA score | 0.79 (0.38–1.64) | 0.54 | / | / | 0.47 (0.18–1.18) | 0.11 | / | / |
| Preop. eGFR | 0.99 (0.99–1.01) | 0.75 | / | / | 1.00 (0.98–1.02) | 0.51 | / | / |
| Preop. Hemoglobin | 1.17 (0.95–1.46) | 0.14 | / | / | 1.19 (0.92–1.54) | 0.17 | / | / |
| R.E.N.A.L. score | 0.71 (0.51–0.97) | 0.03 | 0.90 (0.55–1.29) | 0.58 | 0.84 (0.53–1.33) | 0.46 | 0.83 (0.49–1.42) | 0.50 |
| Tumor size | 0.67 (0.51–0.87) | 0.002 | 0.74 (0.55–0.99) | 0.04 | 0.77 (0.54–1.10) | 0.16 | 0.80 (0.54–1.17) | 0.26 |
| RAPN vs. OPN (ref.) | 5.91 (3.02–11.59) | <0.0001 | 4.48 (2.07–9.68) | 0.0001 | 3.31 (1.45–7.59) | 0.004 | 3.96 (1.60–9.79) | 0.002 |
| Gamma Values (Γ) | Rosenbaum’s Upper Bound |
|---|---|
| Two-Tail p Values | |
| 1.00 | 0.0003 |
| 1.50 | 0.0042 |
| 2.00 | 0.0193 |
| 2.45 | 0.0471 |
| 2.50 | 0.0512 |
| 3.00 | 0.1002 |
| 3.50 | 0.1634 |
| 4.00 | 0.2364 |
| 4.50 | 0.3153 |
| 5.00 | 0.3966 |
| 5.50 | 0.4781 |
| 6.00 | 0.5578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandi, C.; Totaro, A.; Bientinesi, R.; Marino, F.; Pierconti, F.; Russo, A.; Racioppi, M.; Bassi, P.; Sacco, E. Purely Off-Clamp Partial Nephrectomy: Robotic Approach Better than Open Using a Pentafecta Outcome with Propensity Score Matching. J. Clin. Med. 2022, 11, 6241. https://doi.org/10.3390/jcm11216241
Gandi C, Totaro A, Bientinesi R, Marino F, Pierconti F, Russo A, Racioppi M, Bassi P, Sacco E. Purely Off-Clamp Partial Nephrectomy: Robotic Approach Better than Open Using a Pentafecta Outcome with Propensity Score Matching. Journal of Clinical Medicine. 2022; 11(21):6241. https://doi.org/10.3390/jcm11216241
Chicago/Turabian StyleGandi, Carlo, Angelo Totaro, Riccardo Bientinesi, Filippo Marino, Francesco Pierconti, Andrea Russo, Marco Racioppi, Pierfrancesco Bassi, and Emilio Sacco. 2022. "Purely Off-Clamp Partial Nephrectomy: Robotic Approach Better than Open Using a Pentafecta Outcome with Propensity Score Matching" Journal of Clinical Medicine 11, no. 21: 6241. https://doi.org/10.3390/jcm11216241
APA StyleGandi, C., Totaro, A., Bientinesi, R., Marino, F., Pierconti, F., Russo, A., Racioppi, M., Bassi, P., & Sacco, E. (2022). Purely Off-Clamp Partial Nephrectomy: Robotic Approach Better than Open Using a Pentafecta Outcome with Propensity Score Matching. Journal of Clinical Medicine, 11(21), 6241. https://doi.org/10.3390/jcm11216241