Resizing of the Gastric Pouch for Weight Regain after Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: Is It a Valid Option?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
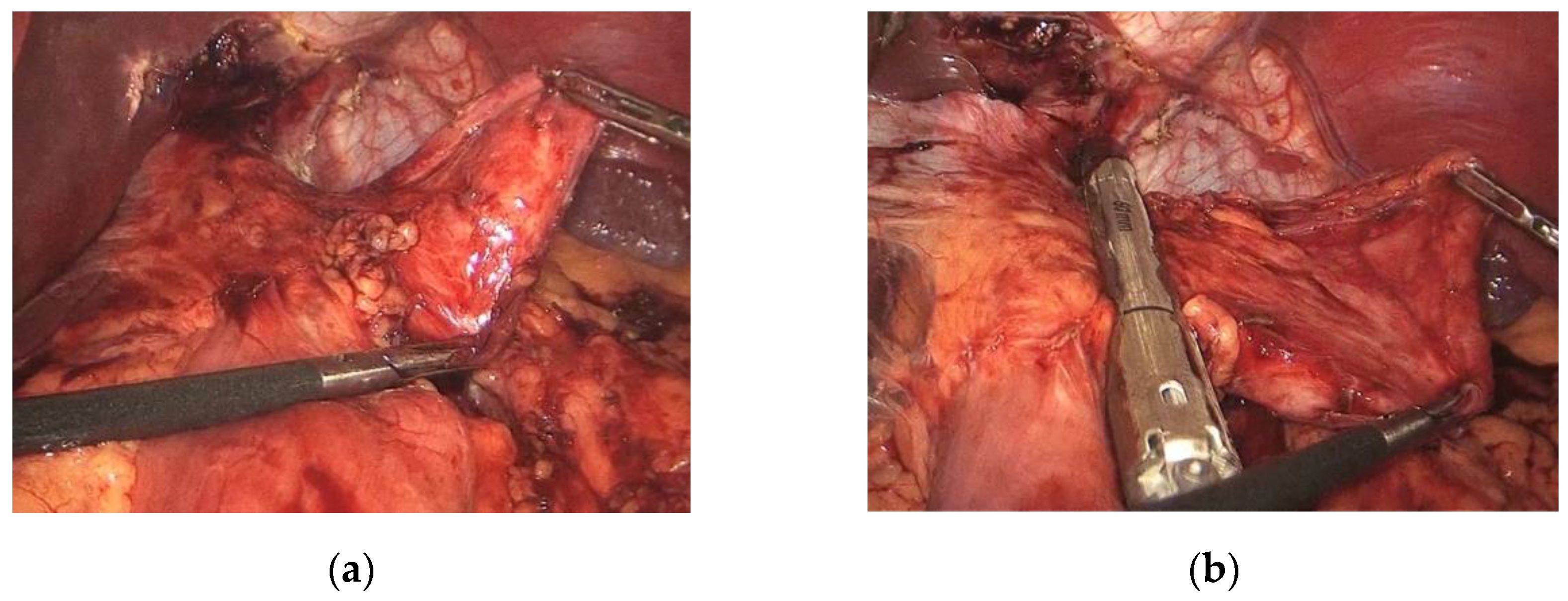
2.2. Surgical Technique
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Ramos, A.; Shikora, S.; Kow, L. Bariatric Surgery Survey 2018: Similarities and Disparities among the 5 IFSO Chapters. Obes. Surg. 2021, 31, 1937–1948. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Jazi, A.H.D.; Shahmiri, S.S.; Eghbali, F.; Valizadeh, R.; Rezvani, M. Revision procedures after initial Roux-en-Y gastric bypass, treatment of weight regain: A systematic review and meta-analysis. Updat. Surg. 2021, 73, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.D.; Nwokeabia, I.D.; Purnell, S.; Zafar, S.N.; Ortega, G.; Hughes, K.; Fullum, T.M. Revision of Roux-En-Y Gastric Bypass for Weight Regain: A Systematic Review of Techniques and Outcomes. Obes. Surg. 2016, 26, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; De Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef] [PubMed]
- Vilallonga, R.; van de Vrande, S.; Himpens, J. Laparoscopic reversal of Roux-en-Y gastric bypass into normal anatomy with or without sleeve gastrectomy. Surg. Endosc. 2013, 27, 4640–4648. [Google Scholar] [CrossRef]
- Dapri, G.; Cadière, G.B.; Himpens, J. Laparoscopic Placement of Non-Adjustable Silicone Ring for Weight Regain after Roux-en-Y Gastric Bypass. Obes. Surg. 2009, 19, 650–654. [Google Scholar] [CrossRef]
- Bessler, M.; Daud, A.; DiGiorgi, M.F.; Inabnet, W.B.; Schrope, B.; Olivero-Rivera, L.; Davis, D. Adjustable gastric banding as revisional bariatric procedure after failed gastric bypass—Intermediate results. Surg. Obes. Relat. Dis. 2010, 6, 31–35. [Google Scholar] [CrossRef]
- Boerboom, A.; Aarts, E.; Lange, V.; Plamper, A.; Rheinwalt, K.; Linke, K.; Peterli, R.; Berends, F.; Hazebroek, E. Banding the Pouch with a Non-adjustable Ring as Revisional Procedure in Patients with Insufficient Results After Roux-en-Y Gastric Bypass: Short-term Outcomes of a Multicenter Cohort Study. Obes. Surg. 2020, 30, 797–803. [Google Scholar] [CrossRef]
- Uittenbogaart, M.; Leclercq, W.K.; Luijten, A.A.; van Dielen, F.M. Laparoscopic Adjustable Gastric Banding after Failed Roux-En-Y Gastric Bypass. Obes. Surg. 2016, 27, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, A.; Sakarellos, P.; Davakis, S.; Vailas, M.; Dimitriou, N.; Papalampros, A.; Schizas, D.; Charalabopoulos, A.; Felekouras, E. Revision of Roux-en-Y Gastric Bypass for Inadequate Weight Loss or Weight Regain. In Vivo 2022, 36, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.B.; de Siqueira, L.T.; Filho, E.N.; Júnior, J.G.C.D.A.; Campos, J.M.; de Barros-Correia, T.X.; Muniz, M.G.; Ferraz, E.M. Revision Surgery for Treatment of Weight Regain after Roux-En-Y Gastric Bypass. Obes. Surg. 2014, 24, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Maleckas, A.; Gudaitytė, R.; Petereit, R.; Venclauskas, L.; Veličkienė, D. Weight regain after gastric bypass: Etiology and treatment options. Gland Surg. 2016, 5, 617–624. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Lautz, D.B.; Thompson, C.C. Gastrojejunal Stoma Diameter Predicts Weight Regain after Roux-en-Y Gastric Bypass. Clin. Gastroenterol. Hepatol. 2011, 9, 228–233. [Google Scholar] [CrossRef]
- Herron, D.M.; Birkett, D.H.; Thompson, C.C.; Bessler, M.; Swanström, L.L. Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: A feasibility study. Surg. Endosc. 2008, 22, 1093–1099. [Google Scholar] [CrossRef]
- Flanagan, L. Measurement of Functional Pouch Volume following the Gastric Bypass Procedure. Obes. Surg. 1996, 6, 38–43. [Google Scholar] [CrossRef]
- Faul, A.; Chevallier, J.-M.; Poghosyan, T. Dilated Gastric Pouch Resizing for Weight Loss Failure after One Anastomosis Gastric Bypass. Obes. Surg. 2019, 29, 3406–3409. [Google Scholar] [CrossRef]
- King, W.C.; Hinerman, A.S.; Belle, S.H.; Wahed, A.S.; Courcoulas, A.P. Comparison of the Performance of Common Measures of Weight Regain after Bariatric Surgery for Association with Clinical Outcomes. JAMA 2018, 320, 1560–1569. [Google Scholar] [CrossRef]
- Voorwinde, V.; Steenhuis, I.H.M.; Janssen, I.M.C.; Monpellier, V.M.; van Stralen, M.M. Definitions of Long-Term Weight Regain and Their Associations with Clinical Outcomes. Obes. Surg. 2020, 30, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Yimcharoen, P.; Heneghan, H.M.; Singh, M.; Brethauer, S.; Schauer, P.; Rogula, T.; Kroh, M.; Chand, B. Endoscopic findings and outcomes of revisional procedures for patients with weight recidivism after gastric bypass. Surg. Endosc. 2011, 25, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Julien, C.; Brown, P.; Woods, I.; Hamdi, A.; Ortega, G.; Fullum, T.; Tran, D. Midterm Outcomes of Revisional Surgery for Gastric Pouch and Gastrojejunal Anastomotic Enlargement in Patients with Weight Regain after Gastric Bypass for Morbid Obesity. Obes. Surg. 2014, 24, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, I.; Petrucciani, N.; Kassir, R.; Malyshev, E.; Mazoyer, C.; Korkmaz, C.; Debs, T.; Gugenheim, J. Midterm Outcomes of Gastric Pouch Resizing for Weight Regain after Roux-en-Y Gastric Bypass. Obes. Surg. 2020, 30, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Yimcharoen, P.; Brethauer, S.A.; Kroh, M.; Chand, B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg. Obes. Relat. Dis. 2012, 8, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Marchesini, J.C.; Bastos, E.L.D.S.; Ramos, M.G.; De Souza, M.D.G.; Campos, J.M.; Ferraz, A.B. The Role of Gastrojejunostomy Size on Gastric Bypass Weight Loss. Obes. Surg. 2017, 27, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Chand, B.; Chen, Y.K.; DeMarco, D.C.; Miller, L.; Schweitzer, M.; Rothstein, R.I.; Lautz, D.B.; Slattery, J.; Ryan, M.B.; et al. Endoscopic Suturing for Transoral Outlet Reduction Increases Weight Loss after Roux-en-Y Gastric Bypass Surgery. Gastroenterology 2013, 145, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Callahan, Z.M.; Su, B.; Kuchta, K.; Linn, J.; Carbray, J.; Ujiki, M. Five-year results of endoscopic gastrojejunostomy revision (transoral outlet reduction) for weight gain after gastric bypass. Surg. Endosc. 2020, 34, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, B.S.; Saghir, S.M.; Naga, Y.; Dhaliwal, A.; Ramai, D.; Cross, C.; Singh, S.; Bhat, I.; Adler, D.G. Efficacy of transoral outlet reduction in Roux-en-Y gastric bypass patients to promote weight loss: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E1332–E1340. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Vantanasiri, K.; Laoveeravat, P.; Matar, R.H.; Vargas, E.J.; Maselli, D.B.; Alkhatry, M.; Fayad, L.; Kumbhari, V.; Fittipaldi-Fernandez, R.J.; et al. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Brunaldi, V.O.; Jirapinyo, P.; de Moura, D.T.H.; Okazaki, O.; Bernardo, W.M.; Neto, M.G.; Campos, J.M.; Santo, M.A.; de Moura, E.G.H. Endoscopic Treatment of Weight Regain following Roux-en-Y Gastric Bypass: A Systematic Review and Meta-analysis. Obes. Surg. 2018, 28, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Bulajic, M.; di Prampero, S.F.V.; Boškoski, I.; Costamagna, G. Endoscopic therapy of weight regain after bariatric surgery. World J. Gastrointest. Surg. 2021, 13, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Elbahrawy, A.; Bougie, A.; Albader, M.; Aggarwal, R.; Demyttenaere, S.; Andalib, A.; Court, O. Laparoscopic Wedge Resection of Gastrojejunostomy for Weight Recidivism after Gastric Bypass. Obes. Surg. 2017, 27, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Borbély, Y.; Winkler, C.; Kröll, D.; Nett, P. Pouch Reshaping for Significant Weight Regain after Roux-en-Y Gastric Bypass. Obes. Surg. 2017, 27, 439–444. [Google Scholar] [CrossRef]
- Wijngaarden, L.H.; Reiber, B.M.M.; Yousufzai, F.; Demirkiran, A.; Klaassen, R.A. Resizing a large pouch after laparoscopic Roux-en-Y gastric bypass: Comparing the effect of two techniques on weight loss. Surg. Endosc. 2021, 36, 3495–3503. [Google Scholar] [CrossRef]
- Spyropoulos, C.; Kehagias, I.; Panagiotopoulos, S. Revisional bariatric surgery: 13-year experience from a tertiary institution. Arch. Surg. 2010, 145, 173–177. [Google Scholar] [CrossRef]
- Iannelli, A.; Schneck, A.S.; Hébuterne, X.; Gugenheim, J. Gastric pouch resizing for Roux-en-Y gastric bypass failure in patients with a dilated pouch. Surg. Obes. Relat. Dis. 2013, 9, 260–267. [Google Scholar] [CrossRef]
- Shimizu, H.; Annaberdyev, S.; Motamarry, I.; Kroh, M.; Schauer, P.R.; Brethauer, S.A. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes. Surg. 2013, 23, 1766–1773. [Google Scholar] [CrossRef]
| Age | 47.1 ± 8.3 years |
| M/F | 22/1 |
| LRYGB/OAGB | 12/11 |
| BMI before bypass | 43.3 ± 5.7 kg/m2 |
| Weight before bypass | 115.5 ± 19.7 |
| Lower BMI after bypass | 27.9 ± 6.2 kg/m2 |
| BMI before resizing | 36.3 ± 4.7 kg/m2 |
| Weight before resizing | 96.5 ± 13.9 kg |
| Comorbidities Hypertension Type II Diabetes Mellitus OSAS Dyslipidemia | 30.4% 17.4% 17.4% 8.7% |
| Previous bariatric Surgery | 34.8% (n.8) |
| Mean operative time | 73.8 ± 21.6 |
| Mean time between bypass and LPR (months) | 77.9 ± 54.5 |
| Mean follow up after LPR (months) | 24.2± 16.1 |
| Mean weight after LPR | 77.9 ± 17.3 kg |
| Mean BMI after LPR | 29.3 ± 5.8 kg/m2 |
| Mean %TWL after LPR | 19.6 ± 9%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, S.; Zulian, V.; De Palma, M.; Sartori, A.; Andreica, A.; Nedelcu, M.; Carandina, S. Resizing of the Gastric Pouch for Weight Regain after Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: Is It a Valid Option? J. Clin. Med. 2022, 11, 6238. https://doi.org/10.3390/jcm11216238
Ferro S, Zulian V, De Palma M, Sartori A, Andreica A, Nedelcu M, Carandina S. Resizing of the Gastric Pouch for Weight Regain after Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: Is It a Valid Option? Journal of Clinical Medicine. 2022; 11(21):6238. https://doi.org/10.3390/jcm11216238
Chicago/Turabian StyleFerro, Silvia, Viola Zulian, Massimiliano De Palma, Andrea Sartori, Anamaria Andreica, Marius Nedelcu, and Sergio Carandina. 2022. "Resizing of the Gastric Pouch for Weight Regain after Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: Is It a Valid Option?" Journal of Clinical Medicine 11, no. 21: 6238. https://doi.org/10.3390/jcm11216238
APA StyleFerro, S., Zulian, V., De Palma, M., Sartori, A., Andreica, A., Nedelcu, M., & Carandina, S. (2022). Resizing of the Gastric Pouch for Weight Regain after Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: Is It a Valid Option? Journal of Clinical Medicine, 11(21), 6238. https://doi.org/10.3390/jcm11216238







