Artificial Intelligence and Corneal Confocal Microscopy: The Start of a Beautiful Relationship
Abstract
:1. Introduction
2. Corneal Confocal Microscopy
2.1. Corneal Anatomy
2.2. Corneal Confocal Microscopy
3. The Diagnostic Efficacy of Corneal Confocal Microscopy in Peripheral Neuropathies
4. Beyond Diabetic Peripheral Neuropathy
4.1. CCM in Other Peripheral Neuropathies
4.2. CCM in Central Neurodegenerative Disease
5. CCM Image Acquisition and Analysis
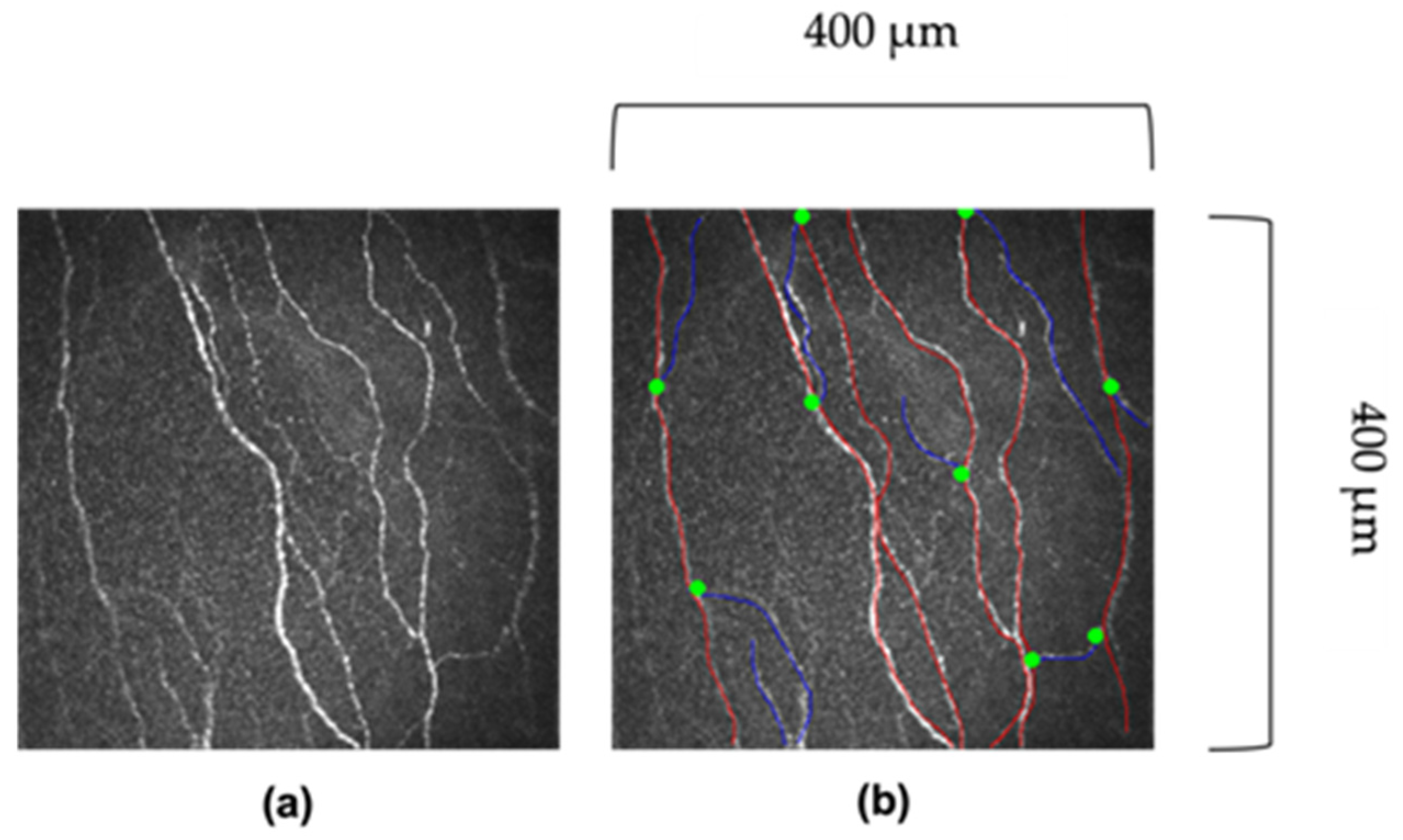
5.1. Manual Analysis
5.2. Automated Analysis
6. Fractal Dimension
6.1. Fractal Dimension in Diabetic Retinopathy
6.2. Fractal Deimention in Corneal Confocal Microscopy Images
7. Artificial Intelligence (AI)
7.1. Artificial Intelligence and Deep Learning
7.2. Artificial Intelligence and Opthalmology
7.3. Artificial Intelligence and Diabetic Retinopathy Screening
8. AI in CCM
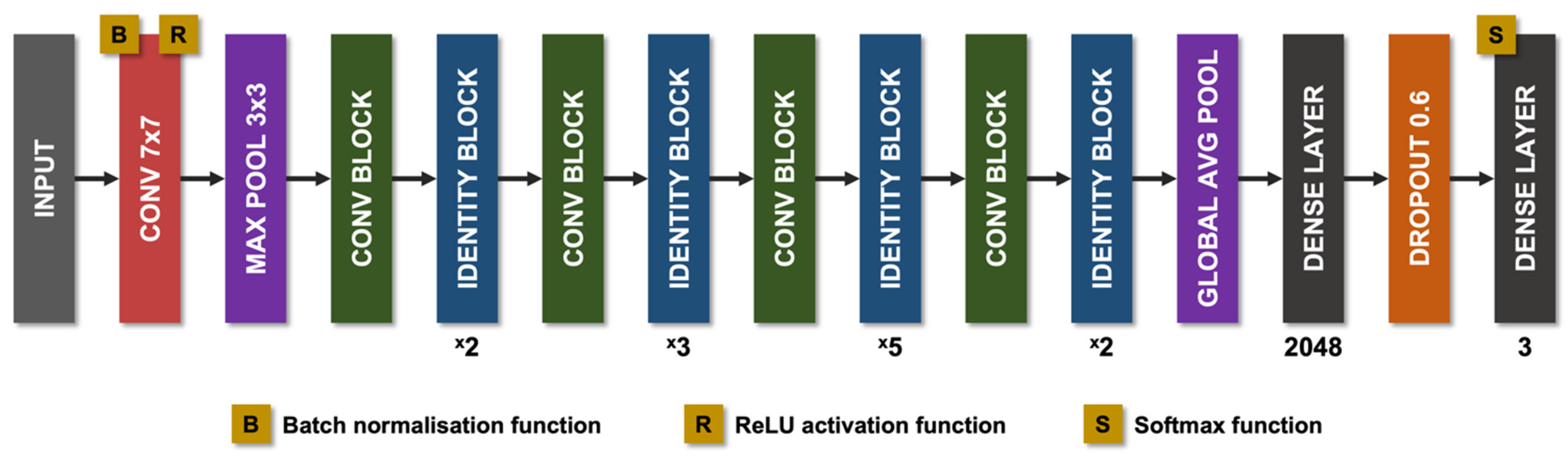
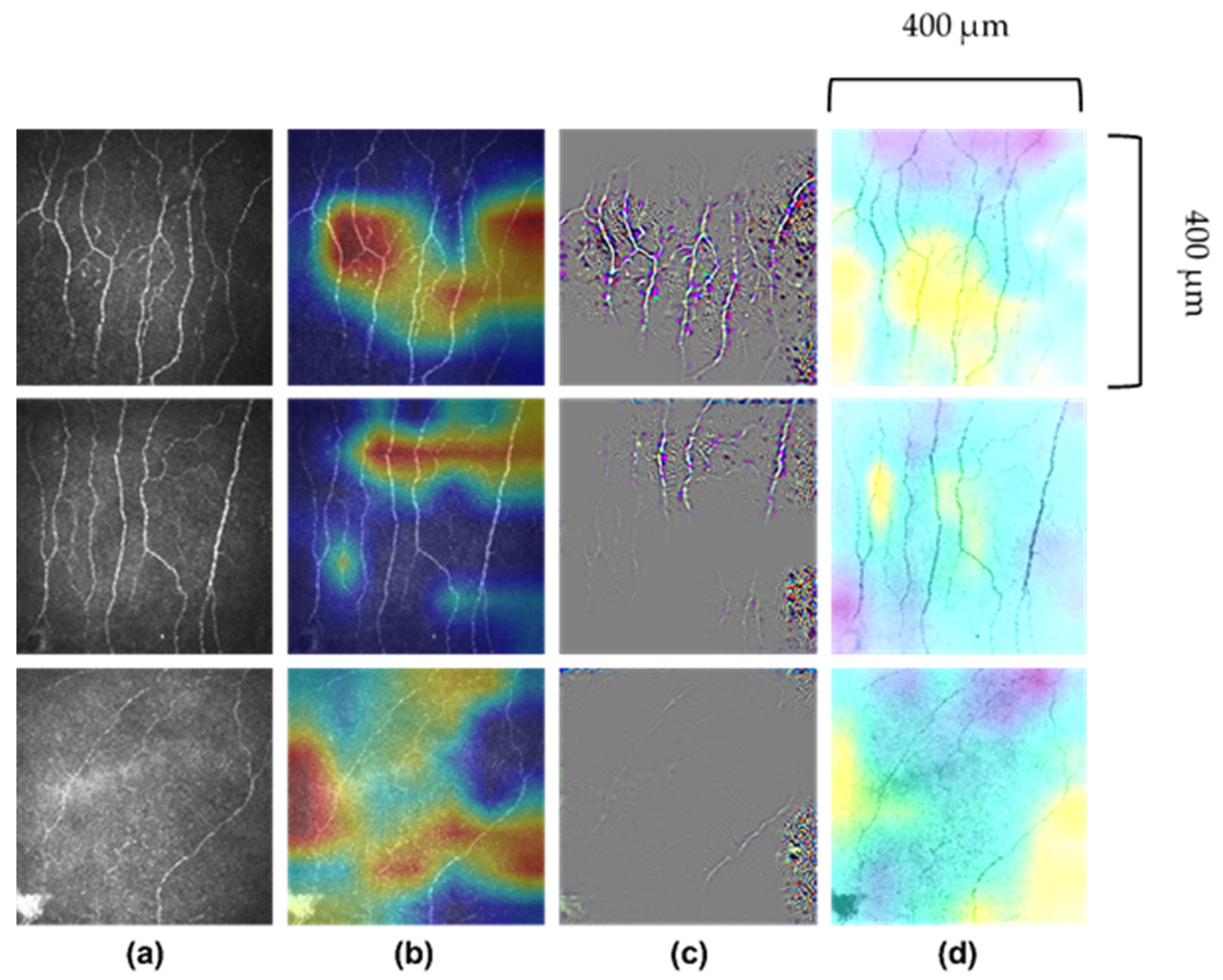
8.1. Technical Aspects of AI in CCM
8.2. AI Models in CCM
8.3. AI in Diabetic Neuropathy
9. Future Clinical Applications
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ANN | artificial neural networks |
| CCM | corneal confocal microscopy |
| CIPD | chronic inflammatory demyelinating polyneuropathy |
| CIPN | chemotherapy-induced peripheral neuropathy |
| CNBD | corneal nerve branch density |
| CNFD | corneal nerve fibre density |
| CNFL | corneal nerve fibre length |
| CNFrD | corneal nerve fractal dimension |
| DLA | deep learning algorithm |
| DPN | diabetic peripheral neuropathy |
| DR | diabetic retinopathy |
| HIV | human immunodeficiency virus |
| IENFD | intra-epidermal nerve fibre density |
| IWL | inferior whorl length |
References
- Watson, J.C.; Dyck, P.J.B. Peripheral Neuropathy: A Practical Approach to Diagnosis and Symptom Management. Mayo Clin. Proc. 2015, 90, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Martyn, C.N.; Hughes, R.A. Epidemiology of peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry 1997, 62, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwathmey, K.G.; Pearson, K.T. Diagnosis and management of sensory polyneuropathy. BMJ 2019, 365, l1108. [Google Scholar] [CrossRef] [Green Version]
- Deli, G.; Bosnyak, E.; Pusch, G.; Komoly, S.; Feher, G. Diabetic Neuropathies: Diagnosis and Management. Neuroendocrinology 2013, 98, 267–280. [Google Scholar] [CrossRef]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J., 3rd; Service, F.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817. [Google Scholar] [CrossRef]
- Partanen, J.; Niskanen, L.; Lehtinen, J.; Mervaala, E.; Siitonen, O.; Uusitupa, M. Natural History of Peripheral Neuropathy in Patients with Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 89–94. [Google Scholar] [CrossRef]
- Geraci, A.P.; Simpson, D.M. Neurological manifestations of HIV-1 infection in the HAART era. Compr. Ther. 2001, 27, 232–241. [Google Scholar] [CrossRef]
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare Costs and Workloss Burden of Patients with Chemotherapy-Associated Peripheral Neuropathy in Breast, Ovarian, Head and Neck, and Nonsmall Cell Lung Cancer. Chemother. Res. Pract. 2012, 2012, 913848. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Perkins, B.A.; Olaleye, D.; Zinman, B.; Bril, V. Simple Screening Tests for Peripheral Neuropathy in the Diabetes Clinic. Diabetes Care 2001, 24, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shy, M.E.; Frohman, E.M.; So, Y.T.; Arezzo, J.C.; Cornblath, D.R.; Giuliani, M.J.; Kincaid, J.C.; Ochoa, J.L.; Parry, G.J.; Weimer, L.H. Quantitative sensory testing: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003, 60, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Nebuchennykh, M.; Løseth, S.; Lindal, S.; Mellgren, S.I. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J. Neurol. 2009, 256, 1067–1075. [Google Scholar] [CrossRef]
- Egenolf, N.; Altenschildesche, C.M.Z.; Kreß, L.; Eggermann, K.; Namer, B.; Gross, F.; Klitsch, A.; Malzacher, T.; Kampik, D.; Malik, R.A.; et al. Diagnosing small fiber neuropathy in clinical practice: A deep phenotyping study. Ther. Adv. Neurol. Disord. 2021, 14, 175628642110043. [Google Scholar] [CrossRef]
- Asghar, O.; Petropoulos, I.N.; Alam, U.; Jones, W.; Jeziorska, M.; Marshall, A.; Ponirakis, G.; Fadavi, H.; Boulton, A.J.M.; Tavakoli, M.; et al. Corneal Confocal Microscopy Detects Neuropathy in Subjects With Impaired Glucose Tolerance. Diabetes Care 2014, 37, 2643–2646. [Google Scholar] [CrossRef] [Green Version]
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Sommer., C.; European Federation of Neurological Societies. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 2005, 12, 747–758. [Google Scholar] [CrossRef]
- Smith, A.G.; Russell, J.; Feldman, E.L.; Goldstein, J.; Peltier, A.; Smith, S.; Hamwi, J.; Pollari, D.; Bixby, B.; Howard, J.; et al. Lifestyle Intervention for Pre-Diabetic Neuropathy. Diabetes Care 2006, 29, 1294–1299. [Google Scholar] [CrossRef] [Green Version]
- Bakkers, M.; Merkies, I.S.J.; Lauria, G.; Devigili, G.; Penza, P.; Lombardi, R.; Hermans, M.C.E.; van Nes, S.I.; De Baets, M.; Faber, C.G. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009, 73, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Rajabally, Y.A.; Stettner, M.; Kieseier, B.C.; Hartung, H.P.; Malik, R.A. CIDP and other inflammatory neuropathies in diabetes —Diagnosis and management. Nat. Rev. Neurol. 2017, 13, 599–611. [Google Scholar] [CrossRef]
- De Felipe, C.; Gonzalez, G.G.; Gallar, J.; Belmonte, C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurons projecting to the cornea: Effect of corneal wounding. Eur. J. Pain 1999, 3, 31–39. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Hyndiuk, R.A.; Kazarian, E.L.; Schultz, R.O.; Seideman, S. Neurotrophic corneal ulcers in diabetes mellitus. Arch. Ophthalmol. 1977, 95, 2193–2196. [Google Scholar] [CrossRef]
- Malik, R.A.; Kallinikos, P.; Abbott, C.A.; van Schie, C.H.M.; Morgan, P.; Efron, N.; Boulton, A.J.M. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003, 46, 683–688. [Google Scholar] [CrossRef]
- Quattrini, C.; Tavakoli, M.; Jeziorska, M.; Kallinikos, P.; Tesfaye, S.; Finnigan, J.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007, 56, 2148–2154. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Al-Mohammedi, A.; Chen, X.; Ferdousi, M.; Ponirakis, G.; Kemp, H.; Chopra, R.; Hau, S.; Schargus, M.; Vollert, J.; et al. The Utility of Corneal Nerve Fractal Dimension Analysis in Peripheral Neuropathies of Different Etiology. Transl. Vis. Sci. Technol. 2020, 9, 43. [Google Scholar] [CrossRef]
- Kallinikos, P.; Berhanu, M.; O’Donnell, C.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Corneal nerve tortuosity in diabetic patients with neuropathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Brines, M.; Culver, D.A.; Ferdousi, M.; Tannemaat, M.R.; van Velzen, M.; Dahan, A.; Malik, R.A. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci. Rep. 2018, 8, 4734. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Gad, H.; Almuhannadi, H.; Brines, M.; Cerami, A.; Malik, R.A. Corneal confocal microscopy: Ready for prime time. Clin. Exp. Optom. 2020, 103, 265–277. [Google Scholar] [CrossRef]
- Kalteniece, A.; Ferdousi, M.; Adam, S.; Schofield, J.; Azmi, S.; Petropoulos, I.; Soran, H.; Malik, R.A. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS ONE 2017, 12, e0183040. [Google Scholar] [CrossRef]
- Tavakoli, M.; Ferdousi, M.; Petropoulos, I.N.; Morris, J.; Pritchard, N.; Zhivov, A.; Ziegler, D.; Pacaud, D.; Romanchuk, K.; Perkins, B.A.; et al. Normative Values for Corneal Nerve Morphology Assessed Using Corneal Confocal Microscopy: A Multinational Normative Data Set. Diabetes Care 2015, 38, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Morphometric Stability of the Corneal Subbasal Nerve Plexus in Healthy Individuals: A 3-Year Longitudinal Study Using Corneal Confocal Microscopy. Investig. Opthalmol. Vis. Sci. 2014, 55, 3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Petropoulos, I.N.; Ponirakis, G.; Menzies, R.A.; Chidiac, O.; Pasquier, J.; Khalil, C.A.; Talal, T.K.; Malik, R.A. Corneal confocal microscopy detects severe small fiber neuropathy in diabetic patients with Charcot neuroarthropathy. J. Diabetes Investig. 2018, 9, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Asghar, O.; Green, P.; Ponirakis, G.; Marshall, A.; Boulton, A.J.M.; Tavkoli, M.; Malik, R.A. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013, 36, 3646–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhivov, A.; Peschel, S.; Schober, H.C.; Stachs, O.; Baltrusch, S.; Bambi, M.T.; Kilangalanga, J.; Winter, K.; Kundt, G.; Guthoff, R.F. Diabetic foot syndrome and corneal subbasal nerve plexus changes in congolese patients with type 2 diabetes. PLoS ONE 2015, 10, e0119842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stem, M.S.; Hussain, M.; Lentz, S.I.; Raval, N.; Gardner, T.W.; Pop-Busui, R.; Shtein, R.M. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J. Diabetes Complicat. 2014, 28, 658–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, M.; Quattrini, C.; Abbott, C.; Kallinikos, P.; Marshall, A.; Finnigan, J.; Morgan, P.; Efron, N.; Boulton, A.J.M.; Malik, R.A. Corneal confocal microscopy: A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010, 33, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.M.; et al. Rapid Automated Diagnosis of Diabetic Peripheral Neuropathy With In Vivo Corneal Confocal Microscopy. Investig. Opthalmol. Vis. Sci. 2014, 55, 2071. [Google Scholar] [CrossRef]
- Alam, U.; Jeziorska, M.; Petropoulos, I.N.; Asghar, O.; Fadavi, H.; Ponirakis, G.; Marshall, A.; Tavakoli, M.; Boulton, A.J.M.; Efron, N.; et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE 2017, 12, e0180175. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Fadavi, H.; Ferdousi, M.; et al. Small Nerve Fiber Quantification in the Diagnosis of Diabetic Sensorimotor Polyneuropathy: Comparing Corneal Confocal Microscopy With Intraepidermal Nerve Fiber Density. Diabetes Care 2015, 38, 1138–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, B.A.; Lovblom, L.E.; Bril, V.; Scarr, D.; Ostrovski, I.; Orszag, A.; Edwards, K.; Pritchard, N.; Russell, A.; Dehghani, C.; et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: A pooled multinational consortium study. Diabetologia 2018, 61, 1856–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhang, C.; Zuo, A.; Li, L.; Chen, L.; Hou, X. Diagnostic utility of corneal confocal microscopy in type 2 diabetic peripheral neuropathy. J. Diabetes Investig. 2021, 12, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.H.; Lovblom, L.E.; Ferdousi, M.; Halpern, E.M.; Jeziorska, M.; Pacaud, D.; Pritchard, N.; Dehghani, C.; Edwards, K.; Srinivasan, S.; et al. Rapid Corneal Nerve Fiber Loss: A Marker of Diabetic Neuropathy Onset and Progression. Diabetes Care 2020, 43, 1829–1835. [Google Scholar] [CrossRef]
- Alam, U.; Ponirakis, G.; Asghar, O.; Petropoulos, I.N.; Azmi, S.; Jeziorska, M.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Corneal Confocal Microscopy Identifies People with Type 1 Diabetes with More Rapid Corneal Nerve Fibre Loss and Progression of Neuropathy. J. Clin. Med. 2022, 11, 2249. [Google Scholar] [CrossRef]
- Ferdousi, M.; Kalteniece, A.; Azmi, S.; Petropoulos, I.N.; Ponirakis, G.; Alam, U.; Ashgar, O.; Marshall, A.; Fullwood, C.; Jeziorska, M.; et al. Diagnosis of Neuropathy and Risk Factors for Corneal Nerve Loss in Type 1 and Type 2 Diabetes: A Corneal Confocal Microscopy Study. Diabetes Care 2021, 44, 150–156. [Google Scholar] [CrossRef]
- Edwards, K.; Pritchard, N.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Standardizing corneal nerve fibre length for nerve tortuosity increases its association with measures of diabetic neuropathy. Diabet. Med. 2014, 31, 1205–1209. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zhang, Y.; Wang, H.; Liu, X.; Zhang, S.; Liu, X.; Fan, D. Corneal sub-basal whorl-like nerve plexus: A landmark for early and follow-up evaluation in transthyretin familial amyloid polyneuropathy. Eur. J. Neurol. 2021, 28, 630–638. [Google Scholar] [CrossRef]
- Che, N.N.; Jiang, Q.H.; Ding, G.X.; Chen, S.Y.; Zhao, Z.X.; Li, X.; Malik, R.A.; Ma, J.J.; Yang, H.Q. Corneal nerve fiber loss relates to cognitive impairment in patients with Parkinson’s disease. NPJ Park. Dis. 2021, 7, 80. [Google Scholar] [CrossRef]
- Fernandes, D.; Luís, M.; Cardigos, J.; Xavier, C.; Alves, M.; Papoila, A.L.; Cunha, J.P.; Ferreira, J.T. Corneal Subbasal Nerve Plexus Evaluation by in Vivo Confocal Microscopy in Multiple Sclerosis: A Potential New Biomarker. Curr. Eye Res. 2021, 46, 1452–1459. [Google Scholar] [CrossRef]
- Tavakoli, M.; Marshall, A.; Pitceathly, R.; Fadavi, H.; Gow, D.; Roberts, M.E.; Malik, R.A. Corneal confocal microscopy: A novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp. Neurol. 2010, 223, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, M.; Marshall, A.; Banka, S.; Petropoulos, I.N.; Fadavi, H.; Kingston, H.; Malik, R.A. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve 2012, 46, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, H.I.; Petropoulos, I.N.; Rice, A.S.C.; Vollert, J.; Maier, C.; Sturm, D.; Schargus, M.; Peto, T.; Hau, S.; Chopra, R.; et al. Use of Corneal Confocal Microscopy to Evaluate Small Nerve Fibers in Patients With Human Immunodeficiency Virus. JAMA Ophthalmol. 2017, 135, 795. [Google Scholar] [CrossRef]
- Bitirgen, G.; Turkmen, K.; Malik, R.A.; Ozkagnici, A.; Zengin, N. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci. Rep. 2018, 8, 12244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, M.; Marshall, A.; Thompson, L.; Kenny, M.; Waldek, S.; Efron, N.; Malik, R.A. Corneal confocal microscopy: A novel noninvasive means to diagnose neuropathy in patients with fabry disease. Muscle Nerve 2009, 40, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tobin, V.; Vas, P.R.J.; Rayman, G. The LDIFLARE and CCM Methods Demonstrate Early Nerve Fiber Abnormalities in Untreated Hypothyroidism: A Prospective Study. J. Clin. Endocrinol. Metab. 2018, 103, 3094–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, D.; Schmidt-Wilcke, T.; Greiner, T.; Maier, C.; Schargus, M.; Tegenthoff, M.; Vorgerd, M. Confocal Cornea Microscopy Detects Involvement of Corneal Nerve Fibers in a Patient with Light-Chain Amyloid Neuropathy Caused by Multiple Myeloma: A Case Report. Case Rep. Neurol. 2016, 8, 134–139. [Google Scholar] [CrossRef]
- Schneider, C.; Bucher, F.; Cursiefen, C.; Fink, G.R.; Heindl, L.M.; Lehmann, H.C. Corneal confocal microscopy detects small fiber damage in chronic inflammatory demyelinating polyneuropathy (CIDP). J. Peripher. Nerv. Syst. 2014, 19, 322–327. [Google Scholar] [CrossRef]
- Stettner, M.; Hinrichs, L.; Guthoff, R.; Bairov, S.; Petropoulos, I.N.; Warnke, C.; Hartung, H.P.; Malik, R.A.; Kieseri, B.C. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann. Clin. Transl. Neurol. 2016, 3, 88–100. [Google Scholar] [CrossRef]
- Bitirgen, G.; Tinkir Kayitmazbatir, E.; Satirtav, G.; Malik, R.A.; Ozkagnici, A. In Vivo Confocal Microscopic Evaluation of Corneal Nerve Fibers and Dendritic Cells in Patients With Behçet’s Disease. Front. Neurol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Evdokimov, D.; Frank, J.; Klitsch, A.; Unterecker, S.; Warrings, B.; Serra, J.; Papagianni, A.; Saffer, N.; Altenschildesche, C.M.Z.; Kampik, D.; et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann. Neurol. 2019, 86, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudejans, L.; He, X.; Niesters, M.; Dahan, A.; Brines, M.; van Velzen, M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci. Rep. 2016, 6, 23573. [Google Scholar] [CrossRef] [Green Version]
- Pagovich, O.E.; Vo, M.L.; Zhao, Z.Z.; Petropoulos, I.N.; Yuan, M.; Lertsuwanroj, B.; Ciralsky, J.; Lai, E.; Kiss, S.; D’Amico, D.J.; et al. Corneal confocal microscopy: Neurologic disease biomarker in Friedreich ataxia. Ann. Neurol. 2018, 84, 893–904. [Google Scholar] [CrossRef]
- Misra, S.L.; Kersten, H.M.; Roxburgh, R.H.; Danesh-Meyer, H.V.; McGhee, C.N.J. Corneal nerve microstructure in Parkinson’s disease. J. Clin. Neurosci. 2017, 39, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Akpinar, Z.; Malik, R.A.; Ozkagnici, A. Use of Corneal Confocal Microscopy to Detect Corneal Nerve Loss and Increased Dendritic Cells in Patients With Multiple Sclerosis. JAMA Ophthalmol. 2017, 135, 777. [Google Scholar] [CrossRef] [PubMed]
- Al-Janahi, E.; Ponirakis, G.; Al Hamad, H.; Vattoth, S.; Elsotouhy, A.; Petropoulos, I.N.; Khan, A.; Gad, H.; Chandran, M.; Sankaranarayanan, A.; et al. Corneal Nerve and Brain Imaging in Mild Cognitive Impairment and Dementia. J. Alzheimer’s Dis. 2020, 77, 1533–1543. [Google Scholar] [CrossRef]
- Ferrari, G.; Grisan, E.; Scarpa, F.; Fazio, R.; Comola, M.; Quattrini, A.; Comi, G.; Rama, P.; Riva, N. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 278. [Google Scholar] [CrossRef] [Green Version]
- Ponirakis, G.; Al Hamad, H.; Sankaranarayanan, A.; Khan, A.; Chandran, M.; Ramadan, M.; Tosino, R.; Gawhale, P.V.; Alobaidi, M.; AlSulati, E.; et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann. Clin. Transl. Neurol. 2019, 6, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczak, J.; Zimmermann, H.; Kheirkhah, A.; Kadas, E.M.; Oberwahrenbrock, T.; Muller, R.; Ren, A.; Kuchling, J.; Dietze, D.; Pruss, H.; et al. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult. Scler. J. 2017, 23, 1847–1853. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Kamran, S.; Li, Y.; Khan, A.; Ponirakis, G.; Akhtar, N.; Deleu, D.; Shuaib, A.; Malik, R.A. Corneal Confocal Microscopy: An Imaging Endpoint for Axonal Degeneration in Multiple Sclerosis. Investig. Opthalmol. Vis. Sci. 2017, 58, 3677. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Fitzgerald, K.C.; Oakley, J.; Ponirakis, G.; Khan, A.; Gad, H.; George, P.; Deleu, D.; Canibano, B.G.; Akhtar, N.; et al. Corneal confocal microscopy demonstrates axonal loss in different courses of multiple sclerosis. Sci. Rep. 2021, 11, 21688. [Google Scholar] [CrossRef]
- Kass-Iliyya, L.; Javed, S.; Gosal, D.; Kobylecki, C.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Tavakoli, M.; Ferdousi, M.; Chaudhuri, K.R.; et al. Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Park. Relat. Disord. 2015, 21, 1454–1460. [Google Scholar] [CrossRef]
- Che, N.N.; Yang, H.Q. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl. Neurodegener. 2020, 9, 28. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Ferdousi, M.; Azmi, S.; Efron, N.; et al. Corneal Nerve Fractal Dimension: A Novel Corneal Nerve Metric for the Diagnosis of Diabetic Sensorimotor Polyneuropathy. Investig. Opthalmol. Vis. Sci. 2018, 59, 1113. [Google Scholar] [CrossRef]
- Vagenas, D.; Pritchard, N.; Edwards, K.; Shahidi, A.M.; Sampson, G.P.; Russell, A.W.; Malik, R.A.; Efron, N. Optimal Image Sample Size for Corneal Nerve Morphometry. Optom. Vis. Sci. 2012, 89, 812–817. [Google Scholar] [CrossRef]
- Chin, J.Y.; Yang, L.W.Y.; Ji, A.J.S.; Nubile, M.; Mastropasqua, L.; Allen, J.C.; Mehta, J.S.; Liu, Y.C. Validation of the Use of Automated and Manual Quantitative Analysis of Corneal Nerve Plexus Following Refractive Surgery. Diagnostics 2020, 10, 493. [Google Scholar] [CrossRef]
- Srinivasan, S.; Dehghani, C.; Pritchard, N.; Edwards, K.; Russell, A.W.; Malik, R.A.; Efron, N. Corneal and Retinal Neuronal Degeneration in Early Stages of Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2017, 58, 6365. [Google Scholar] [CrossRef] [Green Version]
- Takhar, J.S.; Joye, A.S.; Lopez, S.E.; Marneris, A.G.; Tsui, E.; Seitzman, G.D.; Keenan, J.D.; Gonzales, J.A. Validation of a Novel Confocal Microscopy Imaging Protocol With Assessment of Reproducibility and Comparison of Nerve Metrics in Dry Eye Disease Compared With Controls. Cornea 2021, 40, 603–612. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Ferdousi, M.; Marshall, A.; Alam, U.; Ponirakis, G.; Azmi, S.; Fadavi, H.; Efron, N.; Tavakoli, M.; Malik, R.A. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investig. Opthalmol. Vis. Sci. 2015, 56, 2498. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Almuhannadi, H.; Gad, H.; Malik, R.A. Diagnosing Diabetic Neuropathy: Something Old, Something New. Diabetes Metab. J. 2018, 42, 255. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. An Automatic Tool for Quantification of Nerve Fibers in Corneal Confocal Microscopy Images. IEEE Trans. Biomed. Eng. 2017, 64, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Maddaloni, E.; Sabatino, F.; del Toro, R.; Crugliano, S.; Grande, S.; Lauria Pantano, A.; Maurizi, A.R.; Palermo, A.; Bonini, S.; Pozzilli, P.; et al. In Vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet. Med. 2015, 32, 262–266. [Google Scholar] [CrossRef]
- Kowtharapu, B.S.; Winter, K.; Marfurt, C.; Allgeier, S.; Köhler, B.; Hovakimyan, M.; Stahnke, T.; Wree, T.; Stachs, O.; Guthoff, R.F. Comparative quantitative assessment of the human corneal sub-basal nerve plexus by In Vivo confocal microscopy and histological staining. Eye 2017, 31, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med. Image Anal. 2011, 15, 738–747. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Muller, R.; Mikolajczak, J.; Ren, A.; Kadas, E.M.; Zimmermann, H.; Pruess, H.; Paul, F.; Brandt, A.U.; Hamrah, P. Comparison of Standard Versus Wide-Field Composite Images of the Corneal Subbasal Layer by In Vivo Confocal Microscopy. Investig. Opthalmol. Vis. Sci. 2015, 56, 5801. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Manzoor, T.; Morgan, P.; Fadavi, H.; Asghar, O.; Alam, U.; Ponirakis, G.; Dabbah, M.A.; Chen, X.; Graham, J.; et al. Repeatability of In Vivo Corneal Confocal Microscopy to Quantify Corneal Nerve Morphology. Cornea 2013, 32, e83–e89. [Google Scholar] [CrossRef] [Green Version]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.; Tavakoli, M.; Malik, R.A. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med. Image Comput. Comput. Assist. Interv. 2010, 13, 300–307. [Google Scholar]
- Salahuddin, T.; Al-Maadeed, S.A.; Petropoulos, I.N.; Malik, R.A.; Ilyas, S.K.; Qidwai, U. Smart Neuropathy Detection using Machine Intelligence: Filling the Void Between Clinical Practice and Early Diagnosis. In Proceedings of the 2019 Third World Conference on Smart Trends in Systems Security and Sustainablity (WorldS4), London, UK, 30–31 July 2019; pp. 141–146. [Google Scholar]
- Scarpa, F.; Colonna, A.; Ruggeri, A. Multiple-Image Deep Learning Analysis for Neuropathy Detection in Corneal Nerve Images. Cornea 2020, 39, 342–347. [Google Scholar] [CrossRef]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, F.; Colonna, A.; Ruggeri, A. Healthy vs pathological classification of corneal nerves images using deep learning. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2107. [Google Scholar]
- Preston, F.G.; Meng, Y.; Burgess, J.; Ferdousi, M.; Azmi, S.; Petropoulos, I.N.; Kaye, S.; Malik, R.A.; Zheng, Y.; Alam, U. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia 2021, 65, 457–466. [Google Scholar] [CrossRef]
- Losa, G.A. The fractal geometry of life. Riv. Biol. 2014, 102, 29–59. [Google Scholar]
- Zahid, S.; Dolz-Marco, R.; Freund, K.B.; Balaratnasingam, C.; Dansingani, K.; Gilani, F.; Mehta, N.; Young, E.; Klifto, M.R.; Chae, B.; et al. Fractal Dimensional Analysis of Optical Coherence Tomography Angiography in Eyes With Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 4940. [Google Scholar] [CrossRef]
- Cheung, N.; Donaghue, K.C.; Liew, G.; Rogers, S.L.; Wang, J.J.; Lim, S.W.; Jenkins, A.J.; Hsu, W.; Lee, M.L.; Wong, T.Y. Quantitative assessment of early diabetic retinopathy using fractal analysis. Diabetes Care 2009, 32, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Ţălu, Ş.; Călugăru, D.M.; Lupaşcu, C.A. Characterisation of human non-proliferative diabetic retinopathy using the fractal analysis. Int. J. Ophthalmol. 2015, 8, 770–776. [Google Scholar]
- Fan, W.; Nittala, M.G.; Fleming, A.; Robertson, G.; Uji, A.; Wykoff, C.C.; Brown, D.M.; van Hemert, J.; Ip, M.; Wang, K.; et al. Relationship Between Retinal Fractal Dimension and Nonperfusion in Diabetic Retinopathy on Ultrawide-Field Fluorescein Angiography. Am. J. Ophthalmol. 2020, 209, 99–106. [Google Scholar] [CrossRef]
- Torp, T.L.; Kawasaki, R.; Wong, T.Y.; Peto, T.; Grauslund, J. Retinal arteriolar calibre and venular fractal dimension predict progression of proliferative diabetic retinopathy 6 months after panretinal photocoagulation: A prospective, clinical interventional study. BMJ Open Ophthalmol. 2021, 6, e000661. [Google Scholar] [CrossRef]
- Forster, R.B.; Garcia, E.S.; Sluiman, A.J.; Grecian, S.M.; McLachlan, S.; MacGillivray, T.J.; Strachan, M.W.J.; Price, J.F.; Edinburgh Type 2 Diabetes Study (ET2DS) investigators. Retinal venular tortuosity and fractal dimension predict incident retinopathy in adults with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetologia 2021, 64, 1103–1112. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Tan, G.S.W.; Agrawal, R.; Yanagi, Y.; Sie, N.M.; Wong, C.W.; Yeo, I.Y.S.; Lee, S.Y.; Cheung, C.M.G.; Wong, T.Y. Optical Coherence Tomographic Angiography in Type 2 Diabetes and Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 306. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.K.H.; Cheung, C.Y.; Sim, S.S.; Tan, P.C.; Tan, G.S.W.; Wong, T.Y. Retinal Imaging Techniques for Diabetic Retinopathy Screening. J. Diabetes Sci. Technol. 2016, 10, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, H.; Tan, H.C.; Lin, M.T.Y.; Mehta, J.S.; Liu, Y.C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [PubMed]
- Han, S.H.; Kim, K.W.; Kim, S.; Youn, Y.C. Artificial Neural Network: Understanding the Basic Concepts without Mathematics. Dement. Neurocognitive Disord. 2018, 17, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sarvamangala, D.R.; Kulkarni, R.V. Convolutional neural networks in medical image understanding: A survey. Evol. Intell. 2022, 15, 1–22. [Google Scholar] [CrossRef]
- Kapoor, R.; Walters, S.P.; Al-Aswad, L.A. The current state of artificial intelligence in ophthalmology. Surv. Ophthalmol. 2019, 64, 233–240. [Google Scholar] [CrossRef]
- Tognetto, D.; Giglio, R.; Vinciguerra, A.L.; Milan, S.; Rejdak, R.; Rejdak, M.; Zaluska-Ogryzek, K.; Zweifel, S.; Toro, M.D. Artificial intelligence applications and cataract management: A systematic review. Surv. Ophthalmol. 2021, 67, 817–829. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Ladas, J.G.; Lee, J.K. Artificial intelligence in cornea, refractive, and cataract surgery. Curr. Opin. Ophthalmol. 2020, 31, 253–260. [Google Scholar] [CrossRef]
- Dong, L.; Yang, Q.; Zhang, R.H.; Wei, W.B. Artificial intelligence for the detection of age-related macular degeneration in color fundus photographs: A systematic review and meta-analysis. EClinicalMedicine 2021, 35, 100875. [Google Scholar] [CrossRef]
- Muhammad, H.; Fuchs, T.J.; de Cuir, N.; de Moraes, C.G.; Blumberg, D.M.; Liebmann, J.M.; Ritch, R.; Hood, D.C. Hybrid Deep Learning on Single Wide-field Optical Coherence tomography Scans Accurately Classifies Glaucoma Suspects. J. Glaucoma. 2017, 26, 1086–1094. [Google Scholar] [CrossRef]
- Food and Drug Administration. US Food and Drug Administration (2018) FDA Permits Marketing of Artificial Intelligence-Based Device to Detect Certain Diabetes-Related Eye Problems. 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye (accessed on 3 September 2022).
- Styles, C.J. Introducing automated diabetic retinopathy systems: It’s not just about sensitivity and specificity. Eye 2019, 33, 1357–1358. [Google Scholar]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Liew, G.; Michaelides, M.; Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014, 4, e004015. [Google Scholar] [CrossRef] [PubMed]
- Heydon, P.; Egan, C.; Bolter, L.; Chambers, R.; Anderson, J.; Aldington, S.; Stratton, I.M.; Scanlon, P.H.; Webster, L.; Mann, S.; et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br. J. Ophthalmol. 2021, 105, 723–728. [Google Scholar] [CrossRef]
- Rubin, R. Obstacles to Implementing AI Tools in Health Care. JAMA 2021, 325, 333. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Peters, J.; Rogers, M.; Allen, M.; Lowe, J.; Kijauskaite, G.; Wilkinson, E.; Seedat, F.; Hyde, C. Automated grading in the Diabetic Eye Screening Programme External Review against Programme Appraisal Criteria for the UK National Screening Committee [Report]. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1035903/Evidence_summary_AI_in_DESP_2021.pdf (accessed on 3 September 2022).
- Zachariah, S.; Wykes, W.; Yorston, D. The Scottish Diabetic Retinopathy Screening programme. Community Eye Health 2015, 28, s22–s23. [Google Scholar] [PubMed]
- Scotland, G.S.; McNamee, P.; Philip, S.; Fleming, A.D.; Goatman, K.A.; Prescott, G.J.; Fonseca, S.; Sharp, P.F.; Olson, J.A. Cost-effectiveness of implementing automated grading within the national screening programme for diabetic retinopathy in Scotland. Br. J. Ophthalmol. 2007, 91, 1518–1523. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.D.; Goatman, K.A.; Philip, S.; Prescott, G.J.; Sharp, P.F.; Olson, J.A. Automated grading for diabetic retinopathy: A large-scale audit using arbitration by clinical experts. Br. J. Ophthalmol. 2010, 94, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Tampo, H.; Arai, Y.; Inoue, Y.; Kawashima, H. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS ONE 2017, 12, e0179790. [Google Scholar] [CrossRef] [Green Version]
- Bora, A.; Balasubramanian, S.; Babenko, B.; Virmani, S.; Venugopalan, S.; Mitani, A.; de Oliveria Marinho, G.; Cuadros, J.; Ruamviboonsus, P.; Corrado, G.S.; et al. Predicting the risk of developing diabetic retinopathy using deep learning. Lancet Digit. Health 2021, 3, e10–e19. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Cheng, J.; Zheng, Y.; Ghahremani, M.; Chen, H.; Liu, J.; Zhao, Y. Cycle Structure and Illumination Constrained GAN for Medical Image Enhancement. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Lima, Peru, 4–8 October 2020; pp. 667–677. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Mou, L.; Qi, H.; Liu, Y.; Zheng, Y.; Matthew, P.; Su, P.; Liu, J.; Zhang, J.; Zhao, Y. DeepGrading: Deep Learning Grading of Corneal Nerve Tortuosity. IEEE Trans. Med. Imaging 2022, 41, 2079–2091. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Eliceiri, K.W. Dual-stream Multiple Instance Learning Network for Whole Slide Image Classification with Self-supervised Contrastive Learning. Conf. Comput. Vis. Pattern Recognit. Workshops 2021, 2021, 14318–14328. [Google Scholar] [PubMed]
- Campanella, G.; Hanna, M.G.; Geneslaw, L.; Miraflor, A.; Werneck Krauss Silva, V.; Busam, K.J.; Brogi, E.; Reuter, V.E.; Klimstra, D.S.; Fuchs, T.J. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 2019, 25, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Salahouddin, T.; Petropoulos, I.N.; Ferdousi, M.; Ponirakis, G.; Asghar, O.; Alam, U.; Kamran, S.; Mahfoud, Z.R.; Efron, N.; Malik, R.A.; et al. Artificial Intelligence–Based Classification of Diabetic Peripheral Neuropathy From Corneal Confocal Microscopy Images. Diabetes Care 2021, 44, e151–e153. [Google Scholar] [CrossRef]
- Wei, S.; Shi, F.; Wang, Y.; Chou, Y.; Li, X. A Deep Learning Model for Automated Sub-Basal Corneal Nerve Segmentation and Evaluation Using In Vivo Confocal Microscopy. Transl. Vis. Sci. Technol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Regulation and Approval of Medical Devices: 1976–2020. JAMA 2021, 326, 420. [Google Scholar] [CrossRef]
- He, M.; Li, Z.; Liu, C.; Shi, D.; Tan, Z. Deployment of Artificial Intelligence in Real-World Practice: Opportunity and Challenge. Asia-Pac. J. Ophthalmol. 2020, 9, 299–307. [Google Scholar] [CrossRef]
- Devalla, S.K.; Liang, Z.; Pham, T.H.; Boote, C.; Strouthidis, N.G.; Thiery, A.H.; Girard, M.J.A. Glaucoma management in the era of artificial intelligence. Br. J. Ophthalmol. 2020, 104, 301–311. [Google Scholar] [CrossRef]
- Sivaskandarajah, G.A.; Halpern, E.M.; Lovblom, L.E.; Weisman, A.; Orlov, S.; Bril, V.; Perkins, B.A. Structure-Function Relationship Between Corneal Nerves and Conventional Small-Fiber Tests in Type 1 Diabetes. Diabetes Care 2013, 36, 2748–2755. [Google Scholar] [CrossRef] [Green Version]
- Burgess, J.; Frank, B.; Marshall, A.; Khalil, R.S.; Ponirakis, G.; Petropoulos, I.N.; Cuthbertson, D.J.; Malik, R.A.; Alam, U. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics 2021, 11, 165. [Google Scholar] [CrossRef]
- Azmi, S.; Jeziorska, M.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Marshall, A.; Alam, U.; Asghar, O.; Atkinson, A.; Jones, W.; et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019, 62, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brines, M.; Dunne, A.N.; van Velzen, M.; Proto, P.L.; Ostenson, C.G.; Kirk, R.I.; Petropoulos, I.N.; Javed, S.; Malik, R.A.; Cerami, A.; et al. ARA 290, a Nonerythropoietic Peptide Engineered from Erythropoietin, Improves Metabolic Control and Neuropathic Symptoms in Patients with Type 2 Diabetes. Mol. Med. 2014, 20, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, A.; Dunne, A.; Swartjes, M.; Proto, P.L.; Heij, L.; Vogels, O.; van Velzen, M.; Sarton, E.; Niesters, M.; Tannemaat, M.R.; et al. ARA 290 Improves Symptoms in Patients with Sarcoidosis-Associated Small Nerve Fiber Loss and Increases Corneal Nerve Fiber Density. Mol. Med. 2013, 19, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Azmi, S.; Ho, J.H.; Liu, Y.; Ferdousi, M.; Siahmansur, T.; Kalteniece, A.; Marshall, A.; Dhage, S.S.; Iqbal, Z.; et al. Improvements in Diabetic Neuropathy and Nephropathy After Bariatric Surgery: A Prospective Cohort Study. Obes. Surg. 2021, 31, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.; Ferdousi, M.; Liu, Y.; Adam, S.; Iqbal, Z.; Dhage, S.; Ponirakis, G.; Siahmansur, T.; Marshall, A.; Petropoulos, I.; et al. Bariatric surgery leads to an improvement in small nerve fibre damage in subjects with obesity. Int. J. Obes. 2021, 45, 631–638. [Google Scholar] [CrossRef]
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; Megahed, A.; DeFronzo, R.; et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: A substudy of the Qatar Study. BMJ Open Diabetes Res. Care 2020, 88, e001420. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; Perkins, B.A.; Lovblom, L.E.; Bazinet, R.P.; Wolever, T.M.S.; Bril, V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes. Neurology 2017, 88, 2294–2301. [Google Scholar] [CrossRef]
- Britten-Jones, A.C.; Kamel, J.T.; Roberts, L.J.; Braat, S.; Craig, J.P.; MacIsaac, R.J.; Downie, L.E. Investigating the Neuroprotective Effect of Oral Omega-3 Fatty Acid Supplementation in Type 1 Diabetes (nPROOFS1): A Randomized Placebo-Controlled Trial. Diabetes 2021, 70, 1794–1806. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gas, H.; Bashir, B.; Marshall, A.; Boulton, A.J.M.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef]
| Citation | Participants | Reference Standard | Index Test Threshold | Test and Target Condition | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Diabetic Peripheral Neuropathy | |||||||
| Perkins et al., 2018 [42] | Total = 998 T1D = 516 T2D = 482 | Toronto Criteria Confirmed DPN | 12.5 mm/mm2 | Automated CNFL T1D—DPN | 0.77 | 73 | 69 |
| 12.3 mm/mm2 | Automated CNFL T2D—DPN | 0.68 | 69 | 63 | |||
| 12.3 mm/mm2 | Automated CNFL T1D and T2D—DPN | 0.77 | 67 | 66 | |||
| Total < 8.6 mm/mm2 | Automated CNFL T1D and T2D—DPN | - | 88 | 88 | |||
| Alam et al., 2017 [40] | T1D with neuropathy = 31 Control Participants = 27 | Toronto Criteria Confirmed DPN | 25 no/mm2 36.5 no/mm2 16.8 mm/mm2 | CNFD—DPN | 0.81 | 77 | 79 |
| CNBD—DPN | 0.67 | 58 | 79 | ||||
| CNFL—DPN | 0.74 | 61 | 86 | ||||
| Chen et al., 2015 [41] | T1D = 63 Control = 26 | Toronto Criteria Confirmed DPN | 2 SD below the mean of the control group | Manual | |||
| CNFD—DPN | 0.82 | 82 | 71 | ||||
| CNFL—DPN | 0.70 | 59 | 74 | ||||
| CNBD—DPN | 0.59 | 17 | 96 | ||||
| Automated | |||||||
| CNFD—DPN | 0.80 | 60 | 83 | ||||
| CNFL—DPN | 0.77 | 59 | 80 | ||||
| CNBD—DPN | 0.80 | 29 | 98 | ||||
| Edwards et al., 2014 [47] | DM = 231 Control = 61 | Toronto Criteria Confirmed DPN | - | CNFL | 0.64 | 32 | 87 |
| - | Tortuosity-standardised CNFL | 0.67 | 38 | 88 | |||
| Wang et al., 2021 [43] | Total = 220 | Toronto Criteria Confirmed DPN | <15.3 mm/mm2 | CNFL | 0.70 | 80 | 59 |
| Control = 48 | <39 no/mm2 | CNBD | 0.66 | 78 | 52 | ||
| T2D = 172 | <25.68 n/mm2 | CNFD | 0.67 | 85 | 47 | ||
| Other Peripheral Neuropathies | |||||||
| Zhang et al., 2021 [48] | TTR-FAP = 15 Control = 15 | Genetically Confirmed TTR-FAP | <17.99 mm/mm2 | CNFL | 0.88 | 80 | 93 |
| <21.95 mm/mm2 | IWL | 0.89 | 86 | 80 | |||
| Central Peripheral Neuropathies | |||||||
| Che et al., 2021 [49] | Total = 82 | Clinically confirmed PD | <10.08 mm/mm2 | CNFL | 0.67 | 85 | 45 |
| PD = 42 | <22.85 n/mm2 | CNFD | 0.96 | 95 | 88 | ||
| Control = 40 | <26.72 n/mm2 | CNBD | 0.69 | 92 | 52 | ||
| Fernandes et al., 2021 [50] | Total = 82 MS = 60 Control = 22 | Clinically confirmed MS | - | CNFD | 0.84 | - | - |
| - | CNBD | 0.84 | - | - | |||
| - | CNFL | 0.74 | - | - | |||
| - | CNFT | 0.72 | - | - | |||
| Parameter | Description | Unit of Measurement |
|---|---|---|
| Corneal nerve fibre length (CNFL) | Length of all main nerve fibres and branches | mm/mm2 |
| Corneal nerve fibre density (CNFD) | Number of main nerve fibres | no/mm2 |
| Corneal nerve branch density (CNBD) | Number of main nerve fibre branches | no/mm2 |
| Citation | Participants | No. of Images | Study Methodology | Population | AUC | Sensitivity | Specificity | Classification Accuracy | Results Summary |
|---|---|---|---|---|---|---|---|---|---|
| Scarpa et al., 2019 and Scarpa et al., 2020 [89,91] | Total = 100 DPN = 50 Control = 50 | Total = 600; Training = 480; Cross-validation = 600; Evaluation = 120 | CNN | Neuropathy vs. Control (single block) | - | 98 | 96 | 97 | CNN identifies ROI allowing multiple images to be binarised into two separate categories demonstrating diagnostic efficacy |
| Neuropathy vs. Control (whole subject) | - | 98 | 94 | 96 | |||||
| Williams et al., 2020 [90] | Total = 222 DPN+ve = 132 DPN-ve = 90 | Images used for training the Liverpool CNN Total = 1698; | CNN and DLA | DPN+ve vs. DPN-ve | 0.83 | 68 | 87 | - | The Liverpool CNN and Liverpool DLA can quantify corneal nerve morphometrics in participants with confirmed DPN demonstrating diagnostic efficacy |
| External validation of the CNN/DLA Total =1578; Images evaluated using the Liverpool CNN/DLA; | |||||||||
| Participants with and without DPN as per the Toronto expert criteria included. Total images = 2137 | |||||||||
| Salahouddin et al., 2021 [130] | Total = 108 Control = 21 DPN+ve = 25 DPN−ve = 62 | Training = 174; Validation = 534 | DL ANFIS | DPN−ve cs Control | 0.86 (0.77–0.94) | 84 | 71 | - | Based on CCM images alone ANFIS classified 43% of participants as DPN+ve demonstrating diagnostic utility |
| DPN−ve vs. DPN+ve | 0.95 (0.91–0.99) | 92 | 80 | - | |||||
| Control vs. DPN+ve | 1.0 (0.99–1.0) | 100 | 95 | - | |||||
| Preston et al., 2022 [92] | Total = 369 Control = 90 DPN+ve = 130 DPN−ve = 149 | Training = 245; Validation = 84; Test = 40 | DLA | Control | - | 100 | - | 100 | Based on a single CCM image without pre-processing DLA can faithfully classify participants into controls, DPN+ve and DPN−ve categories demonstrating diagnostic utility and accuracy |
| DPN-ve | - | 85 | - | 85 | |||||
| DPN+ve | - | 83 | - | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, U.; Anson, M.; Meng, Y.; Preston, F.; Kirthi, V.; Jackson, T.L.; Nderitu, P.; Cuthbertson, D.J.; Malik, R.A.; Zheng, Y.; et al. Artificial Intelligence and Corneal Confocal Microscopy: The Start of a Beautiful Relationship. J. Clin. Med. 2022, 11, 6199. https://doi.org/10.3390/jcm11206199
Alam U, Anson M, Meng Y, Preston F, Kirthi V, Jackson TL, Nderitu P, Cuthbertson DJ, Malik RA, Zheng Y, et al. Artificial Intelligence and Corneal Confocal Microscopy: The Start of a Beautiful Relationship. Journal of Clinical Medicine. 2022; 11(20):6199. https://doi.org/10.3390/jcm11206199
Chicago/Turabian StyleAlam, Uazman, Matthew Anson, Yanda Meng, Frank Preston, Varo Kirthi, Timothy L. Jackson, Paul Nderitu, Daniel J. Cuthbertson, Rayaz A. Malik, Yalin Zheng, and et al. 2022. "Artificial Intelligence and Corneal Confocal Microscopy: The Start of a Beautiful Relationship" Journal of Clinical Medicine 11, no. 20: 6199. https://doi.org/10.3390/jcm11206199
APA StyleAlam, U., Anson, M., Meng, Y., Preston, F., Kirthi, V., Jackson, T. L., Nderitu, P., Cuthbertson, D. J., Malik, R. A., Zheng, Y., & Petropoulos, I. N. (2022). Artificial Intelligence and Corneal Confocal Microscopy: The Start of a Beautiful Relationship. Journal of Clinical Medicine, 11(20), 6199. https://doi.org/10.3390/jcm11206199







