Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review
Abstract
:1. Introduction
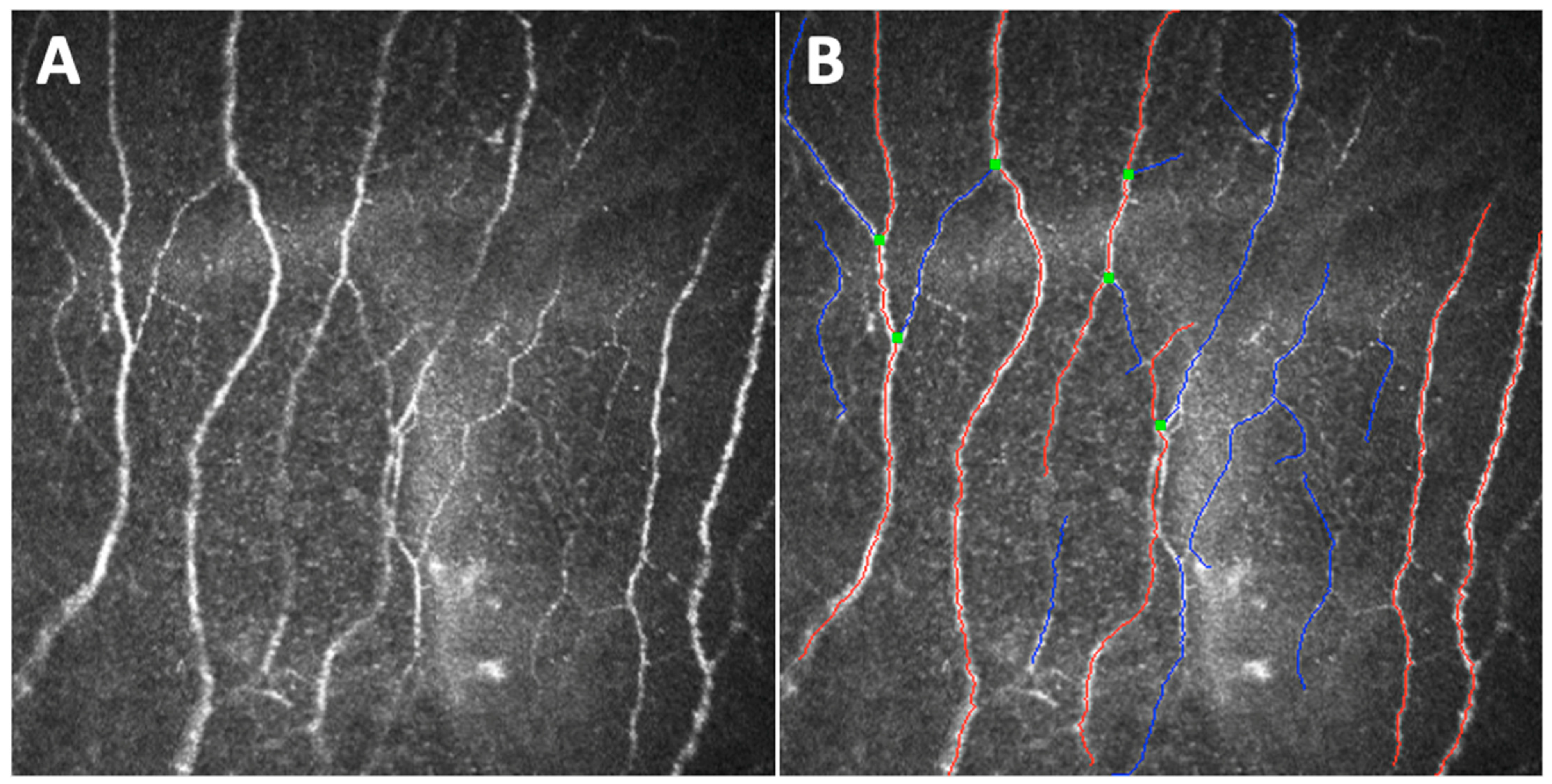
2. Principles of Confocal Microscopy
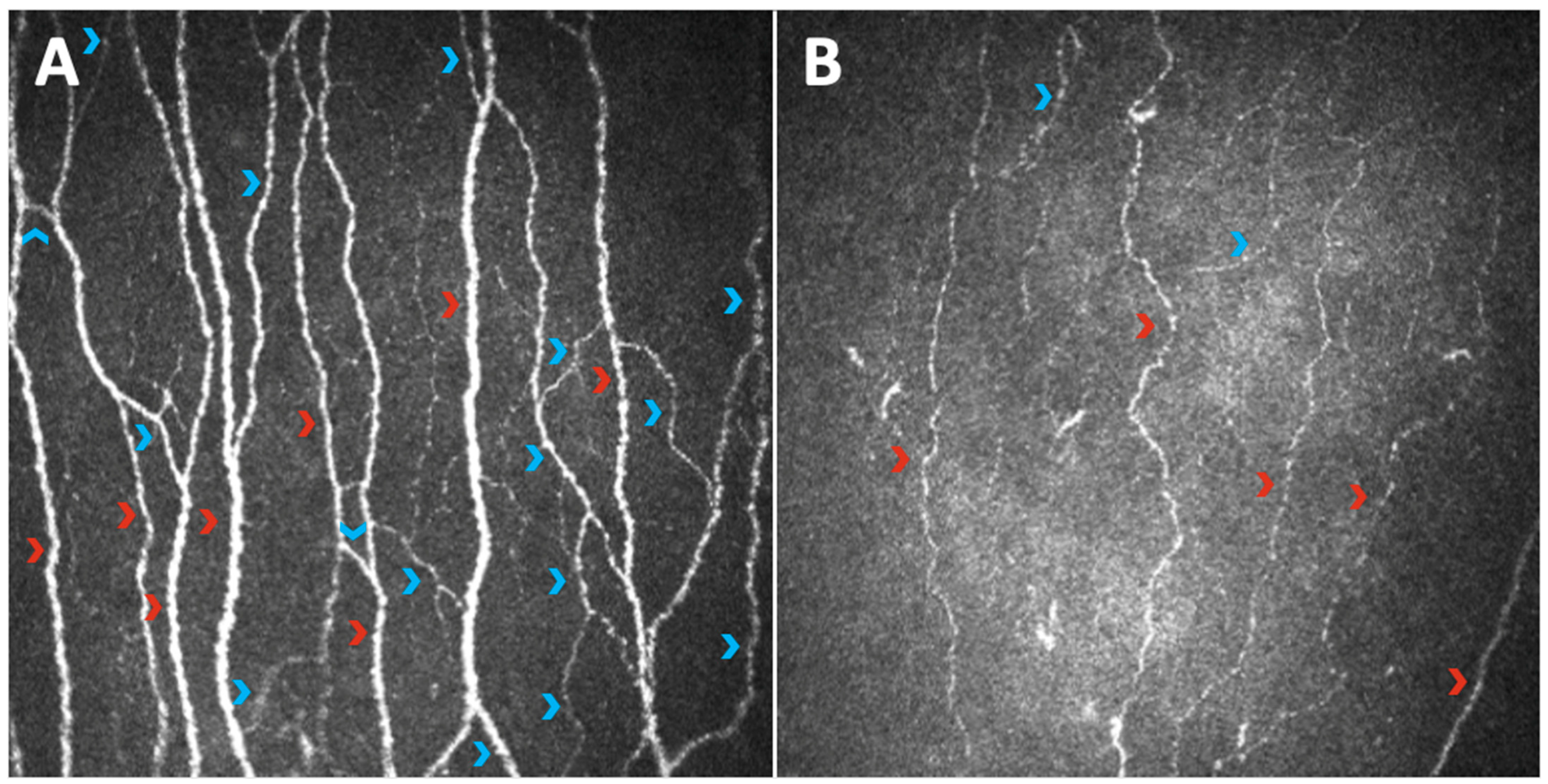
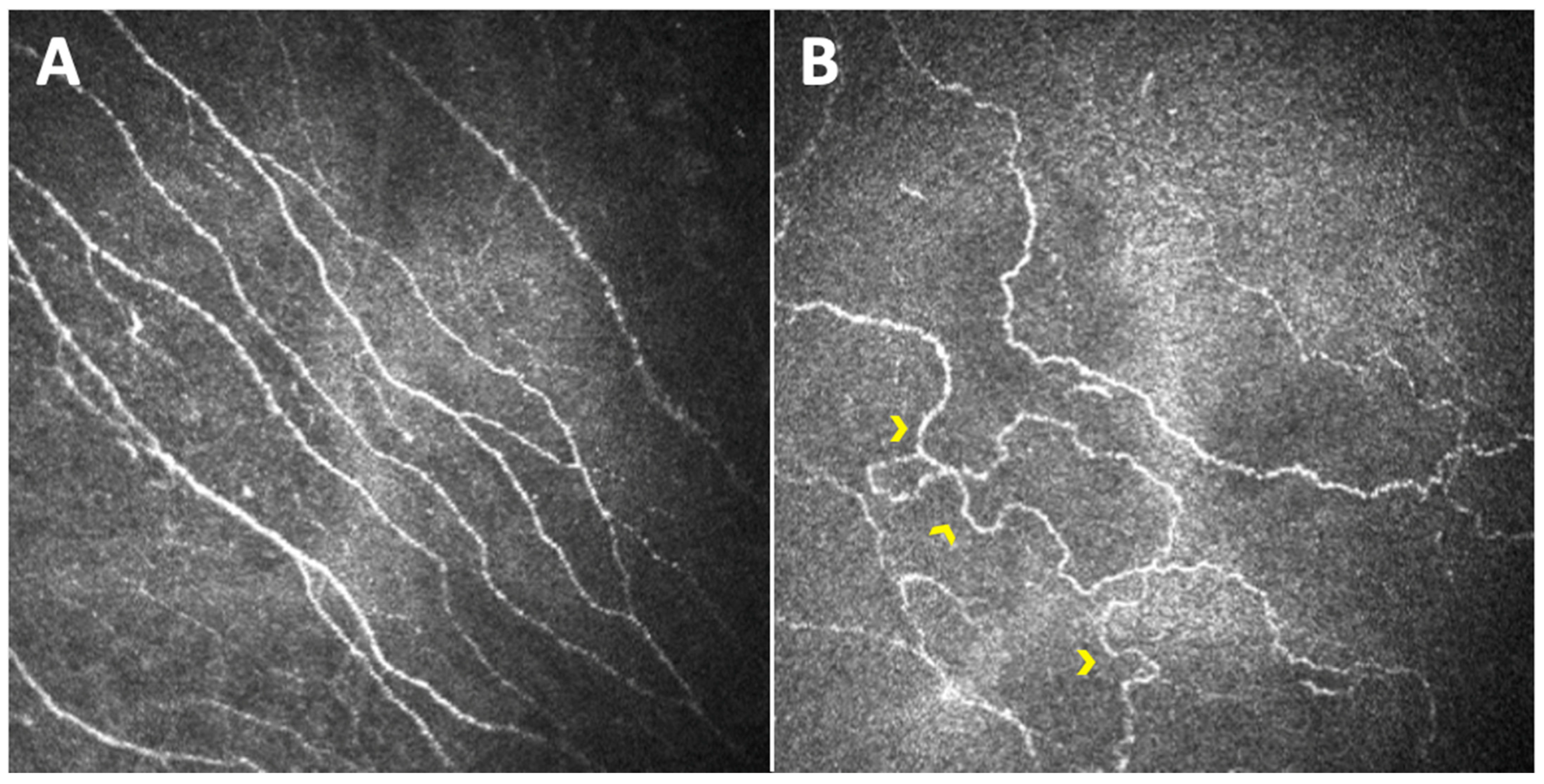
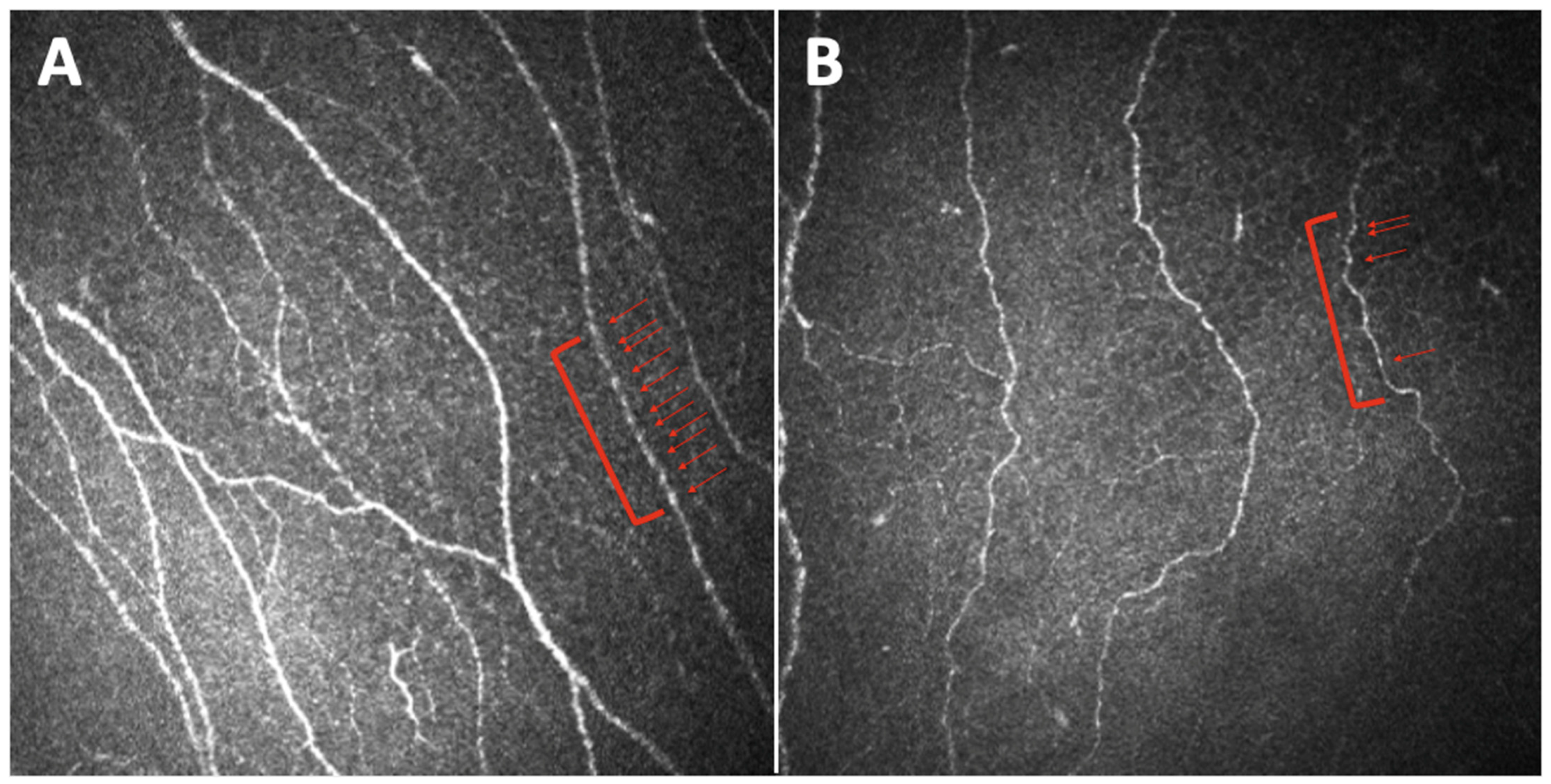
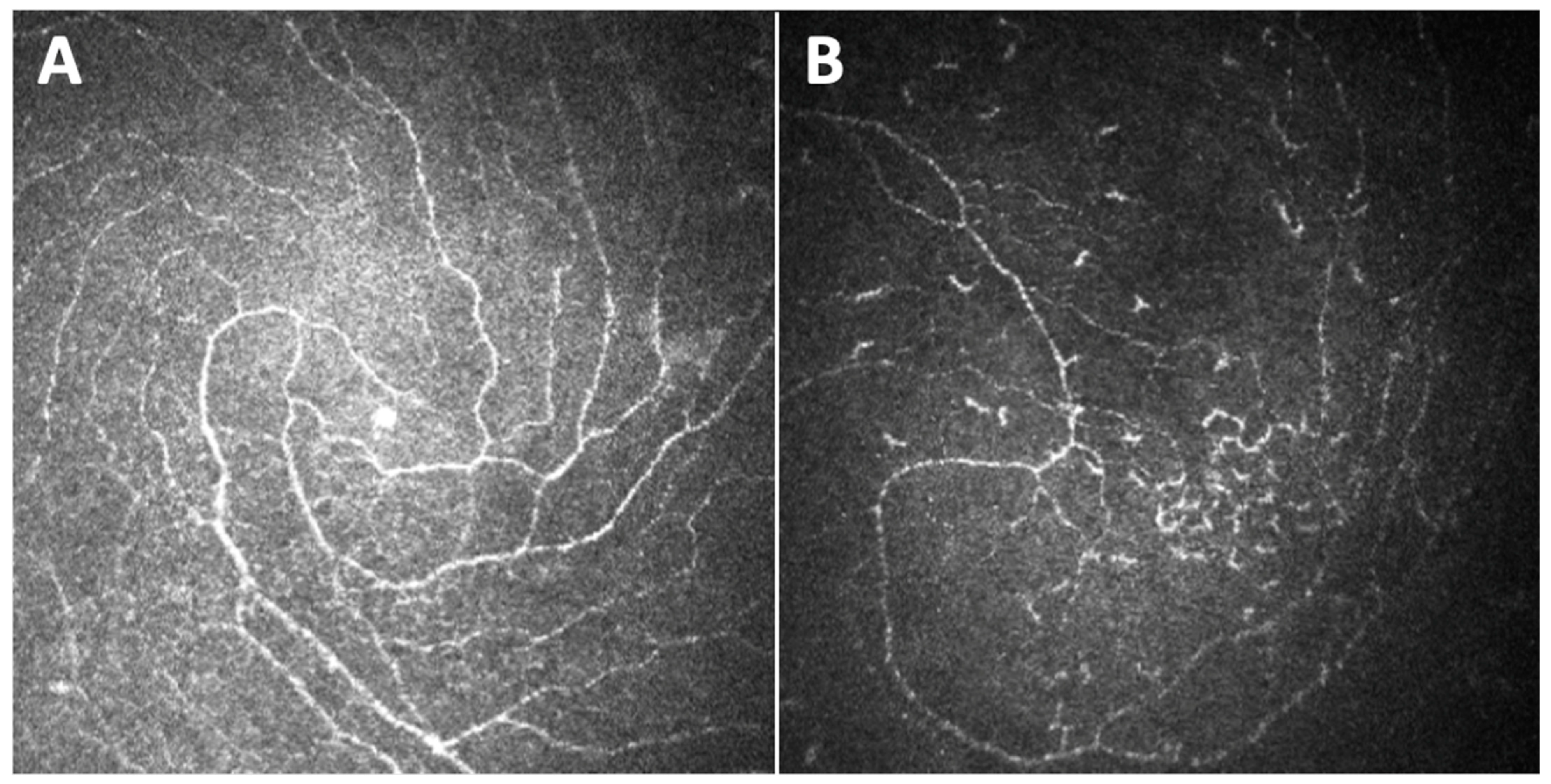
3. The Sub-Basal Nerve Plexus Observed through Different Parameters
4. Comparisons between CCM and other DPN Diagnostic Measures
5. Role of CCM in the Longitudinal Assessment of DPN
6. How Glycemic Parameters Affect Corneal Nerve Fibers
7. CCM Findings Help Defining Diabetic Corneal Neuropathy Pathogenesis
8. CCM in Pediatric Population Studies
9. Limitations and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, L.; Shao, C.; Cai, H.; Wang, Z. The Impact of Diabetes on Vascular Disease: Progress from the Perspective of Epidemics and Treatments. J. Diabetes Res. 2022, 2022, 1531289. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016, 39, S13–S22. [Google Scholar]
- Nathan, D.M. Long-term Complications of Diabetes Mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.K. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997, 46, S54–S57. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Vinik, A.I.; Arezzo, J.C.; Bril, V.; Feldman, E.L.; Freeman, R.; Malik, R.A.; Maser, R.E.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathies. A statement by the American Diabetes Association. Diabetes Care 2005, 28, 956–962. [Google Scholar] [CrossRef]
- Oliveira-Soto, L.; Efron, N. Morphology of corneal nerves using confocal microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef]
- Midena, E.; Cortese, M.; Miotto, S.; Gambato, C.; Cavarzeran, F.; Ghirlando, A. Confocal microscopy of corneal sub-basal nerve plexus: A quantitative and qualitative analysis in healthy and pathologic eyes. J. Refract. Surg. 2009, 25, S125–S130. [Google Scholar] [CrossRef]
- Midena, E.; Gambato, C.; Miotto, S.; Cortese, M.; Salvi, R.; Ghirlando, A. Long-term effects on corneal keratocytes of mitomycin C during photorefractive keratectomy: A randomized contralateral eye confocal microscopy study. J. Refract. Surg. 2007, 23, S1011–S1014. [Google Scholar] [CrossRef]
- Ghirlando, A.; Gambato, C.; Midena, E. LASEK and photorefractive keratectomy for myopia: Clinical and confocal microscopy comparison. J. Refract. Surg. 2007, 23, 694–702. [Google Scholar] [CrossRef]
- Parrozzani, R.; Lazzarini, D.; Alemany-Rubio, E.; Urban, F.; Midena, E. Topical 1% 5-fluorouracil in ocular surface squamous neoplasia: A long-term safety study. Br. J. Ophthalmol. 2011, 95, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Lazzarini, D.; Bortolotti, M.; Piliego, F.; Midena, E.; Fregona, I. Corneal confocal microscopy in patients with vernal keratoconjunctivitis. Ophthalmology 2012, 119, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Lazzarini, D.; Bartolo, O.; Berton, M.; Leonardi, A.; Fregona, I.A.; Parrozzani, R.; Midena, E. Small fiber peripheral neuropathy in Wilson disease: An in vivo documentation by corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1390–1395. [Google Scholar] [CrossRef]
- Campagnolo, M.; Lazzarini, D.; Fregona, I.; Cacciavillani, M.; Bergamo, F.; Parrozzani, R.; Midena, E.; Briani, C. Corneal confocal microscopy in patients with oxaliplatin-induced peripheral neuropathy. J. Peripher. Nerv. Syst. 2013, 18, 269–271. [Google Scholar] [CrossRef]
- Parrozzani, R.; Lombardi, G.; Midena, E.; Leonardi, F.; Londei, D.; Padovan, M.; Caccese, M.; Marchione, G.; Bini, S.; Zagonel, V.; et al. Corneal side effects induced by EGFR-inhibitor antibody–drug conjugate ABT-414 in patients with recurrent glioblastoma: A prospective clinical and confocal microscopy study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907543. [Google Scholar] [CrossRef] [PubMed]
- Parrozzani, R.; Lombardi, G.; Midena, E.; Londei, D.; Padovan, M.; Marchione, G.; Caccese, M.; Midena, G.; Zagonel, V.; Frizziero, L. Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study. Front. Oncol. 2020, 10, 593461. [Google Scholar] [CrossRef]
- Midena, E.; Cosmo, E.; Cattelan, A.M.; Briani, C.; Leoni, D.; Capizzi, A.; Tabacchi, V.; Parrozzani, R.; Midena, G.; Frizziero, L. Small Fibre Peripheral Alterations Following COVID-19 Detected by Corneal Confocal Microscopy. J. Pers. Med. 2022, 12, 563. [Google Scholar] [CrossRef]
- Minsky, M. Memoir on Inventing the Confocal Scanning Microscope. Scanning 1988, 10, 128–138. [Google Scholar] [CrossRef]
- Lemp, M.A.; Dilly, P.N.; Boyde, A. Tandem-scanning (confocal) microscopy of the full-thickness cornea. Cornea 1985, 4, 205–209. [Google Scholar] [CrossRef]
- Cavanagh, H.D.; Petroll, W.M.; Alizadeh, H.; He, Y.G.; McCulley, J.P.; Jester, J.V. Clinical and Diagnostic Use of In Vivo Confocal Microscopy in Patients with Corneal Disease. Ophthalmology 1993, 100, 1444–1454. [Google Scholar] [CrossRef]
- Rio-Cristobal, A.; Martin, R. Corneal assessment technologies: Current status. Surv. Ophthalmol. 2014, 59, 599–614. [Google Scholar] [CrossRef]
- Efron, N.; Perez-Gomez, I.; Mutalib, H.A.; Hollingsworth, J. Confocal microscopy of the normal human cornea. Cont. Lens Anterior Eye 2001, 24, 16–24. [Google Scholar] [CrossRef]
- Niederer, R.L.; McGhee, C.N.J. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog. Retin. Eye Res. 2010, 29, 30–58. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Hossain, P.; Malik, R.A. Clinical applications of corneal confocal microscopy. Clin. Ophthalmol. 2008, 2, 435. [Google Scholar] [PubMed]
- Rosenberg, M.E.; Tervo, T.M.T.; Immonen, I.J.; Muller, L.J.; Gronhagen-Riska, C.; Vesaluoma, M.H. Corneal structure and sensitivity in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Malik, R.A.; Kallinikos, P.; Abbott, C.A.; Van Schie, C.H.M.; Morgan, P.; Efron, N.; Boulton, A.J.M. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003, 46, 683–688. [Google Scholar] [CrossRef]
- Hossain, P.; Sachdev, A.; Malik, R.A. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet 2005, 366, 1340–1343. [Google Scholar] [CrossRef]
- Kallinikos, P.; Berhanu, M.; O’Donnell, C.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Corneal nerve tortuosity in diabetic patients with neuropathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 418–422. [Google Scholar] [CrossRef]
- Midena, E.; Brugin, E.; Ghirlando, A.; Sommavilla, M.; Avogaro, A. Corneal diabetic neuropathy: A confocal microscopy study. J. Refract. Surg. 2006, 22, 1047–1052. [Google Scholar] [CrossRef]
- Ishibashi, F.; Kojima, R.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. The Expanded Bead Size of Corneal C-Nerve Fibers Visualized by Corneal Confocal Microscopy Is Associated with Slow Conduction Velocity of the Peripheral Nerves in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 3653459. [Google Scholar] [CrossRef]
- Mocan, M.C.; Durukan, I.; Irkec, M.; Orhan, M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea 2006, 25, 769–773. [Google Scholar] [CrossRef] [PubMed]
- De Cillà, S.; Ranno, S.; Carini, E.; Fogagnolo, P.; Ceresara, G.; Orzalesi, N.; Rossetti, L.M. Corneal subbasal nerves changes in patients with diabetic retinopathy: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5155–5158. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Culver, D.A.; Ferdousi, M.; Tannemaat, M.R.; Van Velzen, M.; Dahan, A.; Malik, R.A. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci. Rep. 2018, 8, 4734. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Ferdousi, M.; Azmi, S.; Efron, N.; et al. Corneal Nerve Fractal Dimension: A Novel Corneal Nerve Metric for the Diagnosis of Diabetic Sensorimotor Polyneuropathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Hertz, P.; Bril, V.; Orszag, A.; Ahmed, A.; Ng, E.; Nwe, P.; Ngo, M.; Perkins, B.A. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011, 28, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Pritchard, N.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: Baseline findings of the LANDMark study. Clin. Exp. Optom. 2012, 95, 348–354. [Google Scholar] [CrossRef]
- Edwards, K.; Pritchard, N.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Standardizing corneal nerve fibre length for nerve tortuosity increases its association with measuRes. of diabetic neuropathy. Diabet Med. 2014, 31, 1205–1209. [Google Scholar] [CrossRef]
- Ahmed, A.; Bril, V.; Orszag, A.; Paulson, J.; Yeung, E.; Ngo, M.; Orlov, S.; Perkins, B.A. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: A concurrent validity study. Diabetes Care 2012, 35, 821–828. [Google Scholar] [CrossRef]
- Perkins, B.A.; Lovblom, L.E.; Bril, V.; Scarr, D.; Ostrovski, I.; Orszag, A.; Edwards, K.; Pritchard, N.; Russell, A.; Dehghani, C.; et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: A pooled multinational consortium study. Diabetologia 2018, 61, 1856–1861. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Ferdousi, M.; Marshall, A.; Alam, U.; Ponirakis, G.; Azmi, S.; Fadavi, H.; Efron, N.; Tavakoli, M.; Malik, R.A. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2498–2504. [Google Scholar] [CrossRef]
- Pritchard, N.; Dehghani, C.; Edwards, K.; Burgin, E.; Cheang, N.; Kim, H.; Mikhaiel, M.; Stanton, G.; Russell, A.W.; Malik, R.A.; et al. Utility of assessing nerve morphology in central cornea versus whorl area for diagnosing diabetic peripheral neuropathy. Cornea 2015, 34, 756–761. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Nagaoka, T.; Hanada, K.; Omae, T.; Yokota, H.; Abiko, A.; Haneda, M.; Yoshida, A. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5417–5423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalteniece, A.; Ferdousi, M.; Petropoulos, I.; Azmi, S.; Adam, S.; Fadavi, H.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Faber, C.G.; et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci. Rep. 2018, 8, 3283. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, M.; Kalteniece, A.; Petropoulos, I.; Azmi, S.; Dhage, S.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Faber, C.G.; Lauria, G.; et al. Diabetic Neuropathy Is Characterized by Progressive Corneal Nerve Fiber Loss in the Central and Inferior Whorl Regions. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef] [PubMed]
- Mahelková, G.; Burdová, M.Č.; Malá, Š.; Hoskovcová, L.; Dotřelová, D.; Štechová, K. Higher Total Insulin Dose Has Positive Effect on Corneal Nerve Fibers in DM1 Patients. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3800–3807. [Google Scholar] [CrossRef]
- Burdová, M.C.; Kulich, M.; Dotřelová, D.; Mahelková, G. Effect of diabetes mellitus type 1 diagnosis on the corneal cell densities and nerve fibers. Physiol. Res. 2018, 67, 963–974. [Google Scholar] [CrossRef]
- Schiano Lomoriello, D.; Abicca, I.; Parravano, M.; Giannini, D.; Russo, B.; Frontoni, S.; Picconi, F. Early Alterations of Corneal Subbasal Plexus in Uncomplicated Type 1 Diabetes Patients. J. Ophthalmol. 2019, 2019, 9818217. [Google Scholar] [CrossRef]
- Messmer, E.M.; Schmid-Tannwald, C.; Zapp, D.; Kampik, A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1307–1312. [Google Scholar] [CrossRef]
- Gad, H.; Al-Jarrah, B.; Saraswathi, S.; Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Singh, P.; Al Khodor, S.; Elawad, M.; Almasri, W.; et al. Corneal nerve loss in children with type 1 diabetes mellitus without retinopathy or microalbuminuria. J. Diabetes Investig. 2020, 11, 1594–1601. [Google Scholar] [CrossRef]
- Erie, J.C.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. The effect of age on the corneal subbasal nerve plexus. Cornea 2005, 24, 705–709. [Google Scholar] [CrossRef]
- Gambato, C.; Longhin, E.; Catania, A.G.; Lazzarini, D.; Parrozzani, R.; Midena, E. Aging and corneal layers: An in vivo corneal confocal microscopy study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: Development of a potential measure of diabetic peripheral neuropathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7982–7990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Russell, A.W.; Malik, R.A.; Efron, N. Risk factors associated with corneal nerve alteration in type 1 diabetes in the absence of neuropathy: A longitudinal in vivo corneal confocal microscopy study. Cornea 2016, 35, 847–852. [Google Scholar] [CrossRef]
- Andersen, S.T.; Grosen, K.; Tankisi, H.; Charles, M.; Andersen, N.T.; Andersen, H.; Petropoulos, I.N.; Malik, R.A.; Jensen, T.S.; Karlsson, P. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes: ADDITION-Denmark. J. Diabetes Complicat. 2018, 32, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, M.; Kalteniece, A.; Azmi, S.; Petropoulos, I.N.; Ponirakis, G.; Alam, U.; Asghar, O.; Marshall, A.; Fullwood, C.; Jeziorska, M.; et al. Diagnosis of Neuropathy and Risk Factors for Corneal Nerve Loss in Type 1 and Type 2 Diabetes: A Corneal Confocal Microscopy Study. Diabetes Care 2021, 44, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: A 3-year longitudinal study using corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3195–3199. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Ferdousi, M.; Petropoulos, I.N.; Morris, J.; Pritchard, N.; Zhivov, A.; Ziegler, D.; Pacaud, D.; Romanchuk, K.; Perkins, B.A.; et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: A multinational normative data set. Diabetes Care 2015, 38, 838–843. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic somatic neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef]
- Mojaddidi, M.; Quattrini, C.; Tavakoli, M.; Malik, R.A. Recent developments in the assessment of efficacy in clinical trials of diabetic neuropathy. Curr. Diab. Rep. 2005, 5, 417–422. [Google Scholar] [CrossRef]
- Sumner, C.J.; Sheth, S.; Griffin, J.W.; Cornblath, D.R.; Polydefkis, M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003, 60, 108–111. [Google Scholar] [CrossRef]
- Smith, A.G.; Howard, J.R.; Kroll, R.; Ramachandran, P.; Hauer, P.; Singleton, J.R.; McArthur, J. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J. Neurol. Sci. 2005, 228, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, C.; Tavakoli, M.; Jeziorska, M.; Kallinikos, P.; Tesfaye, S.; Finnigan, J.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Surrogate Markers of Small Fiber Damage in Human Diabetic Neuropathy. Diabetes 2007, 56, 2148–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Fadavi, H.; Ferdousi, M.; et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: Comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015, 38, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Jeziorska, M.; Petropoulos, I.N.; Asghar, O.; Fadavi, H.; Ponirakis, G.; Marshall, A.; Tavakoli, M.; Boulton, A.J.M.; Efron, N.; et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE 2017, 12, e0180175. [Google Scholar] [CrossRef]
- Tavakoli, M.; Quattrini, C.; Abbott, C.; Kallinikos, P.; Marshall, A.; Finnigan, J.; Morgan, P.; Efron, N.; Boulton, A.J.M.; Malik, R.A. Corneal confocal microscopy: A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010, 33, 1792–1797. [Google Scholar] [CrossRef]
- Pritchard, N.; Edwards, K.; Dehghani, C.; Fadavi, H.; Jeziorska, M.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Russell, A.W.; Sampson, G.P.; et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): Study design and baseline characteristics. Diabetes Res. Clin. Pract. 2014, 104, 248–256. [Google Scholar] [CrossRef]
- Edwards, K.; Pritchard, N.; Dehghani, C.; Vagenas, D.; Russell, A.; Malik, R.A.; Efron, N. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. J. Diabetes Complicat. 2017, 31, 1325–1327. [Google Scholar] [CrossRef]
- Dhage, S.; Ferdousi, M.; Adam, S.; Ho, J.H.; Kalteniece, A.; Azmi, S.; Alam, U.; Ponirakis, G.; Petropoulos, I.; Atkinson, A.J.; et al. Corneal confocal microscopy identifies small fibre damage and progression of diabetic neuropathy. Sci. Rep. 2021, 11, 1859. [Google Scholar] [CrossRef]
- Pritchard, N.; Edwards, K.; Russell, A.W.; Perkins, B.A.; Malik, R.A.; Efron, N. Corneal confocal microscopy predicts 4-Year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 2015, 38, 671–675. [Google Scholar] [CrossRef]
- Lovblom, L.E.; Halpern, E.M.; Wu, T.; Kelly, D.; Ahmed, A.; Boulet, G.; Orszag, A.; Ng, E.; Ngo, M.; Bril, V.; et al. In Vivo Corneal Confocal Microscopy and Prediction of Future-Incident Neuropathy in Type 1 Diabetes: A Preliminary Longitudinal Analysis. Can. J. Diabetes. 2015, 39, 390–397. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; Perkins, B.A.; Lovblom, L.E.; Bazinet, R.P.; Wolever, T.M.S.; Bril, V. Using in vivo corneal confocal microscopy to identify diabetic sensorimotor polyneuropathy risk profiles in patients with type 1 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000251. [Google Scholar] [CrossRef] [PubMed]
- Perkins, B.A.; Lovblom, L.E.; Lewis, E.J.H.; Bril, V.; Ferdousi, M.; Orszag, A.; Edwards, K.; Pritchard, N.; Russell, A.; Dehghani, C.; et al. Corneal Confocal Microscopy Predicts the Development of Diabetic Neuropathy: A Longitudinal Diagnostic Multinational Consortium Study. Diabetes Care 2021, 44, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.H.; Lovblom, L.E.; Ferdousi, M.; Halpern, E.M.; Jeziorska, M.; Pacaud, D.; Pritchard, N.; Dehghani, C.; Edwards, K.; Srinivasan, S.; et al. Rapid Corneal Nerve Fiber Loss: A Marker of Diabetic Neuropathy Onset and Progression. Diabetes Care 2020, 43, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann. Intern. Med. 1995, 122, 561–568. [Google Scholar] [CrossRef]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.M.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef]
- Mehra, S.; Tavakoli, M.; Kallinikos, P.A.; Efron, N.; Boulton, A.J.M.; Augustine, T.; Malik, R.A. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 2007, 30, 2608–2612. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mitu-Pretorian, M.; Petropoulos, I.N.; Fadavi, H.; Asghar, O.; Alam, U.; Ponirakis, G.; Jeziorska, M.; Marshall, A.; Efron, N.; et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013, 62, 254–260. [Google Scholar] [CrossRef]
- Azmi, S.; Jeziorska, M.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Marshall, A.; Alam, U.; Asghar, O.; Atkinson, A.; Jones, W.; et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019, 62, 1478–1487. [Google Scholar] [CrossRef]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Fadavi, H.; Tavakoli, M.; Alam, U.; Jones, W.; Marshall, A.; Jeziorska, M.; et al. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care 2015, 38, e3–e4. [Google Scholar] [CrossRef]
- Tavakoli, M.; Kallinikos, P.; Iqbal, A.; Herbert, A.; Fadavi, H.; Efron, N.; Boulton, A.J.M.; Malik, R.A. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011, 28, 1261–1267. [Google Scholar] [CrossRef]
- Ishibashi, F.; Okino, M.; Ishibashi, M.; Kawasaki, A.; Endo, N.; Kosaka, A.; Uetake, H. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J. Diabetes Investig. 2012, 3, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Lupachyk, S.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J. mice. J. Peripher. Nerv. Syst. 2014, 19, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J. mice. J. Peripher. Nerv. Syst. 2015, 20, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Slater, J.A.; McGhee, C.N.J.; Pradhan, M.; Braatvedt, G.D. Corneal Confocal Microscopy in Type 1 Diabetes Mellitus: A Six-Year Longitudinal Study. Transl. Vis. Sci. Technol. 2022, 11, 17. [Google Scholar] [CrossRef]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef]
- Chen, D.K.; Frizzi, K.E.; Guernsey, L.S.; Ladt, K.; Mizisin, A.P.; Calcutt, N.A. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J. Peripher. Nerv. Syst. 2013, 18, 306–315. [Google Scholar] [CrossRef]
- Pellegrini, M.; Sebastiani, S.; Tucci, L.; Giannaccare, G.; Moscatiello, S.; Laffi, G.; Pagotto, U.; Di Dalmazi, G.; Versura, P. Association between alterations of corneal sub-basal nerve plexus analyzed with in vivo confocal microscopy and long-term glycemic variability. Eur. J. Ophthalmol. 2021, 31, 2294–2299. [Google Scholar] [CrossRef]
- Ishibashi, F.; Tavakoli, M. Impact of Normoglycemia in Reducing Microvascular Complications in Patients with Type 2 Diabetes: A Follow-Up Study. Front. Endocrinol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Jia, X.; Wang, X.; Wang, X.; Pan, Q.; Xian, T.; Yu, X.; Guo, L. In Vivo Corneal Confocal Microscopy Detects Improvement of Corneal Nerve Parameters following Glycemic Control in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 8516276. [Google Scholar] [CrossRef]
- Dell’Omo, R.; Cifariello, F.; De Turris, S.; Romano, V.; Di Renzo, F.; Di Taranto, D.; Coclite, G.; Agnifili, L.; Mastropasqua, L.; Costagliola, C. Confocal microscopy of corneal nerve plexus as an early marker of eye involvement in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 142, 393–400. [Google Scholar] [CrossRef]
- Pittenger, G.L.; Malik, R.A.; Burcus, N.; Boulton, A.J.; Vinik, A.I. Specific fiber deficits in sensorimotor diabetic polyneuropathy correspond to cytotoxicity against neuroblastoma cells of sera from patients with diabetes. Diabetes Care 1999, 22, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Increased Langerhan cell density and corneal nerve damage in diabetic patients: Role of immune mechanisms in human diabetic neuropathy. Contact Lens Anterior Eye 2011, 34, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppin, K.; Behrendt, A.K.; Reichard, M.; Stachs, O.; Guthoff, R.F.; Baltrusch, S.; Eule, J.C.; Vollmar, B. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3603–3615. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.; Karst, S.; Sacu, S.; Scholda, C.; Pablik, E.; Schmidt-Erfurth, U. Correlation between corneal and retinal neurodegenerative changes and their association with microvascular perfusion in type II diabetes. Acta Ophthalmol. 2019, 97, e545–e550. [Google Scholar] [CrossRef]
- Chang, P.Y.; Carrel, H.; Huang, J.S.; Wang, I.J.; Hou, Y.C.; Chen, W.L.; Wang, J.Y.; Hu, F.R. Decreased Density of Corneal Basal Epithelium and Subbasal Corneal Nerve Bundle Changes in Patients with Diabetic Retinopathy. Am. J. Ophthalmol. 2006, 142, 488–491. [Google Scholar] [CrossRef]
- Nitoda, E.; Kallinikos, P.; Pallikaris, A.; Moschandrea, J.; Amoiridis, G.; Ganotakis, E.S.; Tsilimbaris, M. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr. Eye Res. 2012, 37, 898–906. [Google Scholar] [CrossRef]
- Bitirgen, G.; Ozkagnici, A.; Malik, R.A.; Kerimoglu, H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with Type 2 diabetes mellitus. Diabet Med. 2014, 31, 431–438. [Google Scholar] [CrossRef]
- Hafner, J.; Zadrazil, M.; Grisold, A.; Ricken, G.; Krenn, M.; Kitzmantl, D.; Pollreisz, A.; Gleiss, A.; Schmidt-Erfurth, U. Retinal and Corneal Neurodegeneration and Their Association with Systemic Signs of Peripheral Neuropathy in Type 2 Diabetes. Am. J. Ophthalmol. 2020, 209, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Craig, J.P.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Ellyett, K.; Kilfoyle, D.; Braatvedt, G.D. In vivo confocal microscopy of corneal nerves: An ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5060–5065. [Google Scholar] [CrossRef]
- Zhivov, A.; Winter, K.; Hovakimyan, M.; Peschel, S.; Harder, V.; Schober, H.C.; Kundt, G.; Baltrusch, S.; Guthoff, R.F.; Stachs, O. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS ONE 2013, 8, e52157. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Zhivov, A.; Allgeier, S.; Winter, K.; Ziegler, I.; Brüggemann, J.; Strom, A.; Peschel, S.; Köhler, B.; et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014, 63, 2454–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, I.N.; Green, P.; Chan, A.W.S.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Efron, N.; Tavakoli, M.; Malik, R.A. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS ONE 2015, 10, e0123517. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Qi Wong, N.; Tan, H.; Yu Lin, M.; Yu Lee, I.; Mehta, J.; Liu, Y.C. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen. Res. 2022, 17, 2172–2178. [Google Scholar]
- Ando, A.; Miyamoto, M.; Saito, N.; Kotani, K.; Kamiya, H.; Ishibashi, S.; Tavakoli, M. Small Fibre Neuropathy Is Associated With Impaired Vascular Endothelial Function in Patients With Type 2 Diabetes. Front. Endocrinol. 2021, 12, 653277. [Google Scholar] [CrossRef]
- Ishida, N.; Rao, G.N.; Del Cerro, M.; Aquavella, J.V. Corneal Nerve Alterations in Diabetes Mellitus. Arch. Ophthalmol. 1984, 102, 1380–1384. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Issar, T.; Kwai, N.; Poynten, A.; Krishnan, A.V.; Willcox, M.; Markoulli, M. Association of corneal nerve loss with markers of axonal ion channel dysfunction in type 1 diabetes. Clin. Neurophysiol. 2020, 131, 145–154. [Google Scholar] [CrossRef]
- Yan, A.; Issar, T.; Tummanapalli, S.S.; Markoulli, M.; Kwai, N.C.G.; Poynten, A.M.; Krishnan, A.V. Relationship between Corneal Confocal Microscopy and Markers of Peripheral Nerve Structure and Function in Type 2 Diabetes. Diabet Med. 2020, 37, 326–334. [Google Scholar] [CrossRef]
- Nelson, D.; Mah, J.K.; Adams, C.; Hui, S.; Crawford, S.; Darwish, H.; Stephure, D.; Pacaud, D. Comparison of conventional and non-invasive techniques for the early identification of diabetic neuropathy in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2006, 7, 305–310. [Google Scholar] [CrossRef]
- Pacaud, D.; Romanchuk, K.G.; Tavakoli, M.; Gougeon, C.; Virtanen, H.; Ferdousi, M.; Nettel-Aguirre, A.; Mah, J.K.; Malik, R.A. The reliability and reproducibility of corneal confocal microscopy in children. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Szalai, E.; Deák, E.; Módis, L.; Németh, G.; Berta, A.; Nagy, A.; Felszeghy, E.; Káposzta, R.; Malik, R.A.; Csutak, A. Early Corneal Cellular and Nerve Fiber Pathology in Young Patients With Type 1 Diabetes Mellitus Identified Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deák, E.A.; Szalai, E.; Tóth, N.; Malik, R.A.; Berta, A.; Csutak, A. Longitudinal Changes in Corneal Cell and Nerve Fiber Morphology in Young Patients with Type 1 Diabetes with and without Diabetic Retinopathy: A 2-Year Follow-up Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, M.; Romanchuk, K.; Mah, J.K.; Virtanen, H.; Millar, C.; Malik, R.A.; Pacaud, D. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci. Rep. 2019, 9, 8758. [Google Scholar] [CrossRef]
- Gad, H.; Al-Jarrah, B.; Saraswathi, S.; Mohamed, S.; Kalteniece, A.; Petropoulos, I.N.; Khan, A.; Ponirakis, G.; Singh, P.; Khodor, S.A.; et al. Corneal confocal microscopy identifies a reduction in corneal keratocyte density and sub-basal nerves in children with type 1 diabetes mellitus. Br. J. Ophthalmol. 2021, in press. [CrossRef]
- Götze, A.; Von Keyserlingk, S.; Peschel, S.; Jacoby, U.; Schreiver, C.; Köhler, B.; Allgeier, S.; Winter, K.; Röhlig, M.; Jünemann, A.; et al. The corneal subbasal nerve plexus and thickness of the retinal layers in pediatric type 1 diabetes and matched controls. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef]
- Cozzini, T.; Piona, C.; Marchini, G.; Merz, T.; Brighenti, T.; Bonetto, J.; Marigliano, M.; Olivieri, F.; Maffeis, C.; Pedrotti, E. In vivo confocal microscopy study of corneal nerve alterations in children and youths with Type 1 diabetes. Pediatr. Diabetes. 2021, 22, 780–786. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, D.; Zhang, Y.; Zhang, S.; Wang, H.; Liu, Z.; Wang, H. Using corneal confocal microscopy to compare Mecobalamin intramuscular injections vs oral tablets in treating diabetic peripheral neuropathy: A RCT. Sci. Rep. 2021, 11, 14697. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Sipola, G.; Strom, A.; Herder, C.; Strassburger, K.; Knebel, B.; Reule, C.; Wollmann, J.C.; Icks, A.; Al-Hasani, H.; et al. BOND study: A randomised double-blind, placebo-controlled trial over 12 months to assess the effects of benfotiamine on morphometric, neurophysiological and clinical measuRes. in patients with type 2 diabetes with symptomatic polyneuropathy. BMJ Open 2022, 12, e057142. [Google Scholar] [CrossRef]
- Meijering, E.; Jacob, M.; Sarria, J.C.F.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; McGhee, C.N. Quantitative analysis of invivo confocal microscopy images: A review. Surv. Ophthalmol. 2013, 58, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, P.; Ahmed, S.; James, C.; Hodson, J.; McDonnell, P.J.; Rauz, S.; Williams, G.P. Neuron J. is a rapid and reliable open source tool for evaluating corneal nerve density in herpes simplex keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7312–7320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, A.; Scarpa, F.; Grisan, E. Analysis of corneal images for the recognition of nerve structures. In Proceedings of the 2006 Annual International Conference of the IEEE Engineering in Medicine and Biology, New York, NY, USA, 30 August–3 September 2006; pp. 4739–4742. [Google Scholar]
- Scarpa, F.; Grisan, E.; Ruggeri, A. Automatic recognition of corneal nerve structuRes. in images from confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4801–4807. [Google Scholar] [CrossRef]
- Efron, N.; Edwards, K.; Roper, N.; Pritchard, N.; Sampson, G.P.; Shahidi, A.M.; Vagenas, D.; Russell, A.; Graham, J.; Dabbah, M.A.; et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens 2010, 36, 245–248. [Google Scholar] [CrossRef]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.; Tavakoli, M.; Malik, R.A. Dual-Model Automatic Detection of Nerve-FibRes. In Corneal Confocal Microscopy Images. Med. Image Comput. Comput. Assist. Interv. 2010, 13, 300–307. [Google Scholar]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibRes. in corneal confocal microscopy imaging. Med. Image Anal. 2011, 15, 738–747. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. An Automatic Tool for Quantification of Nerve FibRes. in Corneal Confocal Microscopy Images. IEEE Trans. BioMed. Eng. 2017, 64, 786–794. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.M.; et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2062–2070. [Google Scholar] [CrossRef]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Russell, A.W.; Malik, R.A.; Efron, N. Fully automated, semiautomated, and manual morphometric analysis of corneal subbasal nerve plexus in individuals with and without diabetes. Cornea 2014, 33, 696–702. [Google Scholar] [CrossRef]
- McCarron, M.E.; Weinberg, R.L.; Izzi, J.M.; Queen, S.E.; Tarwater, P.M.; Misra, S.L.; Russakoff, D.B.; Oakley, J.D.; Mankowski, J.L. Combining In Vivo Corneal Confocal Microscopy with Deep Learning-based Analysis Reveals Sensory Nerve Fiber Loss in Acute SIV Infection. Cornea 2021, 40, 635. [Google Scholar] [CrossRef]
- Oakley, J.D.; Russakoff, D.B.; McCarron, M.E.; Weinberg, R.L.; Izzi, J.M.; Misra, S.L.; McGhee, C.N.; Mankowski, J.L. Deep learning-based analysis of macaque corneal sub-basal nerve fibers in confocal microscopy images. Eye Vis. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Salahouddin, T.; Petropoulos, I.N.; Ferdousi, M.; Ponirakis, G.; Asghar, O.; Alam, U.; Kamran, S.; Mahfoud, Z.R.; Efron, N.; Malik, R.A.; et al. Artificial Intelligence-Based Classification of Diabetic Peripheral Neuropathy From Corneal Confocal Microscopy Images. Diabetes Care 2021, 44, e151–e153. [Google Scholar] [CrossRef] [PubMed]
- Mehrgardt, P.; Zandavi, S.M.; Poon, S.K.; Kim, J.; Markoulli, M.; Khushi, M. U-Net Segmented Adjacent Angle Detection (USAAD) for Automatic Analysis of Corneal Nerve Structures. Data 2020, 5, 37. [Google Scholar] [CrossRef]
- Wei, S.; Shi, F.; Wang, Y.; Chou, Y.; Li, X. A Deep Learning Model for Automated Sub-Basal Corneal Nerve Segmentation and Evaluation Using In Vivo Confocal Microscopy. Transl. Vis. Sci. Technol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Fitzgerald, K.C.; Oakley, J.; Ponirakis, G.; Khan, A.; Gad, H.; George, P.; Deleu, D.; Canibano, B.G.; Akhtar, N.; et al. Corneal confocal microscopy demonstrates axonal loss in different courses of multiple sclerosis. Sci. Rep. 2021, 11, 21688. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Pereira, E.; Zheng, Y.; Su, P.; Xie, J.; Zhao, Y.; Shi, Y.; Qi, H.; Liu, J.; et al. Automated Tortuosity Analysis of Nerve Fibers in Corneal Confocal Microscopy. IEEE Trans. Med. Imaging 2020, 39, 2725–2737. [Google Scholar] [CrossRef]
- Scarpa, F.; Colonna, A.; Ruggeri, A. Multiple-Image Deep Learning Analysis for Neuropathy Detection in Corneal Nerve Images. Cornea 2020, 39, 342–347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. https://doi.org/10.3390/jcm11175130
Cosmo E, Midena G, Frizziero L, Bruno M, Cecere M, Midena E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. Journal of Clinical Medicine. 2022; 11(17):5130. https://doi.org/10.3390/jcm11175130
Chicago/Turabian StyleCosmo, Eleonora, Giulia Midena, Luisa Frizziero, Marisa Bruno, Michela Cecere, and Edoardo Midena. 2022. "Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review" Journal of Clinical Medicine 11, no. 17: 5130. https://doi.org/10.3390/jcm11175130
APA StyleCosmo, E., Midena, G., Frizziero, L., Bruno, M., Cecere, M., & Midena, E. (2022). Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. Journal of Clinical Medicine, 11(17), 5130. https://doi.org/10.3390/jcm11175130








