Evaluation of a New, Highly Flexible Radiofrequency Coil for MR Simulation of Patients Undergoing External Beam Radiation Therapy
Abstract
1. Introduction
2. Methods and Materials
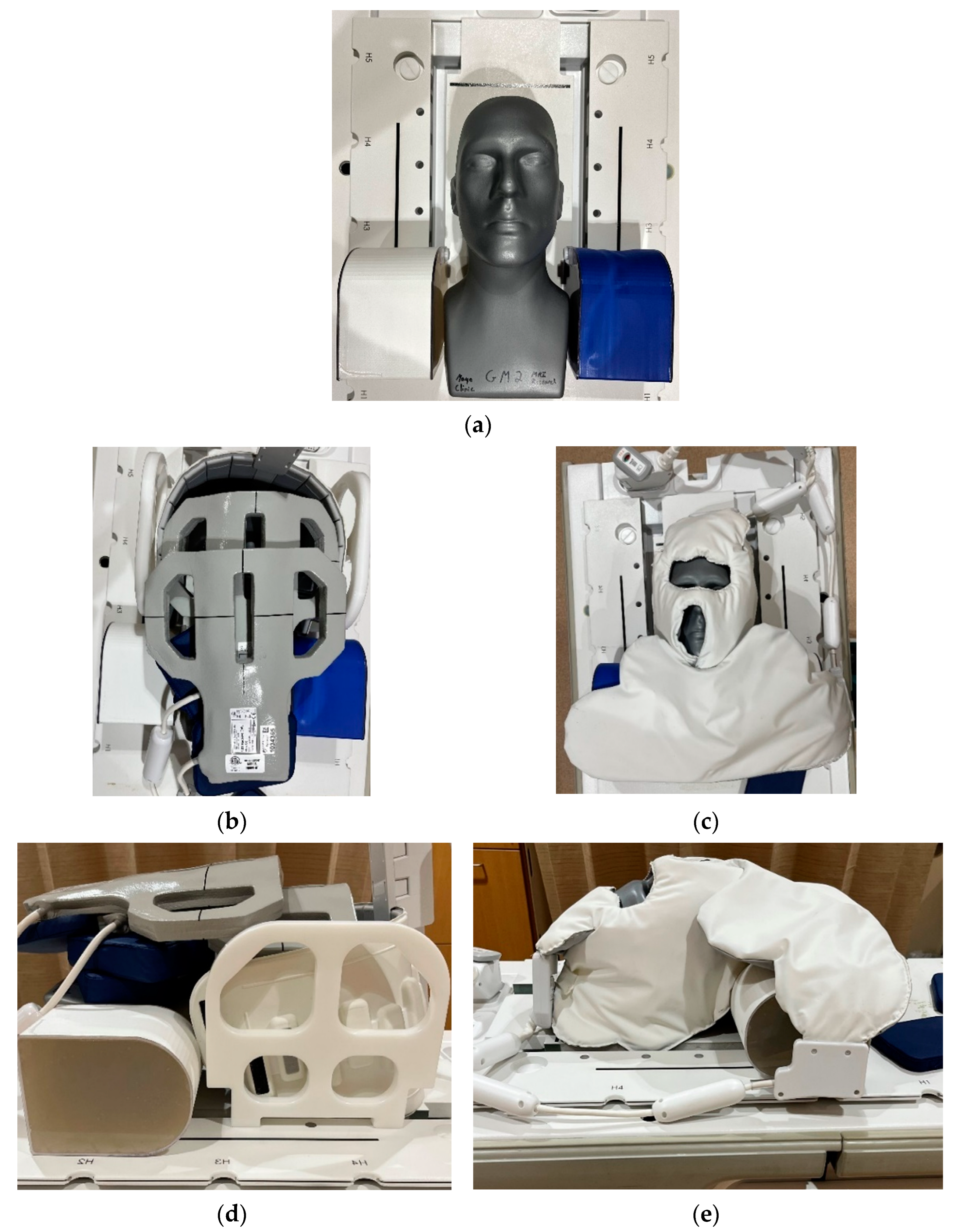
2.1. RF Coil Configurations
2.2. Phantom Data
2.3. In Vivo Data
2.4. Statistical Analysis
3. Results
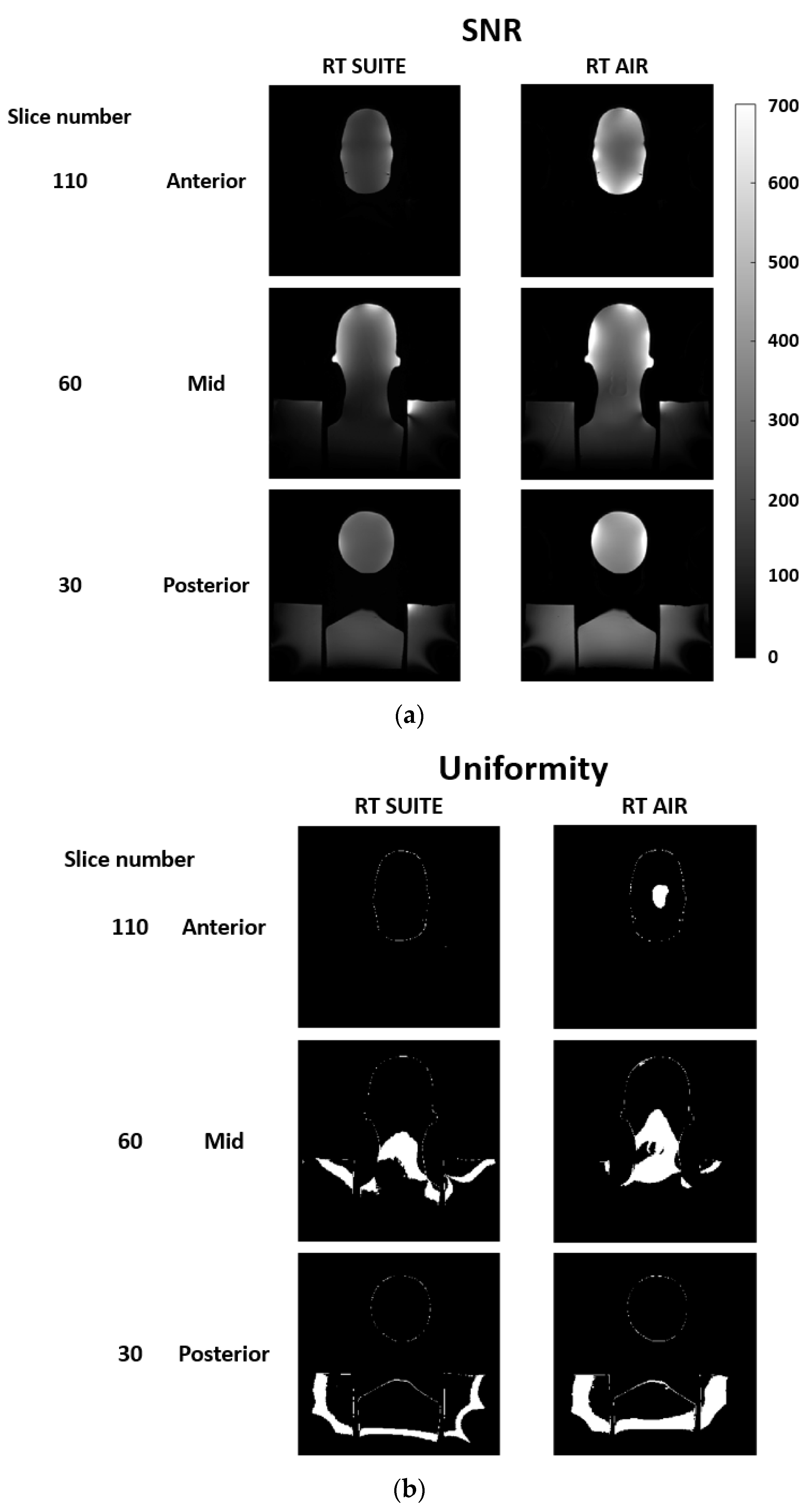
3.1. Phantom
3.2. In Vivo
3.3. In Vivo Illustrative Examples
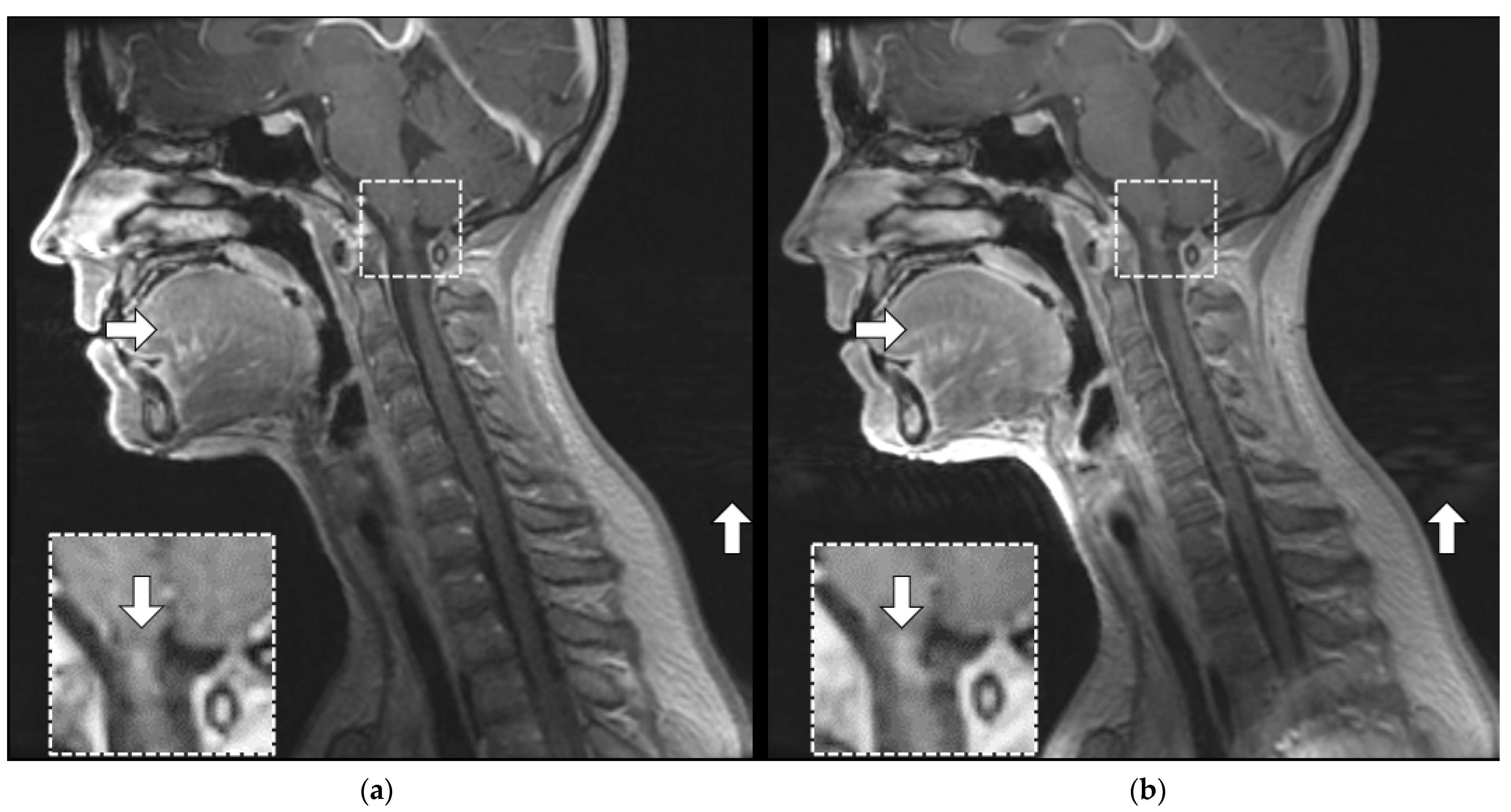
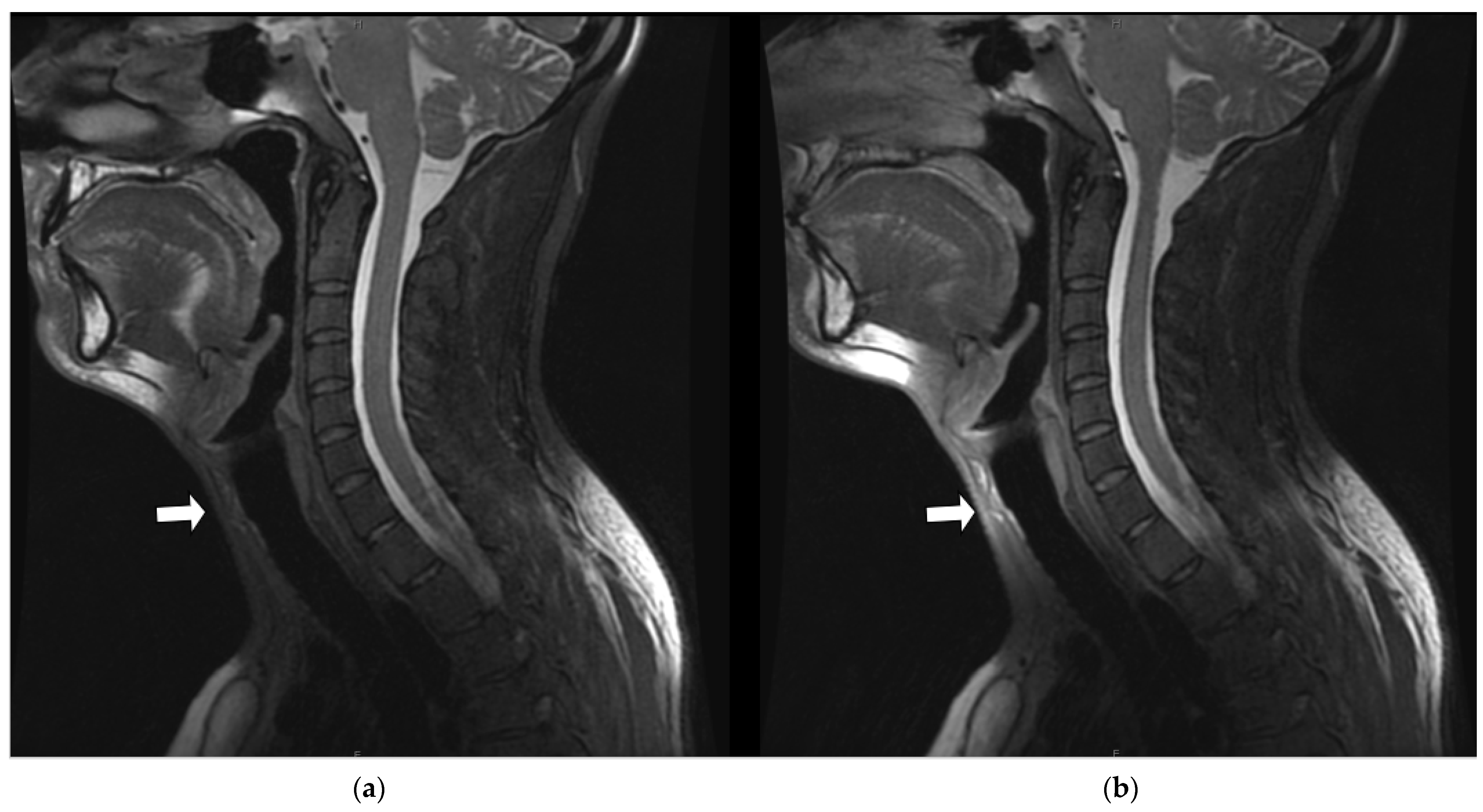
3.3.1. Margin Delineation
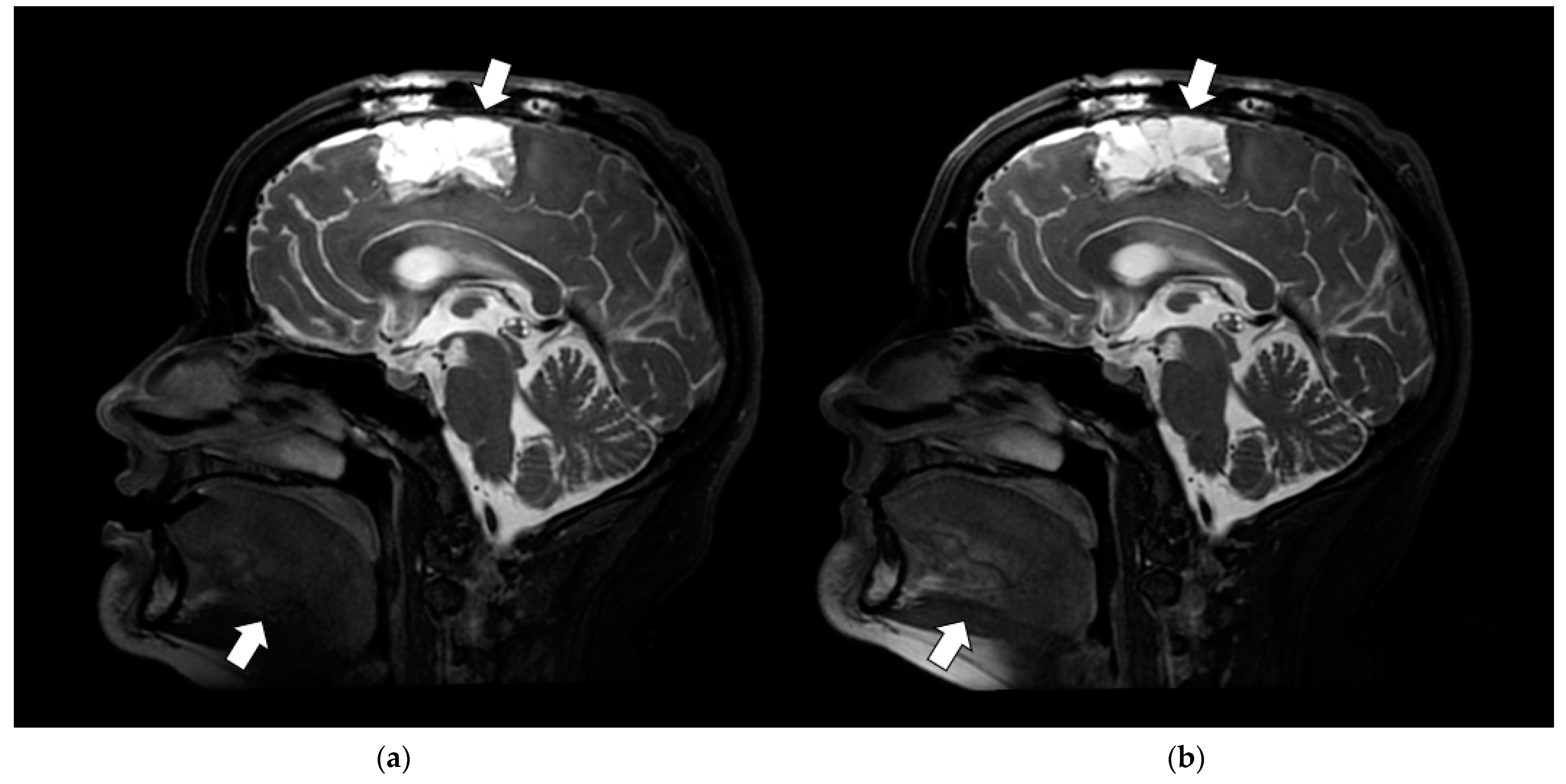
3.3.2. Artifact Enhancement
3.3.3. Coil Placement
3.3.4. SNR and Anatomic Conspicuity
3.3.5. Image Uniformity and Depiction of Fine Anatomic Detail
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| RT SUITE | RT AIR | ||
|---|---|---|---|
| Coil Component | RF Coil Elements | Coil Component | RF Coil Elements |
| RT Open Array | 8 | RT Open Array | 8 |
| License Plate | 16 | Face | 15 |
| Brain Flex | 6 (2 × 3 per paddle) | Anterior Array | 7 |
| Coil Configuration Name (abbreviation) | Number of Coil Elements | Coil Configuration Name (Abbreviation) | Number of Coil Elements |
| RT SUITE (RTS) | 30 | FACE_RT (AF) | 23 (15 Face + 4 RT Open Array) |
| FACE_AA_RT(AF_AA) | 26 (15 Face + 7 Anterior Array + 4 RT Open Array) | ||
| FACE_RT_AA_PA(AF_AA_PA) | 28 (15 Face + 7 Anterior Array + 4 RT Open Array + 2 MR Table) | ||
| Volume SNR Histogram | ||||
| Coil Type & Configuration | ||||
| p-Value | RTS | AF | AF_AA | AF_AA_PA |
| 0.0000 | 225.082 | 275.918 | ||
| 0.0005 | 228.156 | 272.844 | ||
| 0.1100 | 240.184 | 260.816 | ||
| 0.7608 | 252.466 | 248.534 | ||
| 0.0238 | 265.070 | 235.930 | ||
| 0.0475 | 263.274 | 237.726 | ||
| Per Slice SNR | ||||
| Coil Type & Configuration | ||||
| p-Value | RTS | AF | AF_AA | AF_AA_PA |
| 0.0000 | 98.244 | 214.756 | ||
| 0.0000 | 98.538 | 214.462 | ||
| 0.0000 | 99.603 | 213.397 | ||
| 0.4032 | 152.231 | 160.769 | ||
| 0.0006 | 173.994 | 139.006 | ||
| 0.0000 | 188.994 | 124.006 | ||
| Per Slice Uniformity | ||||
| Coil Type & Configuration | ||||
| p-Value | RTS | AF | AF_AA | AF_AA_PA |
| 0.0000 | 130.038 | 182.962 | ||
| 0.0000 | 105.186 | 207.814 | ||
| 0.0000 | 104.692 | 208.308 | ||
| 0.0000 | 111.295 | 201.705 | ||
| 0.0000 | 109.968 | 203.032 | ||
| 0.9880 | 156.577 | 156.423 | ||
| Anatomical Site | Global SNR Histogram | Per Slice SNR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Ranks | Mean Ranks | |||||||||||||||
| Subject ID | Pulse Sequence | Contrast Weighting | Fat Saturation (Y/N)? | Imaging Plane | Gad | p-Value | Coil Type & Configuration | p-Value | Coil Type & Configuration | |||||||
| RTS | AF | AF_AA | AF_AA_PA | RTS | AF | AF_AA | AF_AA_PA | |||||||||
| P1_C1 | Brain | CUBE | T1 | N | Sagittal | Y | 0.1753 | 241.75 | 259.25 | 0.0031 | 145.21 | 175.79 | ||||
| P2_C1 | Brain | CUBE | T1 | Y | Sagittal | Y | 0.0000 | 214.61 | 286.39 | 0.0000 | 94.87 | 218.13 | ||||
| P3_C1 | Brain | CUBE | T2 | Y | Sagittal | Y | 0.0043 | 268.97 | 232.03 | 0.0000 | 206.55 | 98.45 | ||||
| P4_C1 | Brain | CUBE | T1 | N | Sagittal | Y | 0.1327 | 260.21 | 240.79 | 0.3453 | 155.62 | 165.38 | ||||
| P4_C2 | Brain | CUBE | T2 | Y | Sagittal | Y | 0.0034 | 231.63 | 269.37 | 0.0671 | 151.03 | 169.97 | ||||
| P5_C1 | Base of Skull/C Spine | BRAVO | T1 | N | Sagittal | Y | 0.4283 | 255.62 | 245.38 | 0.0000 | 212.03 | 108.98 | ||||
| P5_C2 | Base of Skull/C Spine | CUBE | T1 | Y | Sagittal | Y | 0.0032 | 231.49 | 269.51 | 0.0018 | 140.57 | 172.43 | ||||
| P6_C1 | Brain | CUBE | T1 | N | Sagittal | Y | 0.7130 | 248.12 | 252.88 | 0.0000 | 182.24 | 130.76 | ||||
| P6_C2 | Brain | CUBE | T2 | Y | Sagittal | Y | 0.0809 | 261.78 | 239.22 | 0.0000 | 191.58 | 113.42 | ||||
| V1_C1 | Base of Skull/C Spine | CUBE | T2 | Y | Axial | N | 0.0000 | 172.52 | 328.48 | 0.0000 | 60.50 | 180.50 | ||||
| V2_C1 | Base of Skull/C Spine | CUBE | T2 | Y | Sagittal | N | 0.0137 | 234.64 | 266.36 | 0.0000 | 107.54 | 197.46 | ||||
| V2_C2 | Base of Skull/C Spine | CUBE | T1 | Y | Sagittal | N | 0.0000 | 284.87 | 216.13 | 0.0000 | 193.64 | 119.36 | ||||
| V2_C3 | Base of Skull/C Spine | CUBE | T2 | Y | Axial | N | 0.0001 | 275.85 | 225.15 | 0.0630 | 91.48 | 77.52 | ||||
| V3_C1 | Base of Skull/C Spine | CUBE | T2 | Y | Axial | N | 0.0000 | 178.47 | 322.53 | 0.0000 | 53.17 | 155.83 | ||||
| V3_C2 | Base of Skull/C Spine | CUBE | T1 | N | Axial | N | 0.0000 | 173.85 | 327.15 | 0.0000 | 59.49 | 149.51 | ||||
| V4_C1 | Base of Skull/C Spine | CUBE | T2 | Y | Sagittal | N | 0.4694 | 255.17 | 245.83 | 0.0030 | 137.55 | 167.45 | ||||
| V5_C1 | Base of Skull/C Spine | CUBE | T1 | Y | Sagittal | N | 0.7348 | 248.31 | 252.69 | 0.0000 | 229.86 | 83.14 | ||||
| V5_C2 | Base of Skull/C Spine | CUBE | T2 | Y | Sagittal | N | 0.0000 | 210.94 | 290.06 | 0.0000 | 118.92 | 170.08 | ||||
| Subject Identifier | Site | Post Gad (Y/N) | Sequence | Contrast Weighting | Fat Saturation (Y/N) | Imaging Plane | Coil Config Shorthand | FOV (mm) | Slice (mm) | Frequency (Kx) | Phase (Ky) | Slice (Kz) | TR (msec) | TE (msec) | Signal Averages | BW (Hz/pixel) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1_C1 | Brain | Y | CUBE | T1 | N | Sagittal | AF | 240 | 1.20 | 256 | 256 | 160 | 652.00 | 13.25 | 1 | 488.28 |
| Y | CUBE | T1 | N | Sagittal | RTS | 240 | 1.20 | 256 | 256 | 160 | 652.00 | 13.25 | 1 | 488.28 | ||
| P2_C1 | Brain | Y | CUBE | T1 | Y | Sagittal | AF_AA_PA | 240 | 1.20 | 256 | 256 | 156 | 652.00 | 13.25 | 1 | 488.28 |
| Y | CUBE | T1 | Y | Sagittal | RTS | 240 | 1.20 | 256 | 256 | 156 | 652.00 | 13.25 | 1 | 488.28 | ||
| P3_C1 | Brain | Y | CUBE | T2 | Y | Sagittal | AF | 240 | 1.20 | 288 | 288 | 152 | 1552.00 | 63.97 | 2 | 390.63 |
| Y | CUBE | T2 | Y | Sagittal | RTS | 240 | 1.20 | 288 | 288 | 152 | 1552.00 | 63.97 | 2 | 390.63 | ||
| P4_C2 | Brain | Y | CUBE | T2 | Y | Sagittal | AF | 240 | 1.20 | 288 | 288 | 160 | 1552.00 | 67.06 | 2 | 390.63 |
| Y | CUBE | T2 | Y | Sagittal | RTS | 240 | 1.20 | 288 | 288 | 160 | 1552.00 | 67.06 | 2 | 390.63 | ||
| P4_C1 | Y | CUBE | T1 | N | Sagittal | AF | 240 | 1.20 | 256 | 256 | 160 | 652.00 | 12.98 | 1 | 488.28 | |
| Y | CUBE | T1 | N | Sagittal | RTS | 240 | 1.20 | 256 | 256 | 160 | 652.00 | 12.96 | 1 | 488.28 | ||
| P5_C1 | Base of Skull/C Spine | Y | BRAVO | T1 | N | Sagittal | AF_AA_PA | 260 | 1.20 | 256 | 256 | 160 | 5.88 | 2.47 | 2 | 325.55 |
| Y | BRAVO | T1 | N | Sagittal | RTS | 260 | 1.20 | 256 | 256 | 160 | 5.88 | 2.47 | 2 | 325.55 | ||
| P5_C2 | Y | CUBE | T1 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 12.65 | 1 | 488.28 | |
| Y | CUBE | T1 | Y | Sagittal | RTS | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 12.67 | 1 | 488.28 | ||
| P6_C1 | Brain | Y | CUBE | T1 | N | Sagittal | AF | 240 | 1.20 | 256 | 256 | 156 | 652.00 | 12.84 | 1 | 488.28 |
| Y | CUBE | T1 | N | Sagittal | RTS | 240 | 1.20 | 256 | 256 | 156 | 652.00 | 12.84 | 1 | 488.28 | ||
| P6_C2 | Y | CUBE | T2 | Y | Sagittal | AF | 240 | 1.20 | 288 | 288 | 152 | 1552.00 | 68.86 | 2 | 390.63 | |
| Y | CUBE | T2 | Y | Sagittal | RTS | 240 | 1.20 | 288 | 288 | 152 | 1552.00 | 68.86 | 2 | 390.63 | ||
| V1_C1 | Base of Skull/C Spine | N | CUBE | T2 | Y | Axial | AF_AA_PA | 260 | 1.40 | 288 | 288 | 120 | 1552.00 | 63.70 | 2 | 390.63 |
| N | CUBE | T2 | Y | Axial | RTS | 260 | 1.40 | 288 | 288 | 120 | 1552.00 | 63.70 | 2 | 390.63 | ||
| V2_C1 | Base of Skull/C Spine | N | CUBE | T2 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 288 | 288 | 152 | 1552.00 | 68.41 | 2 | 390.63 |
| N | CUBE | T2 | Y | Sagittal | RTS | 260 | 1.20 | 288 | 288 | 152 | 1552.00 | 68.50 | 2 | 390.63 | ||
| V2_C2 | N | CUBE | T1 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 14.15 | 1 | 488.28 | |
| N | CUBE | T1 | Y | Sagittal | RTS | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 14.29 | 1 | 488.28 | ||
| V2_C3 | N | CUBE | T2 | Y | Axial | AF_AA | 260 | 2.00 | 320 | 300 | 84 | 2202.00 | 192.76 | 2 | 244.14 | |
| N | CUBE | T2 | Y | Axial | RTS | 260 | 2.00 | 320 | 300 | 84 | 2202.00 | 192.18 | 2 | 244.14 | ||
| V3_C1 | Base of Skull/C Spine | N | CUBE | T2 | Y | Axial | AF_AA_PA | 260 | 2.00 | 320 | 300 | 104 | 2202.00 | 179.98 | 2 | 244.14 |
| N | CUBE | T2 | Y | Axial | RTS | 260 | 2.00 | 320 | 300 | 104 | 2202.00 | 179.98 | 2 | 244.14 | ||
| V3_C2 | N | CUBE | T1 | N | Axial | AF_AA_PA | 260 | 2.00 | 320 | 300 | 104 | 652.00 | 17.50 | 2 | 244.14 | |
| N | CUBE | T1 | N | Axial | 260 | 2.00 | 320 | 300 | 104 | 652.00 | 17.50 | 2 | 244.14 | |||
| V4_C1 | Base of Skull/C Spine | N | CUBE | T2 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 288 | 288 | 152 | 1552.00 | 70.66 | 2 | 390.63 |
| N | CUBE | T2 | Y | Sagittal | RTS | 260 | 1.20 | 288 | 288 | 152 | 1552.00 | 70.66 | 2 | 390.63 | ||
| V5_C1 | Base of Skull/C Spine | N | CUBE | T1 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 14.03 | 1 | 488.28 |
| N | CUBE | T1 | Y | Sagittal | RTS | 260 | 1.20 | 256 | 256 | 156 | 652.00 | 14.03 | 1 | 488.28 | ||
| V5_C2 | N | CUBE | T2 | Y | Sagittal | AF_AA_PA | 260 | 1.20 | 288 | 288 | 144 | 1552.00 | 66.75 | 2 | 390.63 | |
| N | CUBE | T2 | Y | Sagittal | RTS | 260 | 1.20 | 288 | 288 | 144 | 1552.00 | 66.75 | 2 | 390.63 |
References
- Devic, S. MRI simulation for radiotherapy treatment planning. Med. Phys. 2012, 39, 6701–6711. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Karlsson, M.G.; Nyholm, T.; Amies, C.; Zackrisson, B. Dedicated Magnetic Resonance Imaging in the Radiotherapy Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Lee, P. MRI-guided radiotherapy: Opening our eyes to the future. Integr. Cancer Sci. Ther. 2016, 3, 420–427. [Google Scholar] [CrossRef]
- Metcalfe, P.; Liney, G.P.; Holloway, L.; Walker, A.; Barton, M.; Delaney, G.P.; Vinod, S.; Tome, W. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol. Cancer Res. Treat. 2013, 12, 429–446. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Payne, G.S. Radiotherapy planning using MRI. Phys. Med. Biol. 2015, 60, R323. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Dasgupta, A.; Chatterjee, A.; Baheti, A.; Engineer, R.; Gupta, T.; Murthy, V. The Promise of Magnetic Resonance Imaging in Radiation Oncology Practice in the Management of Brain, Prostate, and GI Malignancies. JCO Glob. Oncol. 2022, 8, e2100366. [Google Scholar] [CrossRef]
- Beaton, L.; Bandula, S.; Gaze, M.N.; Sharma, R.A. How rapid advances in imaging are defining the future of precision radiation oncology. Br. J. Cancer 2019, 120, 779–790. [Google Scholar] [CrossRef]
- Dirix, P.; Haustermans, K.; Vandecaveye, V. The Value of Magnetic Resonance Imaging for Radiotherapy Planning. Semin. Radiat. Oncol. 2014, 24, 151–159. [Google Scholar] [CrossRef]
- Wiesinger, F.; Bylund, M.; Yang, J.; Kaushik, S.; Shanbhag, D.; Ahn, S.; Jonsson, J.H.; Lundman, J.A.; Hope, T.; Nyholm, T.; et al. Zero TE-based pseudo-CT image conversion in the head and its application in PET/MR attenuation correction and MR-guided radiation therapy planning. Magn. Reson. Med. 2018, 80, 1440–1451. [Google Scholar] [CrossRef]
- Boydev, C.; Demol, B.; Pasquier, D.; Saint-Jalmes, H.; Delpon, G.; Reynaert, N. Zero echo time MRI-only treatment planning for radiation therapy of brain tumors after resection. Phys. Med. 2017, 42, 332–338. [Google Scholar] [CrossRef]
- Dowling, J.A.; Lambert, J.; Parker, J.; Salvado, O.; Fripp, J.; Capp, A.; Wratten, C.; Denham, J.W.; Greer, P.B. An Atlas-Based Electron Density Mapping Method for Magnetic Resonance Imaging (MRI)-Alone Treatment Planning and Adaptive MRI-Based Prostate Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e5–e11. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Steinke, F.; Scheel, V.; Charpiat, G.; Farquhar, J.; Aschoff, P.; Brady, M.; Schölkopf, B.; Pichler, B.J. MRI-Based Attenuation Correction for PET/MRI: A Novel Approach Combining Pattern Recognition and Atlas Registration. J. Nucl. Med. 2008, 49, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Demol, B.; Boydev, C.; Korhonen, J.; Reynaert, N. Dosimetric characterization of MRI-only treatment planning for brain tumors in atlas-based pseudo-CT images generated from standard T1-weighted MR images. Med. Phys. 2016, 43, 6557. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, E.; Wyatt, J.J.; Henry, A.M.; Short, S.C.; Sebag-Montefiore, D.; Murray, L.; Kelly, C.G.; McCallum, H.M.; Speight, R. Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging–Only Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Spadea, M.F.; Maspero, M.; Zaffino, P.; Seco, J. Deep learning based synthetic-CT generation in radiotherapy and PET: A review. Med. Phys. 2021, 48, 6537–6566. [Google Scholar] [CrossRef] [PubMed]
- Glide-Hurst, C.K.; Paulson, E.S.; McGee, K.; Tyagi, N.; Hu, Y.; Balter, J.; Bayouth, J. Task group 284 report: Magnetic resonance imaging simulation in radiotherapy: Considerations for clinical implementation, optimization, and quality assurance. Med. Phys. 2021, 48, e636–e670. [Google Scholar] [CrossRef] [PubMed]
- Rossman, P.; Stormont, R.; Lindsay, S.; Robb, F.; Savitskij, D.; Stanley, D.; Huston, J.; Kaufmann, T.; McGee, K.P. Characterization of a new ultra-flexible, low profile RF receive coil technology. In Proceedings of the International Society of Magnetic Resonance in Medicine 25th Annual Meeting & Exhibition, Honolulu, HI, USA, 22–27 April 2017; p. 763. [Google Scholar]
- Gruber, B.; Froeling, M.; Leiner, T.; Klomp, D.W.J. RF coils: A practical guide for nonphysicists. J. Magn. Reson. Imaging 2018, 48, 590–604. [Google Scholar] [CrossRef]
- McGee, K.P.; Stormont, R.S.; Lindsay, S.A.; Taracila, V.; Savitskij, D.; Robb, F.; Witte, R.J.; Kaufmann, T.J.; Huston, I.J.; Riederer, S.J.; et al. Characterization and evaluation of a flexible MRI receive coil array for radiation therapy MR treatment planning using highly decoupled RF circuits. Phys. Med. Biol. 2018, 63, 08NT02. [Google Scholar] [CrossRef]
- Bernstein, M.A.; King, K.F.; Zhou, X.J. Handbook of MRI Pulse Sequences; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- National Electrical Manufacturers Association. Determination of Signal-to-Noise and Image Uniformity for Single-Channel Non-Volume Coils in Diagnostic MR Imaging; National Electrical Manufacturers Association: Rosslyn, Virginia, 2008; Volume NEMA MS 6-2008 (R2014, R2020). [Google Scholar]
- National Electrical Manufacturers Association. Characterization of Phased Array Coils for Diagnostic Magnetic Resonance Images; National Electrical Manufacturers Association: Rosslyn, Virginia, 2008; Volume NEMA MS 9-2008 (R2014, R2020). [Google Scholar]
- Huang, S.Y.; Seethamraju, R.T.; Patel, P.; Hahn, P.F.; Kirsch, J.E.; Guimaraes, A.R. Body MR Imaging: Artifacts, k-Space, and Solutions. Radiographics 2015, 35, 1439–1460. [Google Scholar] [CrossRef]
- Zhuo, J.; Gullapalli, R.P. MR Artifacts, Safety, and Quality Control. RadioGraphics 2006, 26, 275–297. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Trzasko, J.D.; Gray, E.M.; Campeau, N.G.; Rossman, P.J.; Kang, D.; Robb, F.; Stormont, R.S.; Lindsay, S.A.; Bernstein, M.A.; et al. Application of Adaptive Image Receive Coil Technology for Whole-Brain Imaging. AJR Am. J. Roentgenol. 2021, 216, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Onishi, H.; Nakamoto, A.; Wakayama, T.; Ota, T.; Tsuboyama, T.; Yano, K.; Tarewaki, H.; Koyama, Y.; Tatsumi, M.; et al. Impact of adaptive image receive coil technology for liver MR imaging at 3.0 Tesla: Intraindividual comparison with use of conventional coil. Eur. J. Radiol. 2022, 150, 110271. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jeon, K.N.; Hwang, M.J.; Jung, Y.; Lee, J. Application of Highly Flexible Adaptive Image Receive Coil for Lung MR Imaging Using Zero TE Sequence: Comparison with Conventional Anterior Array Coil. Diagnostics 2022, 12, 148. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGee, K.P.; Campeau, N.G.; Witte, R.J.; Rossman, P.J.; Christopherson, J.A.; Tryggestad, E.J.; Brinkmann, D.H.; Ma, D.J.; Park, S.S.; Rettmann, D.W.; et al. Evaluation of a New, Highly Flexible Radiofrequency Coil for MR Simulation of Patients Undergoing External Beam Radiation Therapy. J. Clin. Med. 2022, 11, 5984. https://doi.org/10.3390/jcm11205984
McGee KP, Campeau NG, Witte RJ, Rossman PJ, Christopherson JA, Tryggestad EJ, Brinkmann DH, Ma DJ, Park SS, Rettmann DW, et al. Evaluation of a New, Highly Flexible Radiofrequency Coil for MR Simulation of Patients Undergoing External Beam Radiation Therapy. Journal of Clinical Medicine. 2022; 11(20):5984. https://doi.org/10.3390/jcm11205984
Chicago/Turabian StyleMcGee, Kiaran P., Norbert G. Campeau, Robert J. Witte, Philip J. Rossman, Jackie A. Christopherson, Erik J. Tryggestad, Debra H. Brinkmann, Daniel J. Ma, Sean S. Park, Dan W. Rettmann, and et al. 2022. "Evaluation of a New, Highly Flexible Radiofrequency Coil for MR Simulation of Patients Undergoing External Beam Radiation Therapy" Journal of Clinical Medicine 11, no. 20: 5984. https://doi.org/10.3390/jcm11205984
APA StyleMcGee, K. P., Campeau, N. G., Witte, R. J., Rossman, P. J., Christopherson, J. A., Tryggestad, E. J., Brinkmann, D. H., Ma, D. J., Park, S. S., Rettmann, D. W., & Robb, F. J. (2022). Evaluation of a New, Highly Flexible Radiofrequency Coil for MR Simulation of Patients Undergoing External Beam Radiation Therapy. Journal of Clinical Medicine, 11(20), 5984. https://doi.org/10.3390/jcm11205984






