Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy
Abstract
1. Introduction
2. Role of Radiotherapy in GBM
3. Role of Systemic Therapy
4. Molecular Basis of GBM
5. Types of Radiotherapy Devices for GBM
6. Imaging Characteristics of GBM on MRI and PET
7. Radiomic and Radiogenomic Differentiation of Molecular Markers, Sex Differences, and Morphologic Subtypes of GBMs
8. Progression of Disease versus Treatment Effect
9. MRI Guided Machines
10. Role of MRI Linac in Assessing Tumor Response during Treatment
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.H.; Pugh, S.L.; McElroy, J.P.; Gilbert, M.R.; Mehta, M.; Klimowicz, A.C.; Magliocco, A.; Bredel, M.; Robe, P.; Grosu, A.L.; et al. Molecular-Based Recursive Partitioning Analysis Model for Glioblastoma in the Temozolomide Era: A Correlative Analysis Based on NRG Oncology RTOG 0525. JAMA Oncol. 2017, 3, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Nie, S.; Zhu, Y.; Yang, J.; Xin, T.; Xue, S.; Sun, J.; Mu, D.; Chen, Z.; Sun, P.; Yu, J.; et al. Clinicopathologic analysis of microscopic tumor extension in glioma for external beam radiotherapy planning. BMC Med. 2021, 19, 269. [Google Scholar] [CrossRef]
- Wick, W.; Weller, M.; van den Bent, M.; Sanson, M.; Weiler, M.; von Deimling, A.; Plass, C.; Hegi, M.; Platten, M.; Reifenberger, G. MGMT testing—The challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Ruzevick, J.; Zlomanczuk, P.; Lukas, R.V. Tumor Treating Fields in Neuro-Oncological Practice. Curr. Oncol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Hottinger, A.F.; Pacheco, P.; Stupp, R. Tumor treating fields: A novel treatment modality and its use in brain tumors. Neuro Oncol. 2016, 18, 1338–1349. [Google Scholar] [CrossRef]
- Walker, M.D.; Strike, T.A.; Sheline, G.E. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int. J. Radiat. Onco.l Biol. Phys. 1979, 5, 1725–1731. [Google Scholar] [CrossRef]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; Leventhal, C.M.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of mithramycin in the treatment of anaplastic gliomas. J. Neurosurg. 1976, 44, 655–667. [Google Scholar] [CrossRef]
- Walker, M.D.; Strike, T.A. Evaluation of methyl-CCNU, BCNU, and radiotherapy in the treatment of malignant glioma (abstr.). Proc. Am. Assoc. Cancer Res. 1976, 17, 652. [Google Scholar]
- Nelson, D.F.; Diener-West, M.; Horton, J.; Chang, C.H.; Schoenfeld, D.; Nelson, J.S. Combined modality approach to treatment of malignant gliomas—Re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988, 6, 279–284. [Google Scholar]
- Murray, K.J.; Nelson, D.F.; Scott, C.; Fischbach, A.J.; Porter, A.; Farnan, N.; Curran, W.J., Jr. Quality-adjusted survival analysis of malignant glioma. Patients treated with twice-daily radiation (RT) and carmustine: A report of Radiation Therapy Oncology Group (RTOG) 83-02. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 453–459. [Google Scholar] [CrossRef]
- Ali, A.N.; Zhang, P.; Yung, W.K.A.; Chen, Y.; Movsas, B.; Urtasun, R.C.; Jones, C.U.; Choi, K.N.; Michalski, J.M.; Fischbach, A.J.; et al. NRG oncology RTOG 9006: A phase III randomized trial of hyperfractionated radiotherapy (RT) and BCNU versus standard RT and BCNU for malignant glioma patients. J. Neurooncol. 2018, 137, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.I.; Brown, D.; Normolle, D.; Schipper, M.; Piert, M.; Junck, L.; Heth, J.; Gomez-Hassan, D.; Ten Haken, R.K.; Chenevert, T.; et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin. Cancer Res. 2012, 18, 273–279. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.; Tsien, C.; Chenevert, T.; Gilbert, M.; Omuro, A.; McDonough, J.; Aldape, K.; Srinivasan, A.; Rogers, C.L.; et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S22–S23. [Google Scholar] [CrossRef]
- Aydin, H.; Sillenberg, I.; von Lieven, H. Patterns of failure following CT-based 3-D irradiation for malignant glioma. Strahlenther Onkol. 2001, 177, 424–431. [Google Scholar] [CrossRef]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409. [Google Scholar] [CrossRef]
- Kruser, T.J.; Bosch, W.R.; Badiyan, S.N.; Bovi, J.A.; Ghia, A.J.; Kim, M.M.; Solanki, A.A.; Sachdev, S.; Tsien, C.; Wang, T.J.C.; et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J. Neurooncol. 2019, 143, 157–166. [Google Scholar] [CrossRef]
- Fine, H.A.; Dear, K.B.; Loeffler, J.S.; Black, P.M.; Canellos, G.P. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 1993, 71, 2585–2597. [Google Scholar] [CrossRef]
- Stewart, L.A. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002, 359, 1011–1018. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef]
- Weller, M.; Tabatabai, G.; Kastner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma—An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Labussiere, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef]
- Network, C.G.A.R. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e46. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Roy, S.; Lahiri, D.; Maji, T.; Biswas, J. Recurrent Glioblastoma: Where we stand. South Asian J. Cancer 2015, 4, 163–173. [Google Scholar] [CrossRef]
- Loeffler, J.S.; Alexander, E.; Hochberg, F.H.; Wen, P.Y.; Morris, J.H.; Schoene, W.C.; Siddon, R.L.; Morse, R.H.; Black, P.M. Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1455–1462. [Google Scholar] [CrossRef]
- Johnson, B.E.; Mazor, T.; Hong, C.; Barnes, M.; Aihara, K.; McLean, C.Y.; Fouse, S.D.; Yamamoto, S.; Ueda, H.; Tatsuno, K.; et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014, 343, 189–193. [Google Scholar] [CrossRef]
- Giordano, F.A.; Brehmer, S.; Murle, B.; Welzel, G.; Sperk, E.; Keller, A.; Abo-Madyan, Y.; Scherzinger, E.; Clausen, S.; Schneider, F.; et al. Intraoperative Radiotherapy in Newly Diagnosed Glioblastoma (INTRAGO): An Open-Label, Dose-Escalation Phase I/II Trial. Neurosurgery 2019, 84, 41–49. [Google Scholar] [CrossRef]
- Sarria, G.R.; Sperk, E.; Han, X.; Sarria, G.J.; Wenz, F.; Brehmer, S.; Fu, B.; Min, S.; Zhang, H.; Qin, S.; et al. Intraoperative radiotherapy for glioblastoma: An international pooled analysis. Radiother. Oncol. 2020, 142, 162–167. [Google Scholar] [CrossRef]
- Gessler, D.J.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Özütemiz, C.; Gencturk, M.; et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neurooncol. Adv. 2022, 4, vdab185. [Google Scholar] [CrossRef]
- Guckenberger, M.; Klement, R.J.; Allgauer, M.; Appold, S.; Dieckmann, K.; Ernst, I.; Ganswindt, U.; Holy, R.; Nestle, U.; Nevinny-Stickel, M.; et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother. Oncol. 2013, 109, 13–20. [Google Scholar] [CrossRef]
- Song, C.W.; Kim, M.S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578. [Google Scholar] [CrossRef]
- Soliman, H.; Ruschin, M.; Angelov, L.; Brown, P.D.; Chiang, V.L.S.; Kirkpatrick, J.P.; Lo, S.S.; Mahajan, A.; Oh, K.S.; Sheehan, J.P.; et al. Consensus Contouring Guidelines for Postoperative Completely Resected Cavity Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 436–442. [Google Scholar] [CrossRef]
- Souhami, L.; Seiferheld, W.; Brachman, D.; Podgorsak, E.B.; Werner-Wasik, M.; Lustig, R.; Schultz, C.J.; Sause, W.; Okunieff, P.; Buckner, J.; et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 853–860. [Google Scholar] [CrossRef]
- Redmond, K.J.; Mehta, M. Stereotactic Radiosurgery for Glioblastoma. Cureus 2015, 7, e413. [Google Scholar] [CrossRef]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef]
- Brown, P.D.; Chung, C.; Liu, D.D.; McAvoy, S.; Grosshans, D.; Al Feghali, K.; Mahajan, A.; Li, J.; McGovern, S.L.; McAleer, M.F.; et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. 2021, 23, 1337–1347. [Google Scholar] [CrossRef]
- Moreau, A.; Febvey, O.; Mognetti, T.; Frappaz, D.; Kryza, D. Contribution of Different Positron Emission Tomography Tracers in Glioma Management: Focus on Glioblastoma. Front. Oncol. 2019, 9, 1134. [Google Scholar] [CrossRef]
- Holzgreve, A.; Albert, N.L.; Galldiks, N.; Suchorska, B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers 2021, 13, 2093. [Google Scholar] [CrossRef]
- van Dijken, B.R.J.; Ankrah, A.O.; Stormezand, G.N.; Dierckx, R.A.J.O.; Jan van Laar, P.; van der Hoorn, A. Prognostic value of 11C-methionine volume-based PET parameters in IDH wild type glioblastoma. PLoS ONE 2022, 17, e0264387. [Google Scholar] [CrossRef]
- Snyder, J.M.; Huang, R.Y.; Bai, H.; Rao, V.R.; Cornes, S.; Barnholtz-Sloan, J.S.; Gutman, D.; Fasano, R.; Van Meir, E.G.; Brat, D.; et al. Analysis of morphological characteristics of IDH-mutant/wildtype brain tumors using whole-lesion phenotype analysis. Neurooncol. Adv. 2021, 3, vdab088. [Google Scholar] [CrossRef]
- Bangalore Yogananda, C.G.; Shah, B.R.; Vejdani-Jahromi, M.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; et al. A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 2020, 22, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yogananda, C.G.B.; Shah, B.R.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; Fei, B.; et al. MRI-Based Deep-Learning Method for Determining Glioma. AJNR Am. J. Neuroradiol. 2021, 42, 845–852. [Google Scholar] [CrossRef]
- Akbari, H.; Bakas, S.; Pisapia, J.M.; Nasrallah, M.P.; Rozycki, M.; Martinez-Lage, M.; Morrissette, J.J.D.; Dahmane, N.; O’Rourke, D.M.; Davatzikos, C. In vivo evaluation of EGFRvIII mutation in primary glioblastoma patients via complex multiparametric MRI signature. Neuro Oncol. 2018, 20, 1068–1079. [Google Scholar] [CrossRef]
- Ishi, Y.; Yamaguchi, S.; Okamoto, M.; Sawaya, R.; Endo, S.; Motegi, H.; Terasaka, S.; Tanei, Z.I.; Hatanaka, K.C.; Tanaka, S.; et al. Clinical and radiological findings of glioblastomas harboring a BRAF V600E mutation. Brain Tumor Pathol. 2022, 39, 162–170. [Google Scholar] [CrossRef]
- Natsumeda, M.; Chang, M.; Gabdulkhaev, R.; Takahashi, H.; Tsukamoto, Y.; Kanemaru, Y.; Okada, M.; Oishi, M.; Okamoto, K.; Rodriguez, F.J.; et al. Predicting BRAF V600E mutation in glioblastoma: Utility of radiographic features. Brain Tumor Pathol. 2021, 38, 228–233. [Google Scholar] [CrossRef]
- Beig, N.; Singh, S.; Bera, K.; Prasanna, P.; Singh, G.; Chen, J.; Saeed Bamashmos, A.; Barnett, A.; Hunter, K.; Statsevych, V.; et al. Sexually dimorphic radiogenomic models identify distinct imaging and biological pathways that are prognostic of overall survival in glioblastoma. Neuro Oncol. 2021, 23, 251–263. [Google Scholar] [CrossRef]
- Choi, S.W.; Cho, H.H.; Koo, H.; Cho, K.R.; Nenning, K.H.; Langs, G.; Furtner, J.; Baumann, B.; Woehrer, A.; Cho, H.J.; et al. Multi-Habitat Radiomics Unravels Distinct Phenotypic Subtypes of Glioblastoma with Clinical and Genomic Significance. Cancers 2020, 12, 1707. [Google Scholar] [CrossRef]
- Gevaert, O.; Mitchell, L.A.; Achrol, A.S.; Xu, J.; Echegaray, S.; Steinberg, G.K.; Cheshier, S.H.; Napel, S.; Zaharchuk, G.; Plevritis, S.K. Glioblastoma multiforme: Exploratory radiogenomic analysis by using quantitative image features. Radiology 2014, 273, 168–174. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Jost, S.; Aghi, M.K.; Heimberger, A.B.; Sampson, J.H.; Wen, P.Y.; Macdonald, D.R.; Van den Bent, M.J.; Chang, S.M. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 2012, 70, 234–243, discussion in 243–234. [Google Scholar] [CrossRef]
- Delgado-Lopez, P.D.; Rinones-Mena, E.; Corrales-Garcia, E.M. Treatment-related changes in glioblastoma: A review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin. Transl. Oncol. 2018, 20, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Le Fèvre, C.; Lhermitte, B.; Ahle, G.; Chambrelant, I.; Cebula, H.; Antoni, D.; Keller, A.; Schott, R.; Thiery, A.; Constans, J.M.; et al. Pseudoprogression versus true progression in glioblastoma patients: A multiapproach literature review: Part 1—Molecular, morphological and clinical features. Crit. Rev. Oncol. Hematol. 2021, 157, 103188. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Melguizo-Gavilanes, I.; Bruner, J.M.; Guha-Thakurta, N.; Hess, K.R.; Puduvalli, V.K. Characterization of pseudoprogression in patients with glioblastoma: Is histology the gold standard? J. Neurooncol. 2015, 123, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; White, M.; Miller-Thomas, M.M.; Fulton, R.S.; Tsien, C.I.; Rich, K.M.; Schmidt, R.E.; Tran, D.D.; Dahiya, S. Molecular and histologic characteristics of pseudoprogression in diffuse gliomas. J. Neurooncol. 2016, 130, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Gahramanov, S.; Raslan, A.M.; Muldoon, L.L.; Hamilton, B.E.; Rooney, W.D.; Varallyay, C.G.; Njus, J.M.; Haluska, M.; Neuwelt, E.A. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: A pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Leung, D.; Lin, E.; Liao, E.; Kurokawa, R.; Kurokawa, M.; Baba, A.; Yokota, H.; Bathla, G.; Moritani, T.; et al. Prognostic Factors of Stroke-Like Migraine Attacks after Radiation Therapy (SMART) Syndrome. AJNR Am. J. Neuroradiol. 2022, 43, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.; Toprak, B.; Yıldırım, H.; Parlak, Ş.; Güven, D.C.; Kertmen, N.; Oğuz, K.K.; Dizdar, Ö. SMART syndrome: A case report. Acta Neurol. Belg. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Boyer, P.N.; Devlin, M.; Boggild, M. Rare and rarer: Co-occurrence of stroke-like migraine attacks after radiation therapy and Charles Bonnet syndromes. Oxf. Med. Case Rep. 2018, 2018, omy077. [Google Scholar] [CrossRef]
- Panigrahy, N.; Aedma, S.; Lee, M. Stroke-Like Migraine Attacks After Radiation Therapy (SMART) Syndrome Presenting with Recurrent Seizures: A Case Study. Cureus 2022, 14, e25691. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Del Mar Alvarez-Torres, M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Zhan, J.; Natarajan, K.; Flintham, R.; Davies, N.; Sanghera, P.; Grist, J.; Duddalwar, V.; Peet, A.; Sawlani, V. Machine learning-based radiomic evaluation of treatment response prediction in glioblastoma. Clin. Radiol. 2021, 76, 628.e617–628.e627. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Prasanna, P.; Wolansky, L.; Pinho, M.; Cohen, M.; Nayate, A.P.; Gupta, A.; Singh, G.; Hatanpaa, K.J.; Sloan, A.; et al. Computer-Extracted Texture Features to Distinguish Cerebral Radionecrosis from Recurrent Brain Tumors on Multiparametric MRI: A Feasibility Study. AJNR Am. J. Neuroradiol. 2016, 37, 2231–2236. [Google Scholar] [CrossRef]
- Jang, B.S.; Park, A.J.; Jeon, S.H.; Kim, I.H.; Lim, D.H.; Park, S.H.; Lee, J.H.; Chang, J.H.; Cho, K.H.; Kim, J.H.; et al. Machine Learning Model to Predict Pseudoprogression Versus Progression in Glioblastoma Using MRI: A Multi-Institutional Study (KROG 18-07). Cancers 2020, 12, 2706. [Google Scholar] [CrossRef]
- Ismail, M.; Hill, V.; Statsevych, V.; Huang, R.; Prasaannna, P.; Correa, R.; Singh, G.; Bera, K.; Beig, N.; Thawani, R.; et al. Shape Features of the Lesion Habitat to Differentiate Brain Tumor Progression from Pseudoprogression on Routine Multiparametric MRI: A Multisite Study. AJNR Am. J. Neuroradiol. 2018, 39, 2187–2193. [Google Scholar] [CrossRef]
- Yun, J.; St Aubin, J.; Rathee, S.; Fallone, B.G. Brushed permanent magnet DC MLC motor operation in an external magnetic field. Med. Phys. 2010, 37, 2131–2134. [Google Scholar] [CrossRef]
- St Aubin, J.; Santos, D.M.; Steciw, S.; Fallone, B.G. Effect of longitudinal magnetic fields on a simulated in-line 6 MV linac. Med. Phys. 2010, 37, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.; Wachowicz, K.; Fallone, B.G.; Rathee, S. Effect of radiation induced current on the quality of MR images in an integrated linac-MR system. Med. Phys. 2012, 39, 6139–6147. [Google Scholar] [CrossRef] [PubMed]
- Liney, G.P.; Whelan, B.; Oborn, B.; Barton, M.; Keall, P. MRI-Linear Accelerator Radiotherapy Systems. Clin. Oncol. (R Coll Radiol.) 2018, 30, 686–691. [Google Scholar] [CrossRef]
- Kluter, S. Technical design and concept of a 0.35 T MR-Linac. Clin. Transl. Radiat. Oncol. 2019, 18, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.; Krayenbuehl, J.; van Timmeren, J.E.; Wilke, L.; Andratschke, N.; Garcia Schuler, H.; Tanadini-Lang, S.; Guckenberger, M.; Balermpas, P. Head and neck radiotherapy on the MR linac: A multicenter planning challenge amongst MRIdian platform users. Strahlenther. Onkol. 2021, 197, 1093–1103. [Google Scholar] [CrossRef]
- Bonert, M.; Schneider, M.; Solyanik, O.; Hellbach, K.; Bondesson, D.; Gaass, T.; Thaens, N.; Ricke, J.; Benkert, T.; Dinkel, J. Diagnostic accuracy of magnetic resonance imaging for the detection of pulmonary nodules simulated in a dedicated porcine chest phantom. PLoS ONE 2020, 15, e0244382. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.W.; Eppinga, W.S.C.; Tijssen, R.H.N.; Kerkmeijer, L.G.W.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Stewart, J.; Sahgal, A.; Lee, Y.; Soliman, H.; Tseng, C.L.; Detsky, J.; Husain, Z.; Ho, L.; Das, S.; Maralani, P.J.; et al. Quantitating Interfraction Target Dynamics During Concurrent Chemoradiation for Glioblastoma: A Prospective Serial Imaging Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 736–746. [Google Scholar] [CrossRef]
- Kim, T.G.; Lim, D.H. Interfractional variation of radiation target and adaptive radiotherapy for totally resected glioblastoma. J. Korean. Med. Sci. 2013, 28, 1233–1237. [Google Scholar] [CrossRef]
- Manon, R.; Hui, S.; Chinnaiyan, P.; Suh, J.; Chang, E.E.; Timmerman, R.; Phan, S.; Das, R.; Mehta, M. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol. Cancer Res. Treat. 2004, 3, 303–307. [Google Scholar] [CrossRef]
- Tsien, C.; Gomez-Hassan, D.; Ten Haken, R.K.; Tatro, D.; Junck, L.; Chenevert, T.L.; Lawrence, T. Evaluating changes in tumor volume using magnetic resonance imaging during the course of radiotherapy treatment of high-grade gliomas: Implications for conformal dose-escalation studies. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Gajjar, S.R.; Padgett, K.R.; Asher, D.; Stoyanova, R.; Ford, J.C.; Mellon, E.A. Daily Tracking of Glioblastoma Resection Cavity, Cerebral Edema, and Tumor Volume with MRI-Guided Radiation Therapy. Cureus 2018, 10, e2346. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.K.; Maziero, D.; Ford, J.C.; Stoyanova, R.; Goryawala, M.; Diwanji, T.; Mellon, E.A. MRI-guided radiotherapy identifies early pseudoprogression of glioblastoma. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Tsien, C.; Galban, C.J.; Chenevert, T.L.; Johnson, T.D.; Hamstra, D.A.; Sundgren, P.C.; Junck, L.; Meyer, C.R.; Rehemtulla, A.; Lawrence, T.; et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J. Clin. Oncol. 2010, 28, 2293–2299. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, M.; Sheng, K.; Gao, Y.; Chen, A.; Kamrava, M.; Lee, P.; Agazaryan, N.; Lamb, J.; Thomas, D.; et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med. Phys. 2016, 43, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Maziero, D.; Straza, M.W.; Ford, J.C.; Bovi, J.A.; Diwanji, T.; Stoyanova, R.; Paulson, E.S.; Mellon, E.A. MR-Guided Radiotherapy for Brain and Spine Tumors. Front. Oncol. 2021, 11, 626100. [Google Scholar] [CrossRef]
- Scott, C.B.; Scarantino, C.; Urtasun, R.; Movsas, B.; Jones, C.U.; Simpson, J.R.; Fischbach, A.J.; Curran, W.J., Jr. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: A report using RTOG 90-06. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 51–55. [Google Scholar] [CrossRef]
- Chan, J.L.; Lee, S.W.; Fraass, B.A.; Normolle, D.P.; Greenberg, H.S.; Junck, L.R.; Gebarski, S.S.; Sandler, H.M. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J. Clin. Oncol. 2002, 20, 1635–1642. [Google Scholar] [CrossRef]
- Ramesh, K.; Mellon, E.A.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; Sheriff, S.A.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neurooncol. Adv. 2022, 4, vdac006. [Google Scholar] [CrossRef]
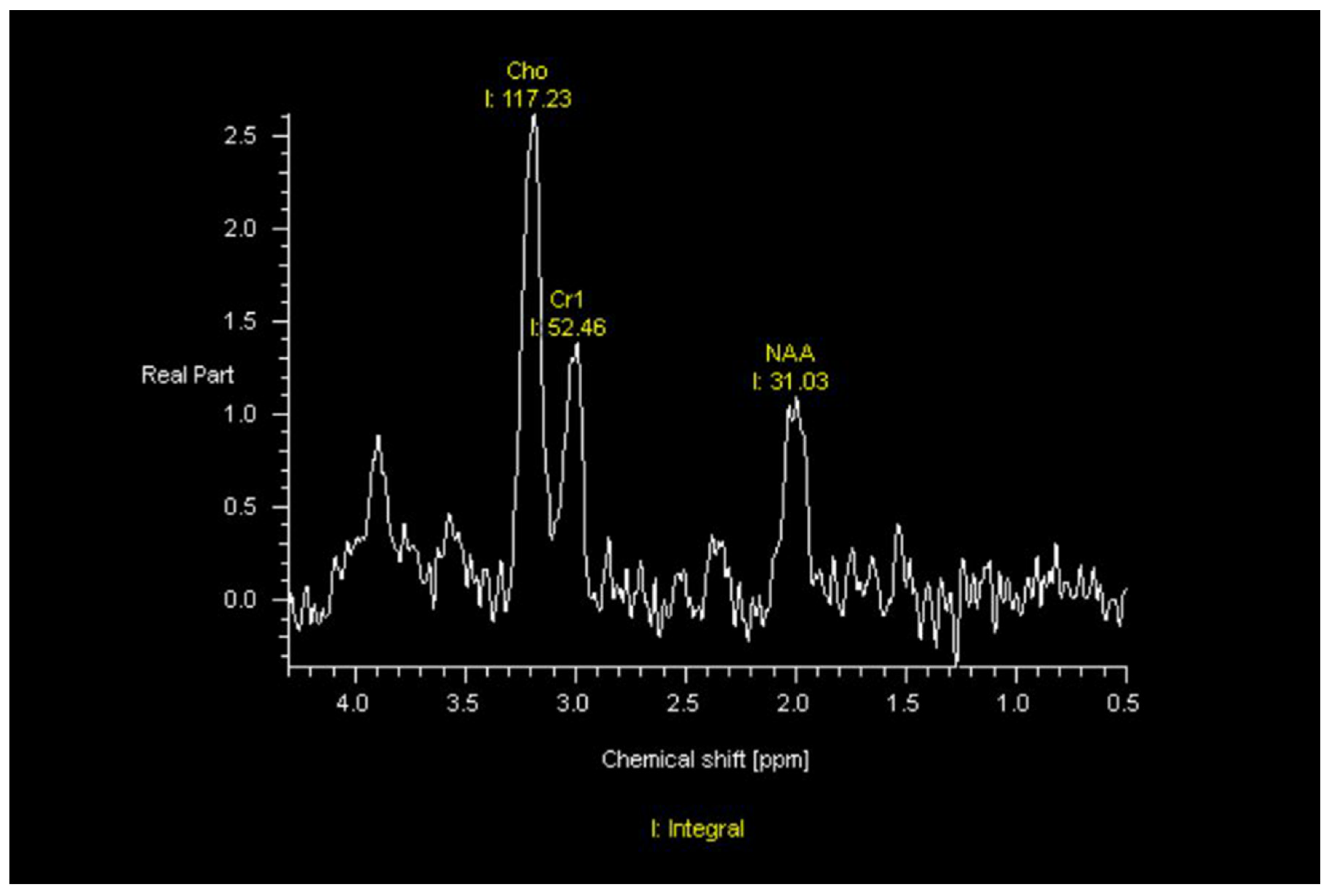
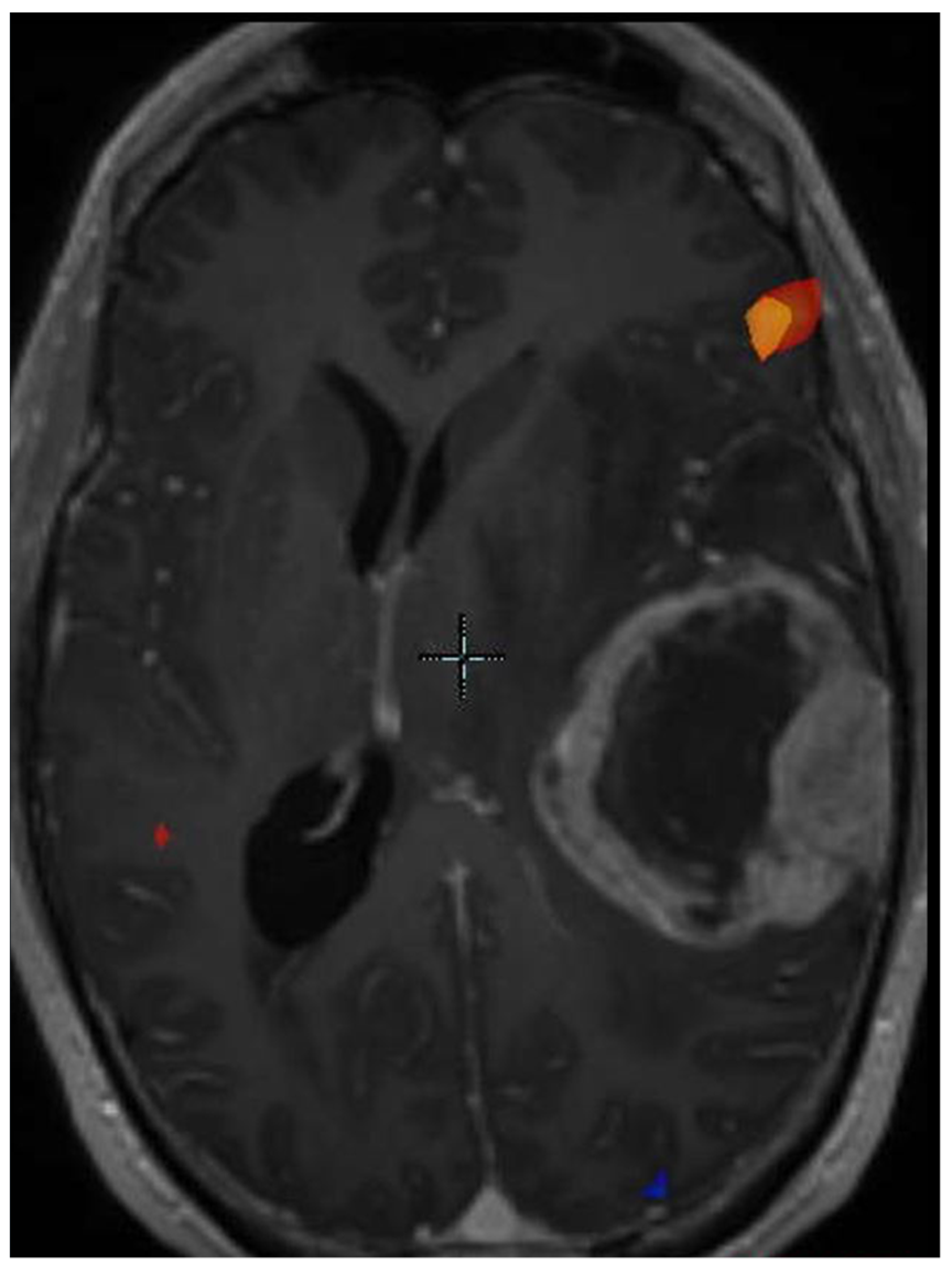
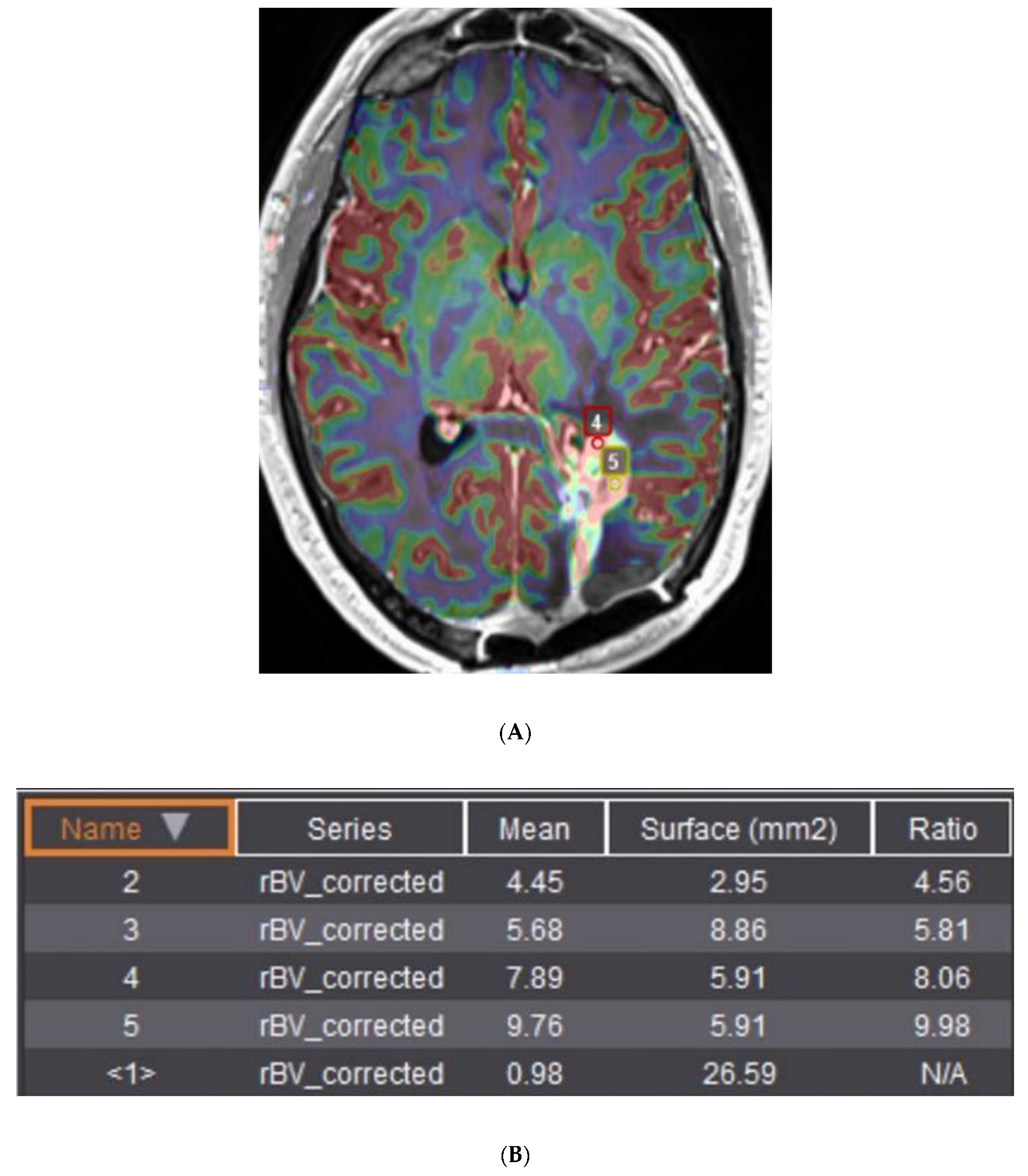
| Response vs. Progression | Change in Sum of Product Diameters | Change in Volumetric Measurement | New Measurable Lesion | Corticosteroids | Clinical Assessment |
|---|---|---|---|---|---|
| Complete Response | 100% Decrease | 100% Decrease | No | Off Corticosteroids or on Physiologic Replacement Dose | Stable or Improved |
| Partial Response | ≥50% Decrease | ≥65% Decrease | No | Corticosteroid Dose Is Same or Lower | Stable or Improved |
| Progressive Disease | ≥25% Increase | ≥40%Increase | Yes | NA | Worse and not attributable to other causes or change in steroid dose |
| Stable Disease | <50% Decrease to <25% Increase | <65% Decrease to <40% Increase | No | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dajani, S.; Hill, V.B.; Kalapurakal, J.A.; Horbinski, C.M.; Nesbit, E.G.; Sachdev, S.; Yalamanchili, A.; Thomas, T.O. Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy. J. Clin. Med. 2022, 11, 5961. https://doi.org/10.3390/jcm11195961
Dajani S, Hill VB, Kalapurakal JA, Horbinski CM, Nesbit EG, Sachdev S, Yalamanchili A, Thomas TO. Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy. Journal of Clinical Medicine. 2022; 11(19):5961. https://doi.org/10.3390/jcm11195961
Chicago/Turabian StyleDajani, Salah, Virginia B. Hill, John A. Kalapurakal, Craig M. Horbinski, Eric G. Nesbit, Sean Sachdev, Amulya Yalamanchili, and Tarita O. Thomas. 2022. "Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy" Journal of Clinical Medicine 11, no. 19: 5961. https://doi.org/10.3390/jcm11195961
APA StyleDajani, S., Hill, V. B., Kalapurakal, J. A., Horbinski, C. M., Nesbit, E. G., Sachdev, S., Yalamanchili, A., & Thomas, T. O. (2022). Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy. Journal of Clinical Medicine, 11(19), 5961. https://doi.org/10.3390/jcm11195961






