In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus
Abstract
1. Introduction
2. Systematic Review Methodology
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Data Extraction and Quality Evaluation of the Studies
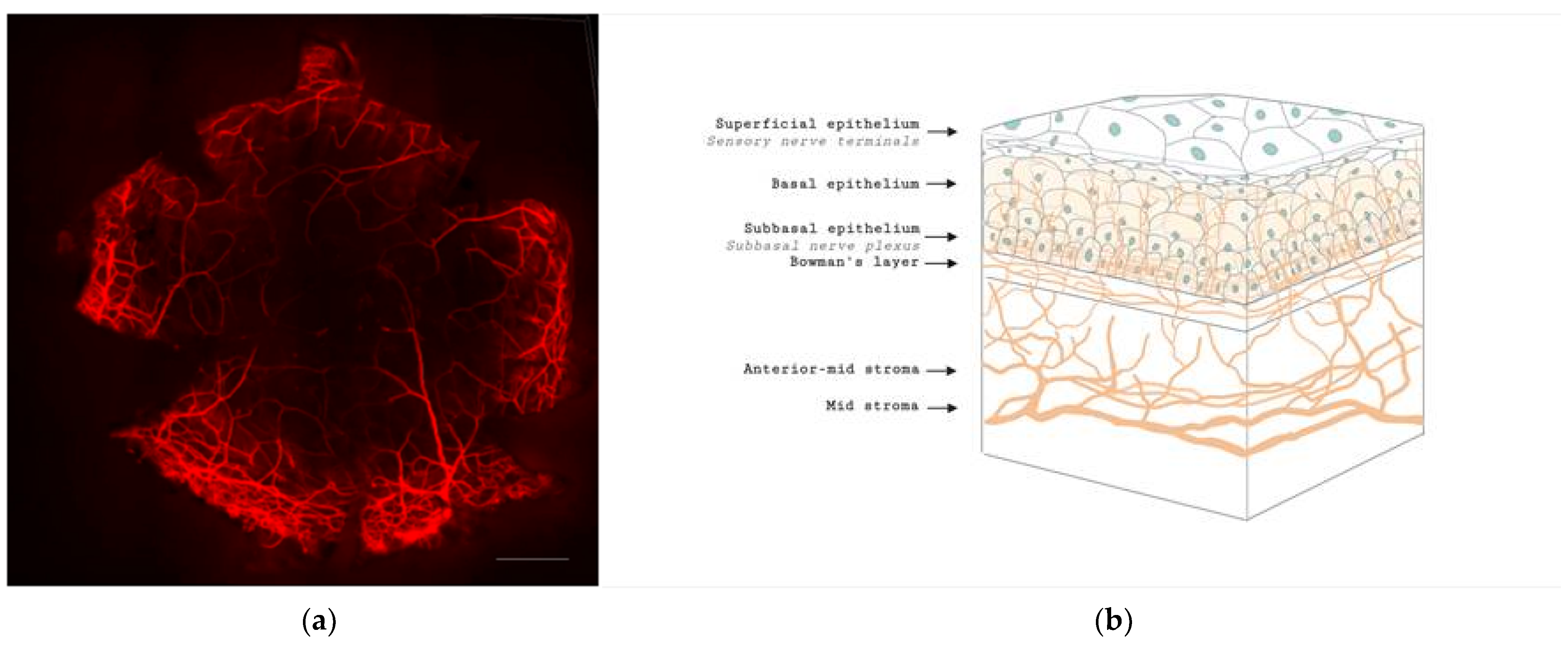
3. Corneal Nerve Function and Anatomy
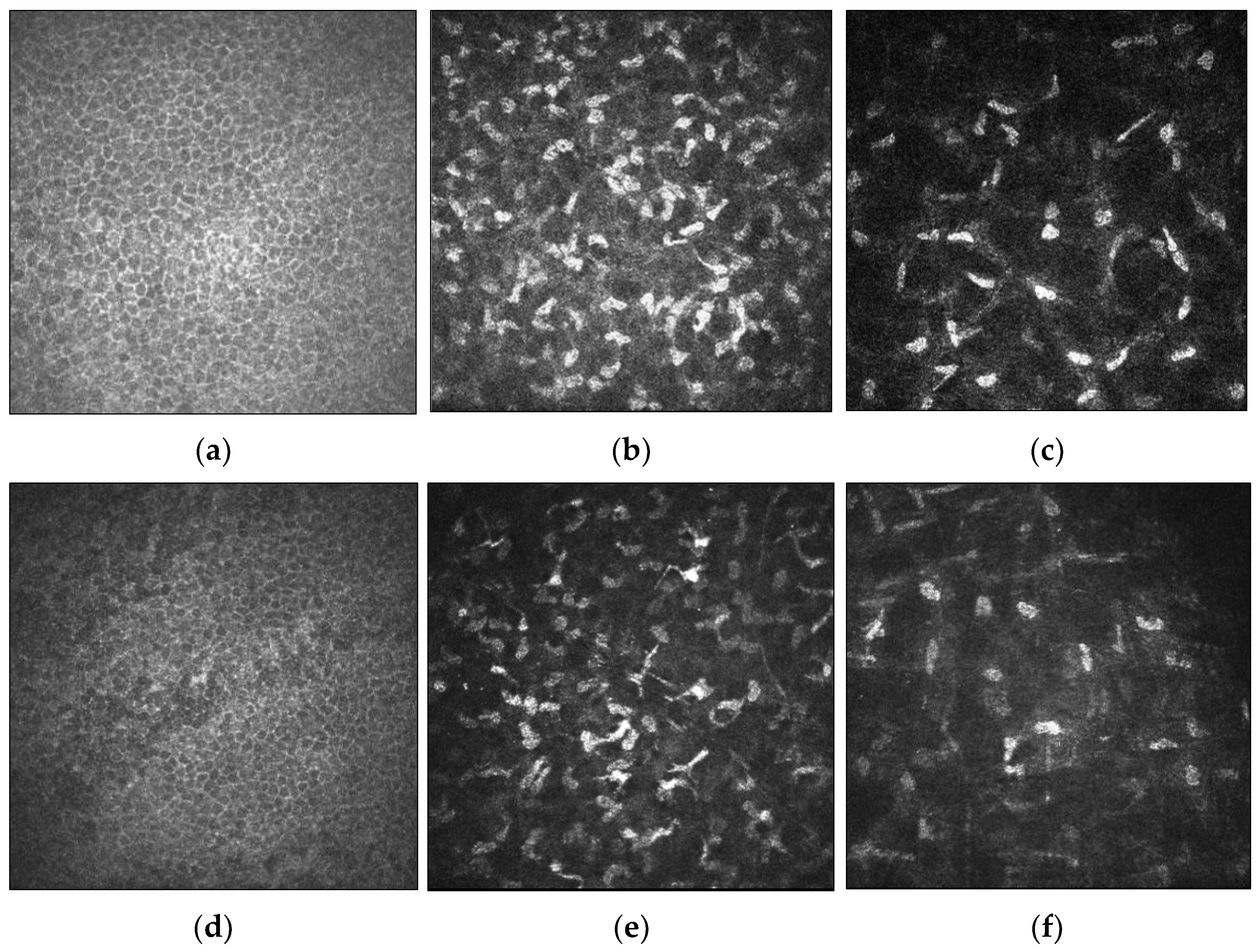
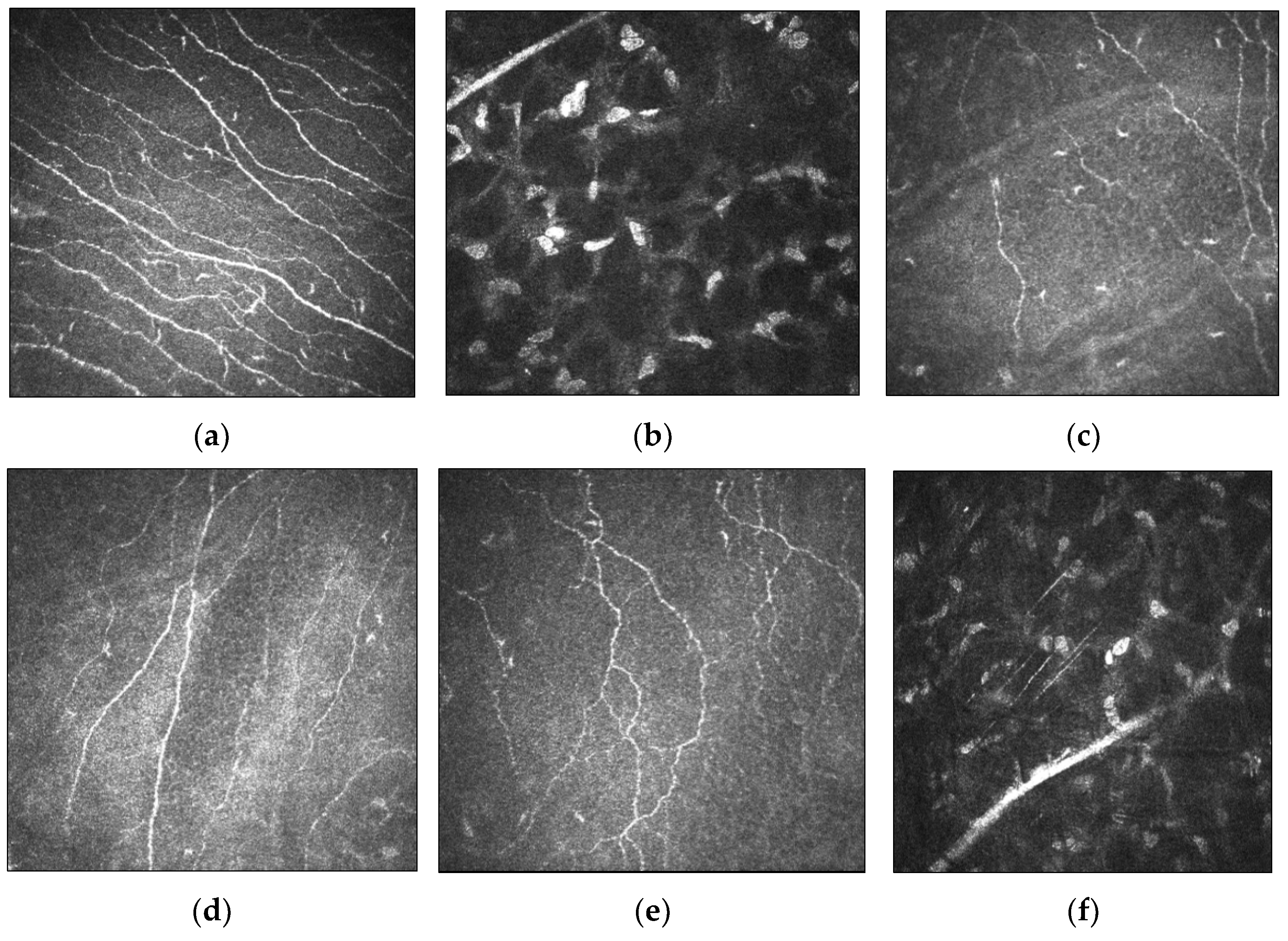
4. Cellular and Corneal Nerve Morphological Changes in Keratoconus
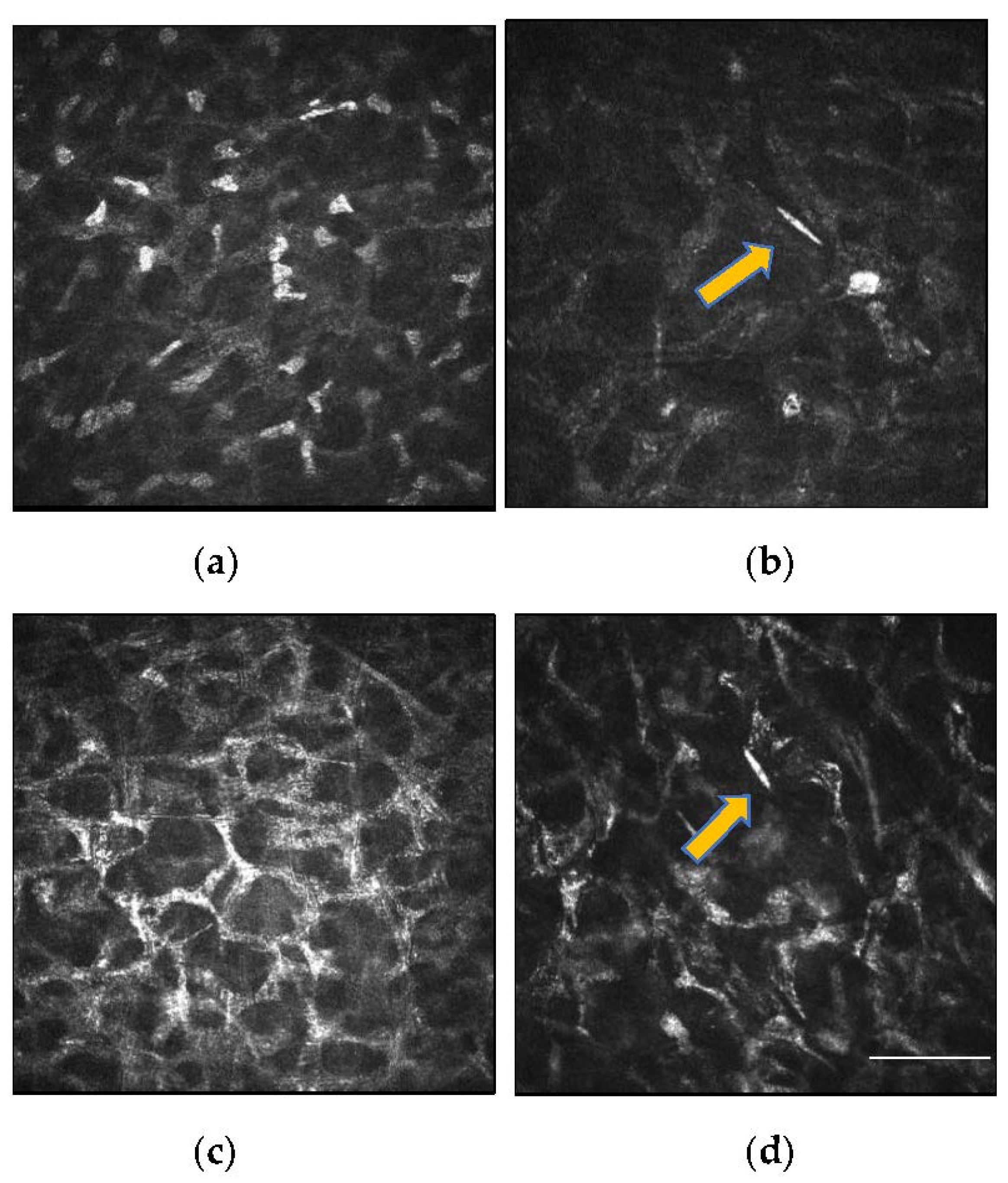
4.1. Microstructural Changes
4.2. Relationship between Corneal Nerves and Corneal Sensitivity in Keratoconus
5. Corneal Nerve and Cellular Changes, and Corneal Sensitivity after Crosslinking
5.1. Corneal Nerve and Cellular Changes
5.2. Changes in Corneal Sensitivity in Relation to Corneal Nerve Status after CXL
6. Future Applications of IVCM in Keratoconus
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Sahebjada, S.; Al-Mahrouqi, H.H.; Moshegov, S.; Panchatcharam, S.M.; Chan, E.; Daniell, M.; Baird, P.N. Eye rubbing in the aetiology of keratoconus: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Sugar, J.; Macsai, M.S. What causes keratoconus? Cornea 2012, 31, 716–719. [Google Scholar] [CrossRef]
- Macsai, M.S.; Varley, G.A.; Krachmer, J.H. Development of keratoconus after contact lens wear. Patient characteristics. Arch. Ophthalmol. 1990, 108, 534–538. [Google Scholar] [CrossRef]
- Nauheim, J.S.; Perry, H.D. A clinicopathologic study of contact-lens-related keratoconus. Am. J. Ophthalmol. 1985, 100, 543–546. [Google Scholar] [CrossRef]
- Ates, K.M.; Estes, A.J.; Liu, Y. Potential underlying genetic associations between keratoconus and diabetes mellitus. Adv. Ophthalmol. Pract. Res. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Dudakova, L.; Liskova, P.; Trojek, T.; Palos, M.; Kalasova, S.; Jirsova, K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Vitart, V.; Burdon, K.P.; Khor, C.C.; Bykhovskaya, Y.; Mirshahi, A.; Hewitt, A.W.; Koehn, D.; Hysi, P.G.; Ramdas, W.D.; et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet. 2013, 45, 155–163. [Google Scholar] [CrossRef]
- Karimian, F.; Aramesh, S.; Rabei, H.M.; Javadi, M.A.; Rafati, N. Topographic evaluation of relatives of patients with keratoconus. Cornea 2008, 27, 874–878. [Google Scholar] [CrossRef]
- Kaya, V.; Utine, C.A.; Altunsoy, M.; Oral, D.; Yilmaz, O.F. Evaluation of corneal topography with Orbscan II in first-degree relatives of patients with keratoconus. Cornea 2008, 27, 531–534. [Google Scholar] [CrossRef]
- Mackiewicz, Z.; Määttä, M.; Stenman, M.; Konttinen, L.; Tervo, T.; Konttinen, Y.T. Collagenolytic proteinases in keratoconus. Cornea 2006, 25, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef] [PubMed]
- Brookes, N.H.; Loh, I.P.; Clover, G.M.; Poole, C.A.; Sherwin, T. Involvement of corneal nerves in the progression of keratoconus. Exp. Eye Res. 2003, 77, 515–524. [Google Scholar] [CrossRef]
- Erie, J.C.; McLaren, J.W.; Patel, S.V. Confocal microscopy in ophthalmology. Am. J. Ophthalmol. 2009, 148, 639–646. [Google Scholar] [CrossRef]
- Guthoff, R.F.; Zhivov, A.; Stachs, O. In vivo confocal microscopy, an inner vision of the cornea—A major review. Clin. Exp. Ophthalmol. 2009, 37, 100–117. [Google Scholar] [CrossRef]
- Tavakoli, M.; Hossain, P.; Malik, R.A. Clinical applications of corneal confocal microscopy. Clin. Ophthalmol. 2008, 2, 435–445. [Google Scholar] [CrossRef]
- Stewart, S.; Liu, Y.C.; Lin, M.T.; Mehta, J.S. Clinical Applications of In Vivo Confocal Microscopy in Keratorefractive Surgery. J. Refract. Surg. 2021, 37, 493–503. [Google Scholar] [CrossRef]
- Allgeier, S.; Bartschat, A.; Bohn, S.; Peschel, S.; Reichert, K.M.; Sperlich, K.; Walckling, M.; Hagenmeyer, V.; Mikut, R.; Stachs, O.; et al. 3D confocal laser-scanning microscopy for large-area imaging of the corneal subbasal nerve plexus. Sci. Rep. 2018, 8, 7468. [Google Scholar] [CrossRef]
- So, W.Z.; Wong, S.Q.; Tan, H.C.; Mehta, J.S.; Liu, Y.C. Diabetic cornea neuropathy as the surrogate marker for diabetic peripheral neuropathy. Neural Regen Res. 2021, 9, 3956. [Google Scholar] [CrossRef]
- Mansoor, H.; Tan, H.C.; Lin, M.T.; Mehta, J.S.; Liu, Y.C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef]
- Chin, J.Y.; Yang, L.W.Y.; Ji, A.J.S.; Nubile, M.; Mastropasqua, L.; Allen, J.C.; Mehta, J.S.; Liu, Y.C. Validation of the Use of Automated and Manual Quantitative Analysis of Corneal Nerve Plexus Following Refractive Surgery. Diagnostics 2020, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Jung, A.S.J.; Chin, J.Y.; Yang, L.W.Y.; Mehta, J.S. Cross-sectional Study on Corneal Denervation in Contralateral Eyes Following SMILE Versus LASIK. J. Refract. Surg. 2020, 36, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Marafon, S.B.; Kwitko, S.; Marinho, D.R. Long-term results of accelerated and conventional corneal cross-linking. Int. Ophthalmol. 2020, 40, 2751–2761. [Google Scholar] [CrossRef]
- Meiri, Z.; Keren, S.; Rosenblatt, A.; Sarig, T.; Shenhav, L.; Varssano, D. Efficacy of Corneal Collagen Cross-Linking for the Treatment of Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2016, 35, 417–428. [Google Scholar] [CrossRef]
- Raiskup-Wolf, F.; Hoyer, A.; Spoerl, E.; Pillunat, L.E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J. Cataract Refract. Surg. 2008, 34, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Fares, U.; Suleman, H.; Lowe, J.; Dua, H.S. Architecture and distribution of human corneal nerves. Br. J. Ophthalmol. 2010, 94, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Dua, H.S.; Gomes, J.A. Clinical course of hurricane keratopathy. Br. J. Ophthalmol. 2000, 84, 285–288. [Google Scholar] [CrossRef][Green Version]
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069. [Google Scholar]
- Chung, E.S.; Lee, K.H.; Kim, M.; Chang, E.J.; Chung, T.Y.; Kim, E.K.; Lee, H.K. Expression of neurotrophic factors and their receptors in keratoconic cornea. Curr. Eye Res. 2013, 38, 743–750. [Google Scholar] [CrossRef]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.; Nistri, A.; et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: Implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Hin-FaiYam, G.; Tzu-YuLin, M.; EriciaTeo; Koh, S.-K.; LuDeng; LeiZhou; LouisTong; Mehta, J.S. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J. Adv. Res. 2021, 29, 67–81. [Google Scholar] [CrossRef]
- Yang LW, Y.; Mehta, J.S.; Liu, Y.C. Corneal neuromediator profiles following laser refractive surgery. Neural Regen. Res. 2021, 16, 2177. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Scorcia, V.; Lambiase, A.; Bonini, S. Preliminary evidence of neuropeptides involvement in keratoconus. Acta Ophthalmol. 2015, 93, e315–e316. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Faraj, L.; Fares, U.; Otri, A.M.; Dua, H.S. The morphologic characteristics of corneal nerves in advanced keratoconus as evaluated by acetylcholinesterase technique. Am. J. Ophthalmol. 2011, 152, 364–376.e361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lin, M.T.; Mehta, J.S. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen. Res. 2021, 16, 690–691. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Fuest, M.; Zhou, L.; Liu, Y.C.; Deng, L.; Chan, A.S.; Ong, H.S.; Khor, W.B.; Ang, M.; Mehta, J.S. Differential epithelial and stromal protein profiles in cone and non-cone regions of keratoconus corneas. Sci. Rep. 2019, 9, 2965. [Google Scholar] [CrossRef]
- Hollingsworth, J.G.; Efron, N.; Tullo, A.B. In vivo corneal confocal microscopy in keratoconus. Ophthalmic Physiol. Opt. 2005, 25, 254–260. [Google Scholar] [CrossRef]
- Mocan, M.C.; Yilmaz, P.T.; Irkec, M.; Orhan, M. In vivo confocal microscopy for the evaluation of corneal microstructure in keratoconus. Curr. Eye Res. 2008, 33, 933–939. [Google Scholar] [CrossRef]
- Uçakhan, O.O.; Kanpolat, A.; Ylmaz, N.; Ozkan, M. In vivo confocal microscopy findings in keratoconus. Eye Contact Lens 2006, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Rabinowitz, Y.S.; Meisler, D.M.; Wilson, S.E. Keratocyte apoptosis associated with keratoconus. Exp. Eye Res. 1999, 69, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Ozkagnici, A.; Malik, R.A.; Oltulu, R. Evaluation of contact lens-induced changes in keratoconic corneas using in vivo confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Mannion, L.S.; Tromans, C.; O’Donnell, C. Corneal nerve structure and function in keratoconus: A case report. Eye Contact Lens 2007, 33, 106–108. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1348–1351. [Google Scholar] [CrossRef]
- Ozgurhan, E.B.; Kara, N.; Yildirim, A.; Bozkurt, E.; Uslu, H.; Demirok, A. Evaluation of corneal microstructure in keratoconus: A confocal microscopy study. Am. J. Ophthalmol. 2013, 156, 885–893.e882. [Google Scholar] [CrossRef]
- Kawabuchi, M.; Chongjian, Z.; Islam, A.T.; Hirata, K.; Nada, O. The effect of aging on the morphological nerve changes during muscle reinnervation after nerve crush. Restor. Neurol. Neurosci. 1998, 13, 117–127. [Google Scholar]
- Flockerzi, E.; Daas, L.; Seitz, B. Structural changes in the corneal subbasal nerve plexus in keratoconus. Acta Ophthalmol. 2020, 98, e928–e932. [Google Scholar] [CrossRef]
- Koh, S.; Inoue, R.; Maeda, N.; Kabata, D.; Shintani, A.; Jhanji, V.; Klyce, S.D.; Maruyama, K.; Nishida, K. Long-term Chronological Changes in Very Asymmetric Keratoconus. Cornea 2019, 38, 605–611. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Rabinowitz, Y.S. Longitudinal study of keratoconus progression. Exp. Eye Res. 2007, 85, 502–507. [Google Scholar] [CrossRef]
- Pahuja, N.K.; Shetty, R.; Nuijts, R.M.; Agrawal, A.; Ghosh, A.; Jayadev, C.; Nagaraja, H. An In Vivo Confocal Microscopic Study of Corneal Nerve Morphology in Unilateral Keratoconus. Biomed. Res. Int. 2016, 2016, 5067853. [Google Scholar] [CrossRef]
- Simo Mannion, L.; Tromans, C.; O’Donnell, C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Cont. Lens Anterior Eye 2005, 28, 185–192. [Google Scholar] [CrossRef]
- Patel, D.V.; Ku, J.Y.; Johnson, R.; McGhee, C.N. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye 2009, 23, 586–592. [Google Scholar] [CrossRef]
- Millodot, M.; Owens, H. Sensitivity and fragility in keratoconus. Acta Ophthalmol. 1983, 61, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Karakaya, H.; Ozçetin, H.; Ertürk, H.; Yücel, A.; Ozmen, A.; Baykara, M.; Tsubota, K. Tear function and ocular surface changes in keratoconus. Ophthalmology 2003, 110, 1110–1118. [Google Scholar] [CrossRef]
- Lum, E.; Murphy, P.J. Effects of ambient humidity on the Cochet-Bonnet aesthesiometer. Eye 2018, 32, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, B.; Papas, E.; Stapleton, F. Assessing the sensory function of the ocular surface: Implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp. Eye Res. 2011, 92, 408–413. [Google Scholar] [CrossRef]
- Dienes, L.; Kiss, H.J.; Perényi, K.; Nagy, Z.Z.; Acosta, M.C.; Gallar, J.; Kovács, I. Corneal Sensitivity and Dry Eye Symptoms in Patients with Keratoconus. PLoS ONE 2015, 10, e0141621. [Google Scholar] [CrossRef]
- Murphy, P.J.; Patel, S.; Marshall, J. The effect of long-term, daily contact lens wear on corneal sensitivity. Cornea 2001, 20, 264–269. [Google Scholar] [CrossRef]
- Mandathara, P.S.; Stapleton, F.J.; Kokkinakis, J.; Willcox, M.D. Pilot Study of Corneal Sensitivity and Its Association in Keratoconus. Cornea 2017, 36, 163–168. [Google Scholar] [CrossRef]
- Xia, Y.; Chai, X.; Zhou, C.; Ren, Q. Corneal nerve morphology and sensitivity changes after ultraviolet A/riboflavin treatment. Exp. Eye Res. 2011, 93, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Hafezi, F.; Kymionis, G.; Caragiuli, S.; Jacob, S.; Traversi, C.; Barabino, S.; Randleman, J.B. In Vivo Confocal Microscopy after Corneal Collagen Crosslinking. Ocul. Surf. 2015, 13, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Traversi, C.; Baiocchi, S.; Caporossi, O.; Bovone, C.; Sparano, M.C.; Balestrazzi, A.; Caporossi, A. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: Early and late modifications. Am. J. Ophthalmol. 2008, 146, 527–533. [Google Scholar] [CrossRef]
- Mazzotta, C.; Caporossi, T.; Denaro, R.; Bovone, C.; Sparano, C.; Paradiso, A.; Baiocchi, S.; Caporossi, A. Morphological and functional correlations in riboflavin UV A corneal collagen cross-linking for keratoconus. Acta Ophthalmol. 2012, 90, 259–265. [Google Scholar] [CrossRef]
- Mazzotta, C.; Balestrazzi, A.; Traversi, C.; Baiocchi, S.; Caporossi, T.; Tommasi, C.; Caporossi, A. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: Ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea 2007, 26, 390–397. [Google Scholar] [CrossRef]
- Zare, M.A.; Mazloumi, M.; Farajipour, H.; Hoseini, B.; Fallah, M.R.; Mahrjerdi, H.Z.; Abtahi, M.A.; Abtahi, S.H. Effects of Corneal Collagen Crosslinking on Confocal Microscopic Findings and Tear Indices in Patients with Progressive Keratoconus. Int. J. Prev. Med. 2016, 7, 132. [Google Scholar] [CrossRef]
- Kymionis, G.D.; Diakonis, V.F.; Kalyvianaki, M.; Portaliou, D.; Siganos, C.; Kozobolis, V.P.; Pallikaris, A.I. One-year follow-up of corneal confocal microscopy after corneal cross-linking in patients with post laser in situ keratosmileusis ectasia and keratoconus. Am. J. Ophthalmol. 2009, 147, 774–778.e771. [Google Scholar] [CrossRef]
- Jordan, C.; Patel, D.V.; Abeysekera, N.; McGhee, C.N. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmology 2014, 121, 469–474. [Google Scholar] [CrossRef]
- Parissi, M.; Randjelovic, S.; Poletti, E.; Guimarães, P.; Ruggeri, A.; Fragkiskou, S.; Wihlmark, T.B.; Utheim, T.P.; Lagali, N. Corneal Nerve Regeneration After Collagen Cross-Linking Treatment of Keratoconus: A 5-Year Longitudinal Study. JAMA Ophthalmol. 2016, 134, 70–78. [Google Scholar] [CrossRef]
- Mazzotta, C.; Jacob, S.; Agarwal, A.; Kumar, D.A. In Vivo Confocal Microscopy After Contact Lens-Assisted Corneal Collagen Cross-linking for Thin Keratoconic Corneas. J. Refract. Surg. 2016, 32, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sufi, A.R.; Soundaram, M.; Gohil, N.; Keenan, J.D.; Prajna, N.V. Structural Changes in Thin Keratoconic Corneas Following Crosslinking with Hypotonic Riboflavin: Findings on In Vivo Confocal Microscopy. J. Ophthalmic Vis. Res. 2021, 16, 325–337. [Google Scholar] [CrossRef]
- Raiskup, F.; Spoerl, E. Corneal cross-linking with hypo-osmolar riboflavin solution in thin keratoconic corneas. Am. J. Ophthalmol. 2011, 152, 28–32.e21. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Ramovecchi, V. Customized epithelial debridement for thin ectatic corneas undergoing corneal cross-linking: Epithelial island cross-linking technique. Clin. Ophthalmol. 2014, 8, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, H.; Jabbarvand, M.; Khodaparast, M.; Ameli, K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: A randomized controlled trial. J. Refract. Surg. 2014, 30, 837–842. [Google Scholar] [CrossRef]
- Caporossi, A.; Mazzotta, C.; Baiocchi, S.; Caporossi, T.; Paradiso, A.L. Transepithelial corneal collagen crosslinking for keratoconus: Qualitative investigation by in vivo HRT II confocal analysis. Eur. J. Ophthalmol. 2012, 22 (Suppl. S7), S81–S88. [Google Scholar] [CrossRef]
- Al-Aqaba, M.; Calienno, R.; Fares, U.; Otri, A.M.; Mastropasqua, L.; Nubile, M.; Dua, H.S. The effect of standard and transepithelial ultraviolet collagen cross-linking on human corneal nerves: An ex vivo study. Am. J. Ophthalmol. 2012, 153, 258–266.e252. [Google Scholar] [CrossRef]
- Jouve, L.; Borderie, V.; Sandali, O.; Temstet, C.; Basli, E.; Laroche, L.; Bouheraoua, N. Conventional and Iontophoresis Corneal Cross-Linking for Keratoconus: Efficacy and Assessment by Optical Coherence Tomography and Confocal Microscopy. Cornea 2017, 36, 153–162. [Google Scholar] [CrossRef]
- Filippello, M.; Stagni, E.; Buccoliero, D.; Bonfiglio, V.; Avitabile, T. Transepithelial cross-linking in keratoconus patients: Confocal analysis. Optom. Vis. Sci. 2012, 89, e1–e7. [Google Scholar] [CrossRef]
- Bouheraoua, N.; Jouve, L.; El Sanharawi, M.; Sandali, O.; Temstet, C.; Loriaut, P.; Basli, E.; Borderie, V.; Laroche, L. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7601–7609. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Serrao, S.; Rosati, M.; Ducoli, P.; Lombardo, G. Biomechanical changes in the human cornea after transepithelial corneal crosslinking using iontophoresis. J. Cataract Refract. Surg. 2014, 40, 1706–1715. [Google Scholar] [CrossRef]
- Ziaei, M.; Vellara, H.; Gokul, A.; Patel, D.; McGhee, C.N.J. Prospective 2-year study of accelerated pulsed transepithelial corneal crosslinking outcomes for Keratoconus. Eye 2019, 33, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Sgheri, A.; Bagaglia, S.A.; Rechichi, M.; Di Maggio, A. Customized corneal crosslinking for treatment of progressive keratoconus: Clinical and OCT outcomes using a transepithelial approach with supplemental oxygen. J. Cataract Refract. Surg. 2020, 46, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Ozgurhan, E.B.; Celik, U.; Bozkurt, E.; Demirok, A. Evaluation of subbasal nerve morphology and corneal sensation after accelerated corneal collagen cross-linking treatment on keratoconus. Curr. Eye Res. 2015, 40, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, M.; Yüksel, E.; Bilgihan, K. Effect of corneal cross-linking on contact lens tolerance in keratoconus. Clin. Exp. Optom. 2017, 100, 369–374. [Google Scholar] [CrossRef]
- Wasilewski, D.; Mello, G.H.; Moreira, H. Impact of collagen crosslinking on corneal sensitivity in keratoconus patients. Cornea 2013, 32, 899–902. [Google Scholar] [CrossRef]
- dell’Omo, R.; Cifariello, F.; De Turris, S.; Romano, V.; Di Renzo, F.; Di Taranto, D.; Coclite, G.; Agnifili, L.; Mastropasqua, L.; Costagliola, C. Confocal microscopy of corneal nerve plexus as an early marker of eye involvement in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 142, 393–400. [Google Scholar] [CrossRef]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef]
- Henriquez, M.A.; Hadid, M.; Izquierdo, L., Jr. A Systematic Review of Subclinical Keratoconus and Forme Fruste Keratoconus. J. Refract. Surg. 2020, 36, 270–279. [Google Scholar] [CrossRef]
- Kolozsvári, B.L.; Petrovski, G.; Gogolák, P.; Rajnavölgyi, É.; Tóth, F.; Berta, A.; Fodor, M. Association between mediators in the tear fluid and the severity of keratoconus. Ophthalmic Res. 2014, 51, 46–51. [Google Scholar] [CrossRef] [PubMed]
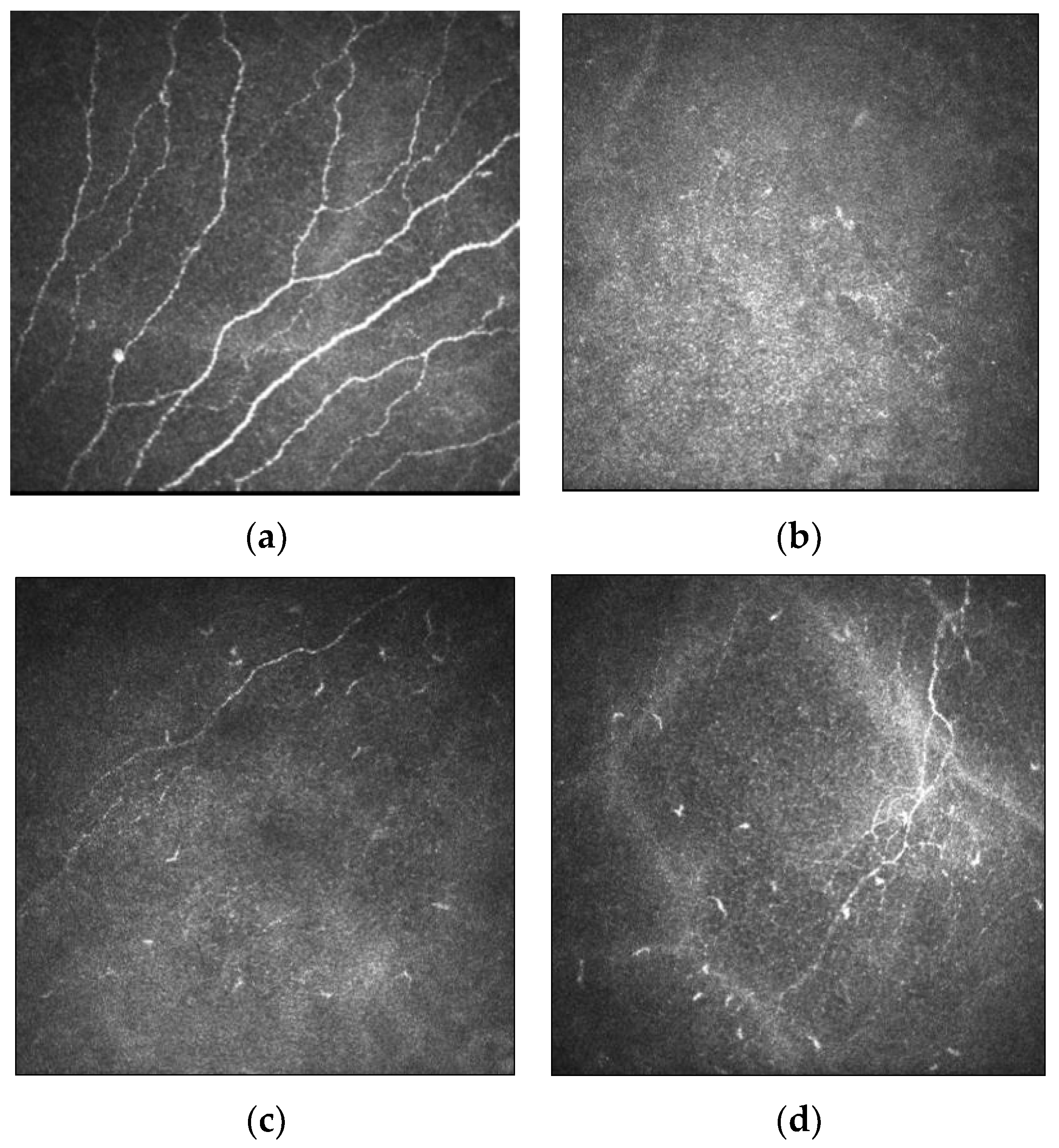
| Author | Assessment | Number of Eyes | Findings |
|---|---|---|---|
| Brookes et al. [13] 2003 | Excised corneas | 10 KCN, 3 controls |
|
| Aqaba et al. [37] 2011 | Excised corneas | 14 KCN, 6 controls |
|
| Mocan et al. [42] 2008 | IVCM assessment | 68 KCN, 22 controls |
|
| Patel et al. [47] 2006 | IVCM assessment | 4 KCN |
|
| Flockerzi et al. [50] 2020 | IVCM assessment | 23 KCN |
|
| Mannion et al. [46] 2007 | IVCM assessment | 1 KCN |
|
| Mannion et al. [54] 2005 | IVCM assessment | 13 KCN, 13 controls |
|
| Ozgurhan et al. [48] 2013 | IVCM assessment | 30 KCN, 32 subclinical KCN, 53 KCN relatives, 30 controls |
|
| Patel et al. [55] 2009 | IVCM assessment | 27 KCN, 31 controls |
|
| Pahuja et al. [53] 2016 | IVCM assessment | 33 normal eyes of KCN, 30 controls |
|
| Author | Study and CXL Protocol | N. of Eyes | Follow Up | Findings |
|---|---|---|---|---|
| Xia et al. [63] 2011 | Longitudinal study, transepithelial or epithelium-off conventional CXL | 108 rabbit eyes | 180 days |
|
| Mazzotta et al. [64] 2015 | Longitudinal study; epithelium-off CXL | 84 eyes | 12 months |
|
| Mazzotta et al. [65] 2008 | Longitudinal study; epithelium-off CXL | 44 eyes | 3 years |
|
| Parissi et al. [71] 2016 | Longitudinal study; epithelium-off CXL | 19 eyes | 5 years |
|
| Al-aqaba et al. [78] 2012 | Cross-sectional study; transepithelial or epithelium-off CXL | 8 eyes | N/A |
|
| Zare et al. [68] 2016 | Longitudinal study; epithelium-off CXL | 32 eyes | 6 months |
|
| Jordan et al. [70] 2014 | Longitudinal study; epithelium-off CXL | 38 eyes | 12 months |
|
| Mazzotta et al. [72] 2016 | Longitudinal study; contact lens assisted epithelium-off CXL | 10 eyes | 6 months |
|
| Sufi et al. [73] 2021 | Longitudinal study; epithelium-off CXL with hypotonic riboflavin | 10 eyes | 6 months |
|
| Mazzotta et al. [75] 2014 | Longitudinal study; epithelial island CXL | 10 eyes | 12 months |
|
| Kymionis et al. [69] 2009 | Longitudinal study; epithelium-off CXL | 5 eyes | 12 months |
|
| Hashemian et al. [76] 2014 | Longitudinal study; epithelium-off or AXL | 153 eyes | 15 months |
|
| Caporossi et al. [77] 2012 | Longitudinal study; transepithelial CXL | 10 eyes | 6 months |
|
| Bouheraoua et al. [81] 2014 | Longitudinal study; transepithelial CXL, epithelium-off CXL or AXL | 45 eyes | 6 months |
|
| Filippello et al. [80] 2012 | Longitudinal study; transepithelial CXL | 20 eyes | 18 months |
|
| Jouve et al. [79]. 2017 | Longitudinal study; transepithelial CXL using iontophoresis or Epithelium-off CXL | 80 eyes | 24 months |
|
| Ozgurhan et al. [85] 2015 | Longitudinal study epithelium-off AXL | 30 eyes | 12 months |
|
| Unlu et al. [86] 2017 | Longitudinal study; epithelium-off CXL | 30 eyes | 6 months |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, A.W.J.; Mansoor, H.; Sim, N.; Lin, M.T.-Y.; Liu, Y.-C. In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. J. Clin. Med. 2022, 11, 393. https://doi.org/10.3390/jcm11020393
Teo AWJ, Mansoor H, Sim N, Lin MT-Y, Liu Y-C. In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. Journal of Clinical Medicine. 2022; 11(2):393. https://doi.org/10.3390/jcm11020393
Chicago/Turabian StyleTeo, Alvin Wei Jun, Hassan Mansoor, Nigel Sim, Molly Tzu-Yu Lin, and Yu-Chi Liu. 2022. "In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus" Journal of Clinical Medicine 11, no. 2: 393. https://doi.org/10.3390/jcm11020393
APA StyleTeo, A. W. J., Mansoor, H., Sim, N., Lin, M. T.-Y., & Liu, Y.-C. (2022). In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. Journal of Clinical Medicine, 11(2), 393. https://doi.org/10.3390/jcm11020393






