Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Lentiviral Packaging and Cell Infection
2.3. Cell Counting Kit-8 (CCK-8)
2.4. Osteogenic Differentiation Protocol
2.5. Measurement of Alkaline Phosphatase (ALP) Activity
2.6. Alizarin Red Staining (ARS)
2.7. RNA Isolation and qPCR
2.8. Western Blot Analysis
2.9. Cell Seeding
2.10. In Vivo Evaluation in Animals
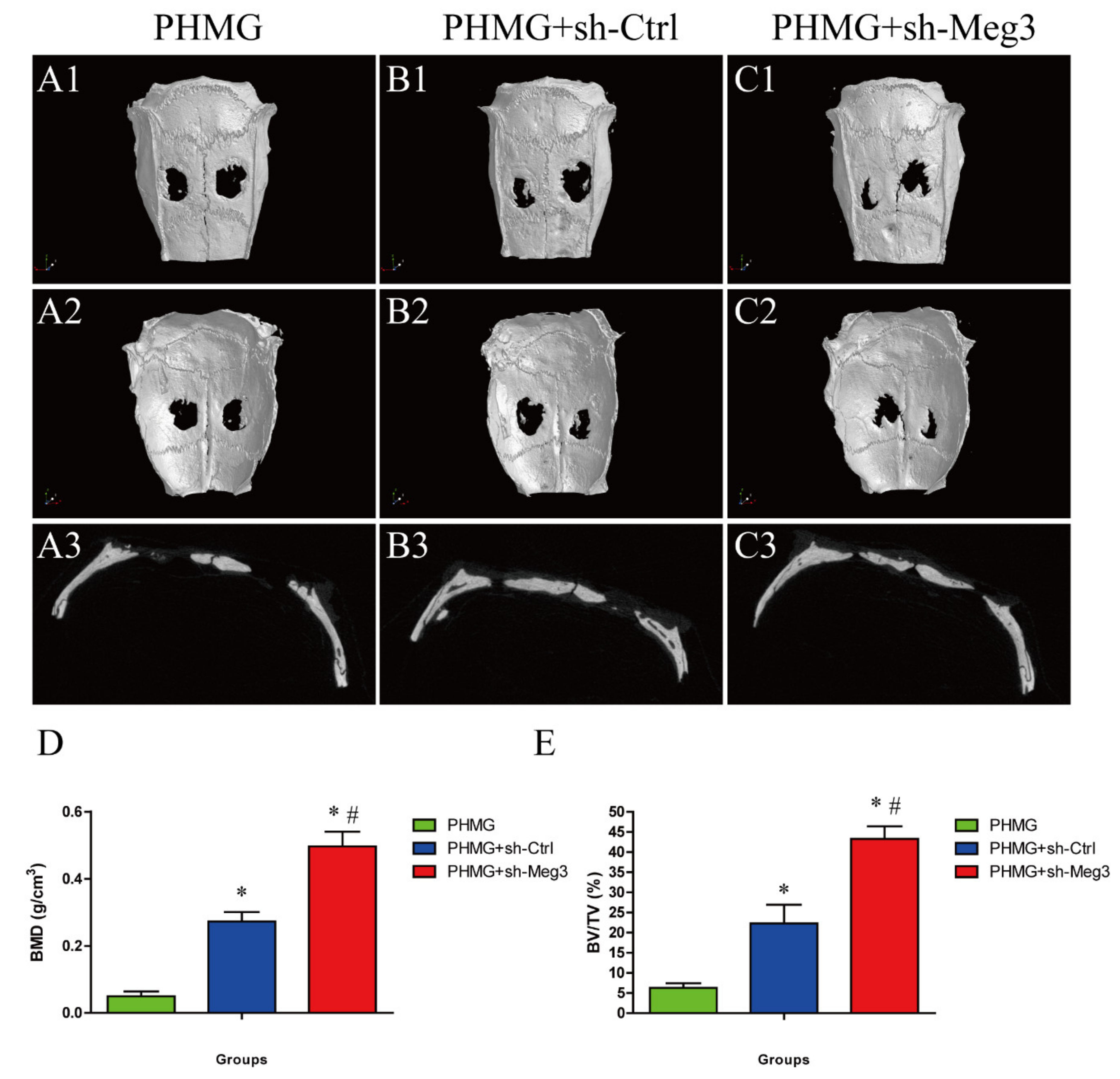
2.11. Micro-Computed Tomography (CT) Evaluation
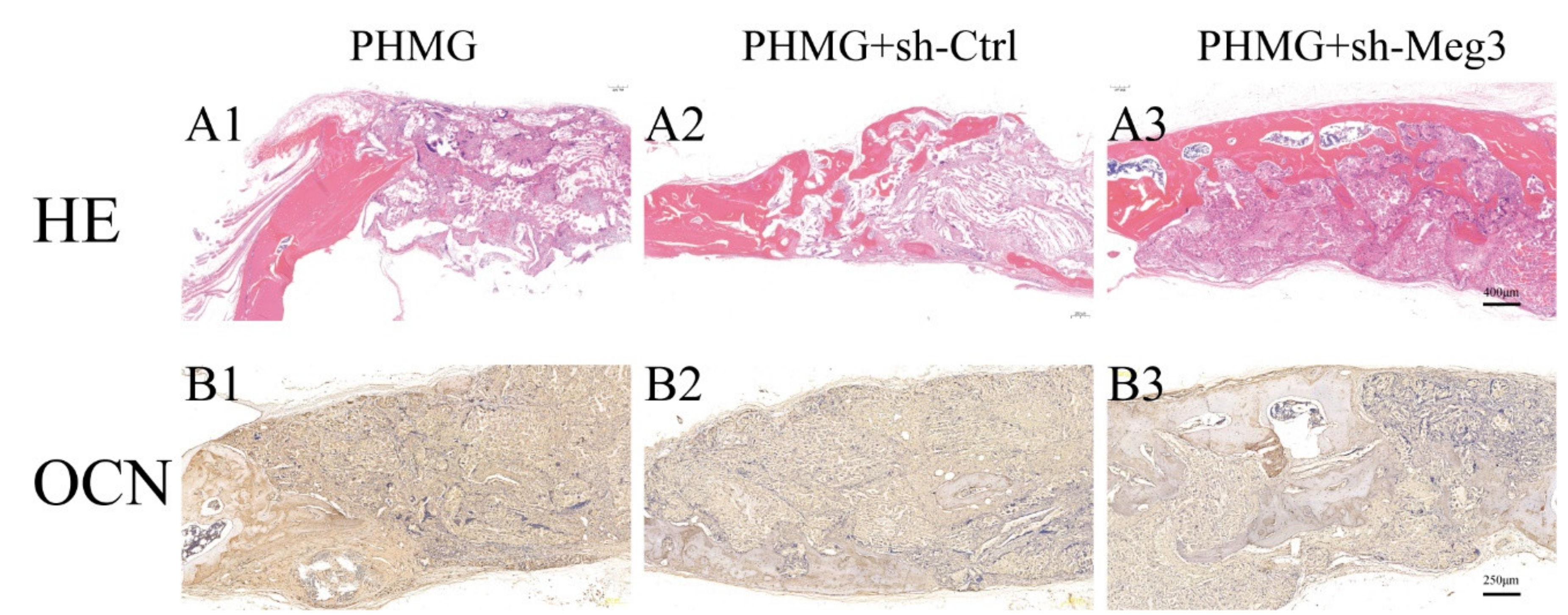
2.12. Histological and Immunohistochemical (IHC) Analysis
2.13. Statistical Analysis
3. Results
3.1. Endogenous MEG3 Was Increased during Osteogenic Differentiation of BMSCs
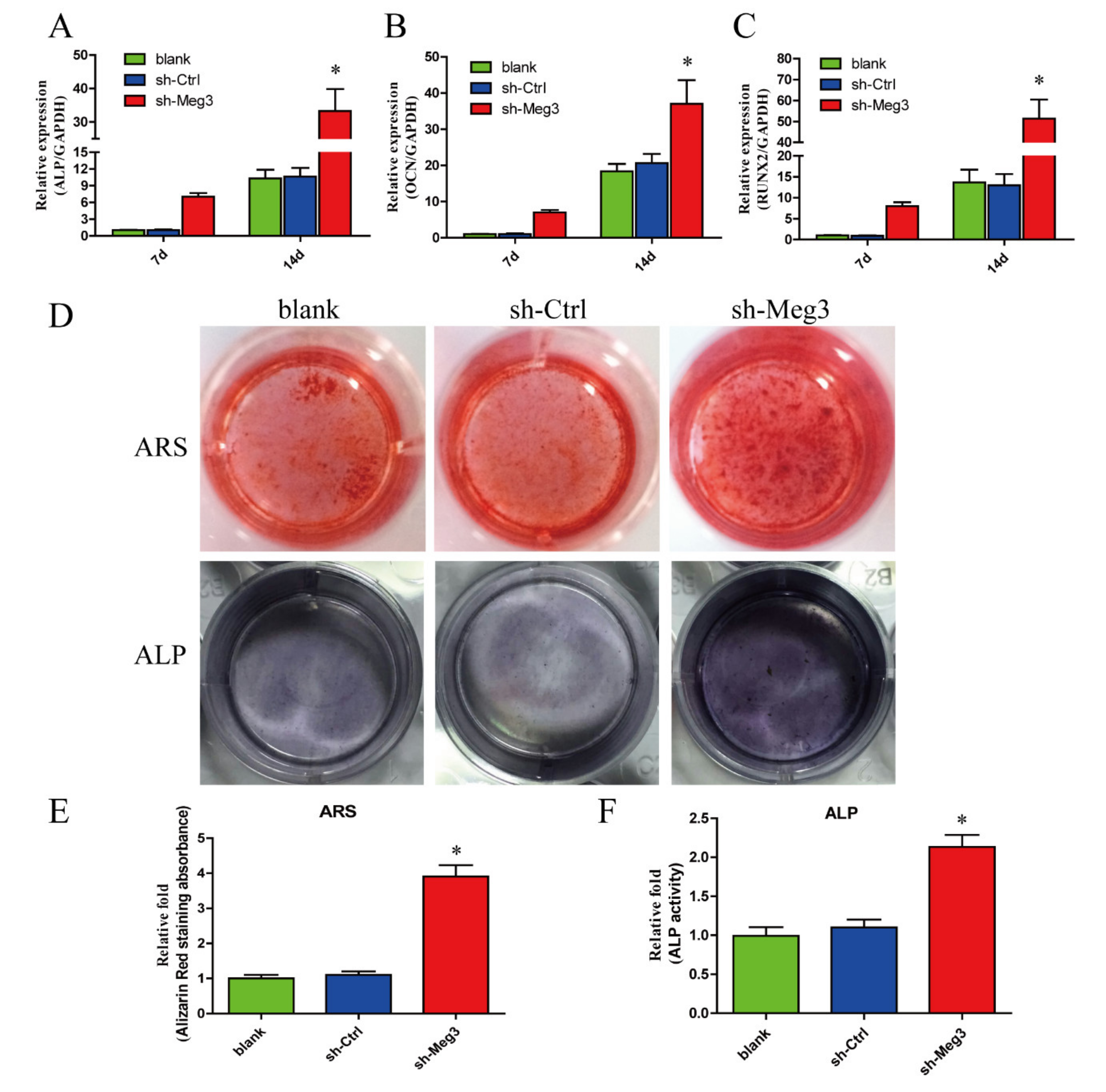
3.2. MEG3 Knockdown Promotes Osteogenic Differentiation of BMSCs In Vitro
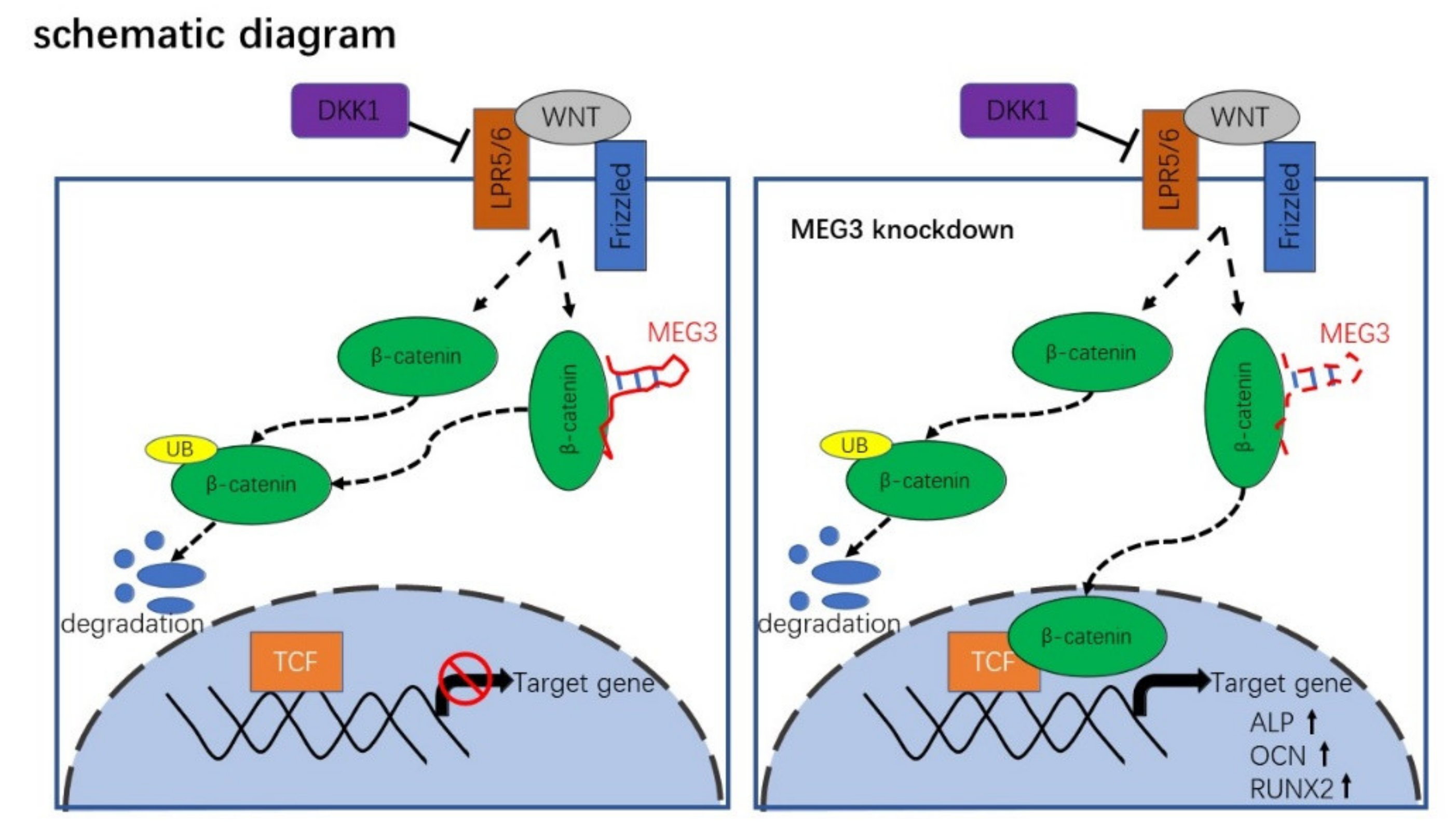
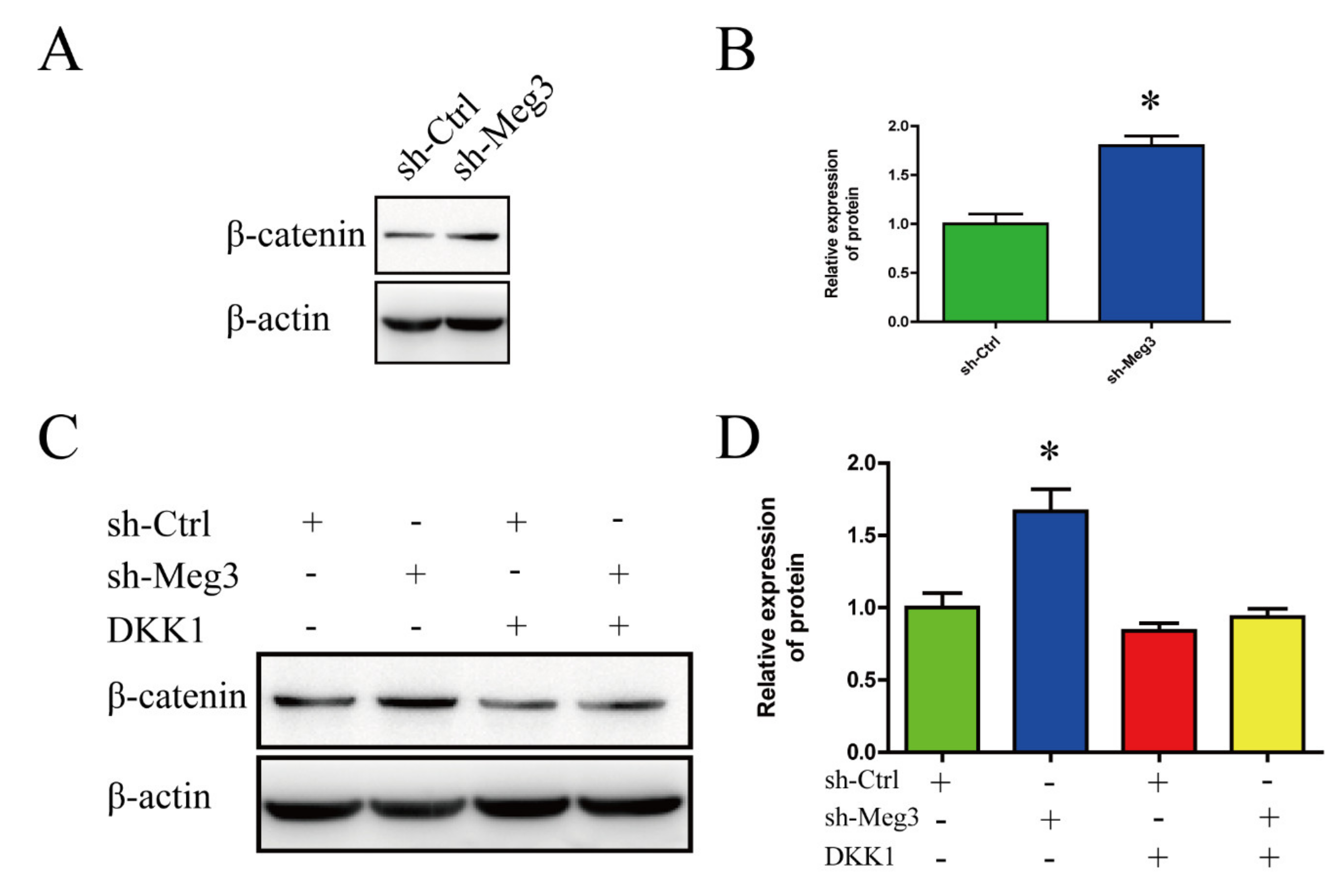
3.3. MEG3 Knockdown Activates Wnt/β-Catenin Signaling Pathway in BMSCs
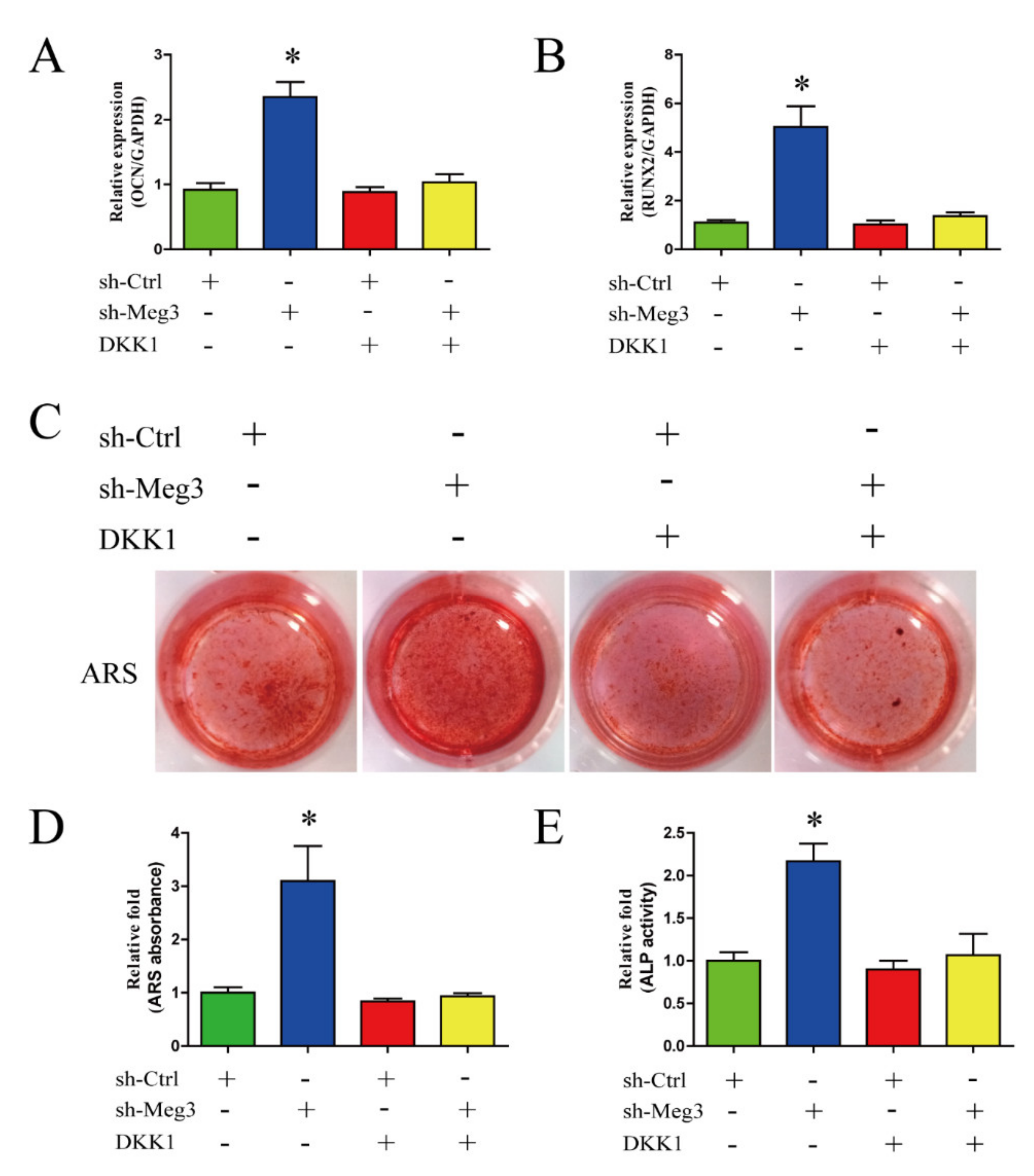
3.4. Wnt/β-Catenin Inhibition Impairs MEG3-Induced Osteogenic Differentiation of BMSCs
3.5. MEG3 Knockdown Accelerated Bone Repairing in a Rat Critical-Sized Skull Defect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yeap, M.C.; Tu, P.H.; Liu, Z.H.; Hsieh, P.C.; Liu, Y.T.; Lee, C.Y.; Lai, H.Y.; Chen, C.T.; Huang, Y.C.; Wei, K.C.; et al. Long-Term Complications of Cranioplasty Using Stored Autologous Bone Graft, Three-Dimensional Polymethyl Methacrylate, or Titanium Mesh After Decompressive Craniectomy: A Single-Center Experience After 596 Procedures. World Neurosurg. 2019, 128, e841–e850. [Google Scholar] [CrossRef] [PubMed]
- Giese, H.; Meyer, J.; Unterberg, A.; Beynon, C. Long-term complications and implant survival rates after cranioplastic surgery: A single-center study of 392 patients. Neurosurg. Rev. 2021, 44, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Cabbad, N.C.; Stalder, M.W.; Arroyave, A.; Wolfe, E.M.; Wolfe, S.A. Autogenous Bone Cranioplasty: Review of a 42-Year Experience by a Single Surgeon. Plast. Reconstr. Surg. 2019, 143, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bao, C.; Xu, H.H.K.; Pan, J.; Hu, J.; Wang, P.; Luo, E. Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats. Acta Biomater. 2016, 42, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Liu, Y.; Ding, Z.Y.; Cao, J.Q.; Huang, J.H.; Zhang, J.Y.; Jia, W.T.; Wang, J.; Liu, C.S.; Li, X.L. Synergistic effects of dimethyloxallyl glycine and recombinant human bone morphogenetic protein-2 on repair of critical-sized bone defects in rats. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Huang, Y.; Han, D.; Zhang, J.; Cao, J.; Jin, X.; Huang, J.; Li, X.; Wang, T. Three-dimensional poly (epsilon-caprolactone)/hydroxyapatite/collagen scaffolds incorporating bone marrow mesenchymal stem cells for the repair of bone defects. Biomed. Mater. 2016, 11, 025005. [Google Scholar] [CrossRef]
- Mondal, T.; Subhash, S.; Vaid, R.; Enroth, S.; Uday, S.; Reinius, B.; Mitra, S.; Mohammed, A.; James, A.R.; Hoberg, E.; et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Gordon, F.E.; Nutt, C.L.; Cheunsuchon, P.; Nakayama, Y.; Provencher, K.A.; Rice, K.A.; Zhou, Y.; Zhang, X.; Klibanski, A. Increased expression of angiogenic genes in the brains of mouse meg3-null embryos. Endocrinology 2010, 151, 2443–2452. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Q.; Zhang, K.S.; Hu, B.; Niu, X.; Zhou, S.M.; Li, S.G.; Luo, Y.P.; Wang, Y.; Deng, Z.F. Downregulation of the Long Non-Coding RNA Meg3 Promotes Angiogenesis After Ischemic Brain Injury by Activating Notch Signaling. Mol. Neurobiol. 2017, 54, 8179–8190. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zeng, X.; Miao, J.; Liu, C.; Wei, F.; Liu, D.; Zheng, Z.; Ting, K.; Wang, C.; Guo, J. Upregulation of long noncoding RNA MEG3 inhibits the osteogenic differentiation of periodontal ligament cells. J. Cell. Physiol. 2019, 234, 4617–4626. [Google Scholar] [CrossRef]
- Zhao, L.D.; Xu, W.C.; Cui, J.; Liang, Y.C.; Cheng, W.Q.; Xin, B.C.; Song, J. Long non-coding RNA maternally expressed gene 3 inhibits osteogenic differentiation of human dental pulp stem cells via microRNA-543/smad ubiquitin regulatory factor 1/runt-related transcription factor 2 axis. Arch. Oral Biol. 2020, 118, 104838. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zhang, Y.; Ma, L.; Lin, L.; Meng, J.; Jiang, L.; Wang, L.; Zhou, P.; Zhang, Y. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed. Pharmacother. 2017, 89, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hong, H.; Zhang, X.; Chen, D.; Chen, Z.; Ling, J.; Wu, L. Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2061–2067. [Google Scholar] [CrossRef]
- Li, Z.; Jin, C.; Chen, S.; Zheng, Y.; Huang, Y.; Jia, L.; Ge, W.; Zhou, Y. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol. Cell. Biochem. 2017, 433, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Ge, X.; Yang, S.; Huang, M.; Zhuang, W.; Chen, P.; Zhang, X.; Fu, J.; Qu, J.; Li, B. Upregulation of lncRNA MEG3 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells From Multiple Myeloma Patients By Targeting BMP4 Transcription. Stem Cells 2015, 33, 1985–1997. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Wang, D.; Zhang, Y.; Chen, J.; Li, B.; Su, S.; Wei, L.; You, H.; Fang, Y.; et al. Hypermethylation-mediated downregulation of long non-coding RNA MEG3 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells and promotes pediatric aplastic anemia. Int. Immunopharmacol. 2021, 93, 107292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Lin, L.P.; Zou, R.; Zhao, Q.H.; Lin, F.Q. Silencing long non-coding RNA MEG3 accelerates tibia fraction healing by regulating the Wnt/beta-catenin signalling pathway. J. Cell. Mol. Med. 2019, 23, 3855–3866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.W.; Zhao, M. Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadeh, A.; Norozi, F.; Shahrabi, S.; Shahjahani, M.; Saki, N. Wnt/beta-catenin signaling in bone marrow niche. Cell Tissue Res. 2016, 363, 321–335. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; He, X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a007880. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, D.; Yin, H.; Wang, S.; Li, C.; Chen, E.; Hu, D.; Tao, Y.; Yu, J.; Zheng, Q.; et al. Overexpression of HSPA1A enhances the osteogenic differentiation of bone marrow mesenchymal stem cells via activation of the Wnt/beta-catenin signaling pathway. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Han, H.; Tian, T.; Huang, G.; Li, D.; Yang, S. The lncRNA H19/miR-541-3p/Wnt/beta-catenin axis plays a vital role in melatonin-mediated osteogenic differentiation of bone marrow mesenchymal stem cells. Aging 2021, 13, 18257–18273. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, W.; Sun, S.; Zhang, T.; Zhang, T.; Huang, H.; Wang, M. Microfibrillar-associated protein 5 regulates osteogenic differentiation by modulating the Wnt/beta-catenin and AMPK signaling pathways. Mol. Med. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Song, L.; Liu, M.; Ono, N.; Bringhurst, F.R.; Kronenberg, H.M.; Guo, J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J. Bone Miner. Res. 2012, 27, 2344–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Huang, M. Long non-coding RNA MEG3 promotes the proliferation of glioma cells through targeting Wnt/beta-catenin signal pathway. Cancer Gene Ther. 2017, 24, 381–385. [Google Scholar] [CrossRef]
- Li, L.; Pei, S.; Sun, N. MEG3 targets miR-184 and Wnt/beta-catenin and modulates properties of osteosarcoma. Front. Biosci. 2020, 25, 1901–1912. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, S.C.; Qiao, X.F.; Kong, Y.; Liu, J.G.; Peng, X.M.; Wang, Y.X.; Abdulkarim Mohammed Al-Mohana, R.A. LncRNA MEG3 promotes proliferation and differentiation of osteoblasts through Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4521–4529. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/beta-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Xie, W.; Shang, Q.; Su, B. The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. BioMed Res. Int. 2015, 2015, 356893. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, H.; Zheng, M.Q.; Dong, Q.R. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage 2019, 13, 1274S–1284S. [Google Scholar] [CrossRef]
- Chen, S.; Jia, L.; Zhang, S.; Zheng, Y.; Zhou, Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res. Ther. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jia, L. Long noncoding RNAs related to the odontogenic potential of dental mesenchymal cells in mice. Arch. Oral Biol. 2016, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.A.; Williams, B.O. Wnt signaling in bone and muscle. Bone 2015, 80, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Ashrafizaveh, S.; Ashrafizadeh, M.; Zarrabi, A.; Husmandi, K.; Zabolian, A.; Shahinozzaman, M.; Aref, A.R.; Hamblin, M.R.; Nabavi, N.; Crea, F.; et al. Long non-coding RNAs in the doxorubicin resistance of cancer cells. Cancer Lett. 2021, 508, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-kappaB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef] [PubMed]

| Gene | Direction | Primer Sequence (5′-3′) |
|---|---|---|
| human GAPDH | Forward | ATCCCATCACCATCTTCC |
| Reverse | GAGTCCTTCCACGATACCA | |
| human ALP | Forward | GTTTTCTGTTCTGTAAGACGGG |
| Reverse | GCCGTTAATTGACGTTCCGA | |
| human RUNX-2 | Forward | CCGAGCTACGAAATGCCTCT |
| Reverse | GGACCGTCCACTGTCACTTT | |
| human OCN | Forward | CCCCCTCTAGCCTAGGACC |
| Reverse | ACCAGGTAATGCCAGTTTGC | |
| human MEG3 | Forward | GTTGAGCCTTCAGTGTCTGCAT |
| Reverse | GCTTTGGAACCGCATCACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qi, X.; Wang, X.-H.; Miao, H.-S.; Xue, Z.-C.; Zhang, L.-L.; Zhao, S.-H.; Wu, L.-H.; Gao, G.-Y.; Lou, M.-Q.; et al. Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/β-Catenin Signaling Pathway. J. Clin. Med. 2022, 11, 395. https://doi.org/10.3390/jcm11020395
Liu J, Qi X, Wang X-H, Miao H-S, Xue Z-C, Zhang L-L, Zhao S-H, Wu L-H, Gao G-Y, Lou M-Q, et al. Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/β-Catenin Signaling Pathway. Journal of Clinical Medicine. 2022; 11(2):395. https://doi.org/10.3390/jcm11020395
Chicago/Turabian StyleLiu, Juan, Xin Qi, Xiao-Hong Wang, Hong-Sheng Miao, Zi-Chao Xue, Le-Le Zhang, San-Hu Zhao, Liang-Hao Wu, Guo-Yi Gao, Mei-Qing Lou, and et al. 2022. "Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/β-Catenin Signaling Pathway" Journal of Clinical Medicine 11, no. 2: 395. https://doi.org/10.3390/jcm11020395
APA StyleLiu, J., Qi, X., Wang, X.-H., Miao, H.-S., Xue, Z.-C., Zhang, L.-L., Zhao, S.-H., Wu, L.-H., Gao, G.-Y., Lou, M.-Q., & Yi, C.-Q. (2022). Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/β-Catenin Signaling Pathway. Journal of Clinical Medicine, 11(2), 395. https://doi.org/10.3390/jcm11020395







