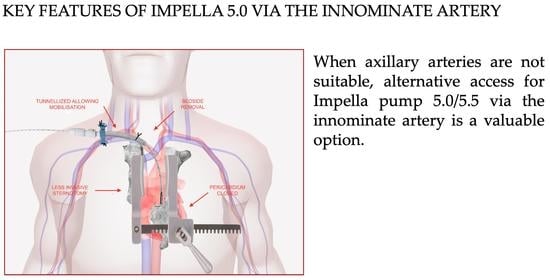
Impella 5.0/5.5 Implantation via Innominate Artery: Further Expanding the Opportunities for Temporary Mechanical Circulatory Support
Abstract
:1. Introduction
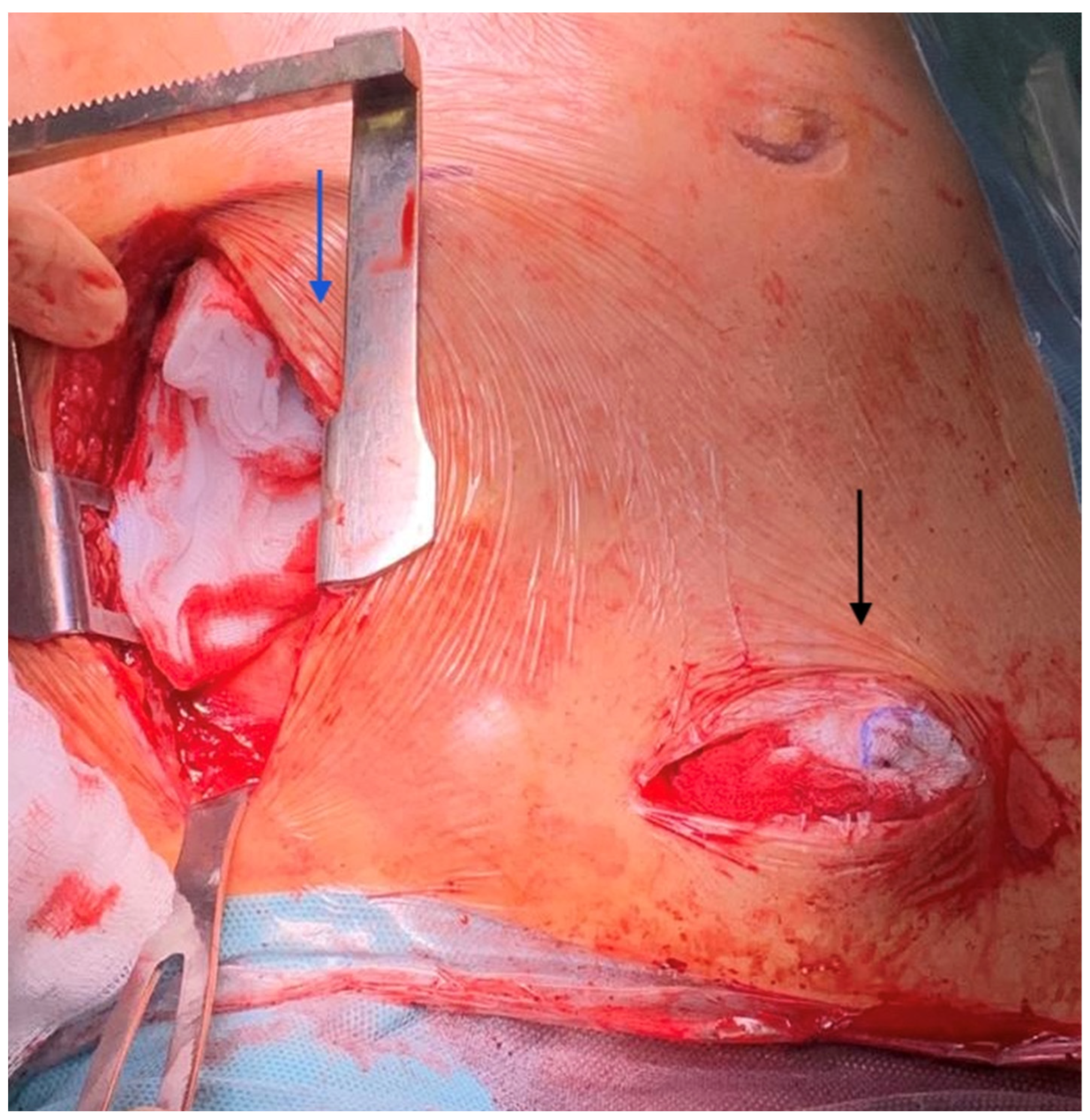
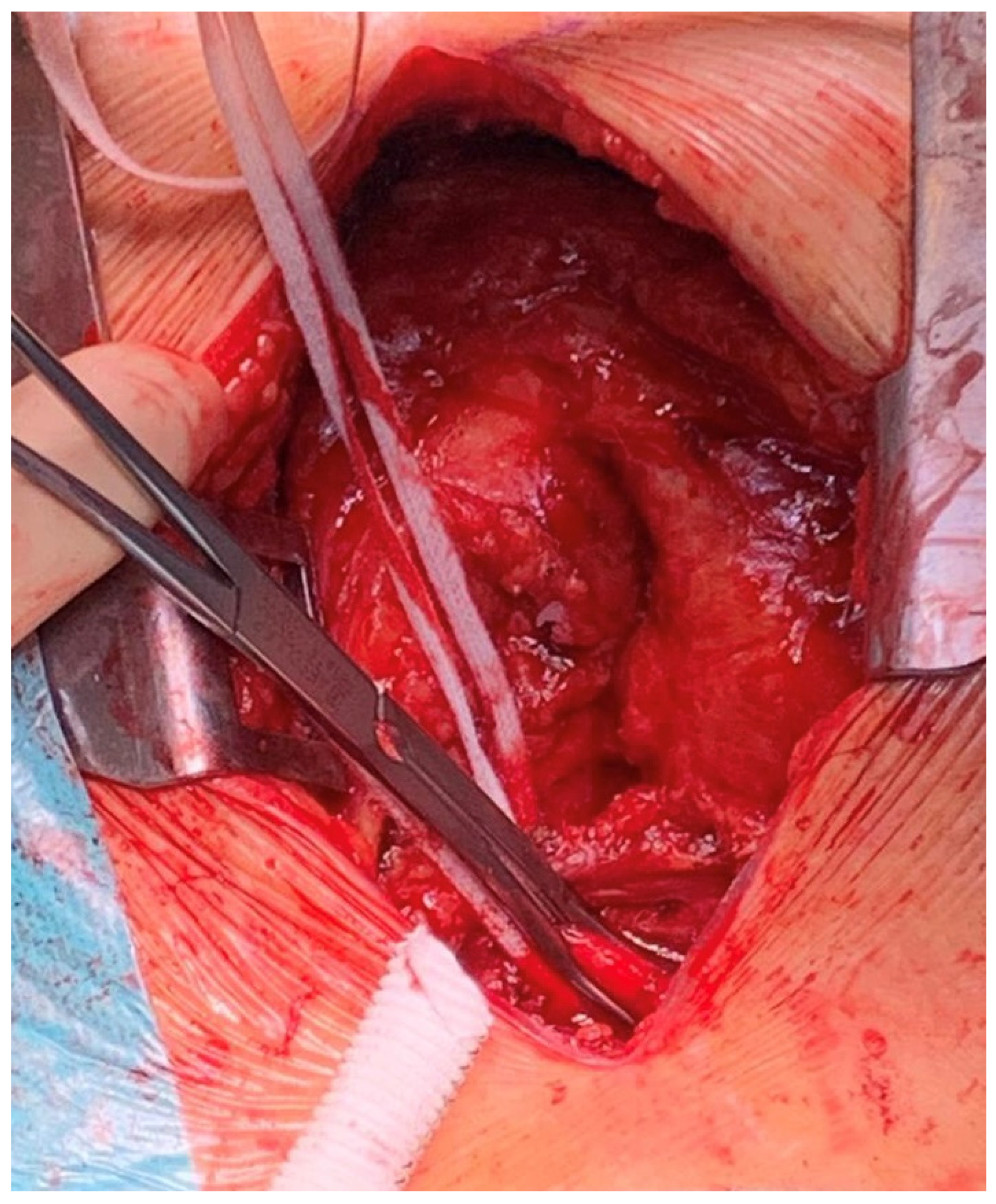
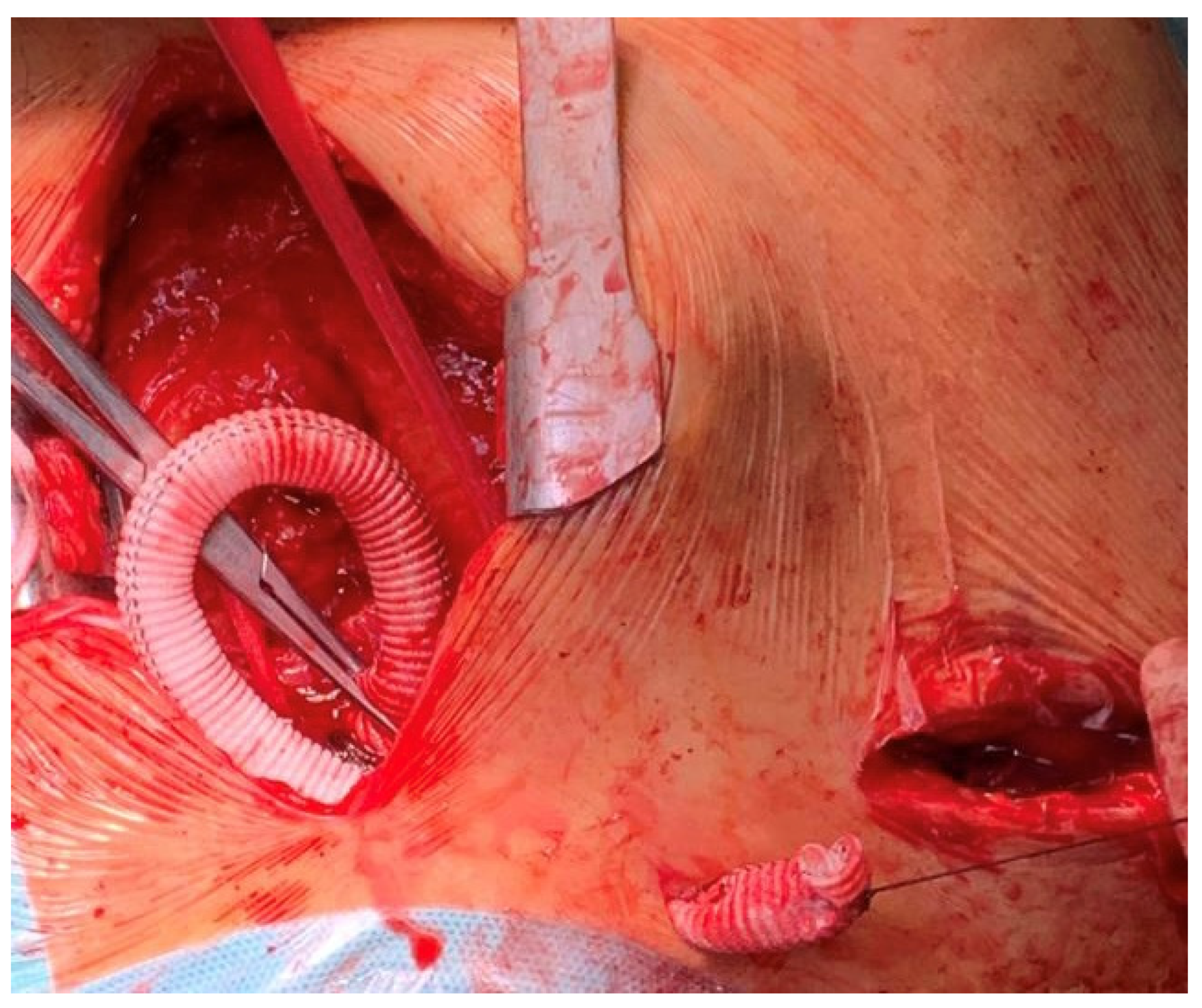
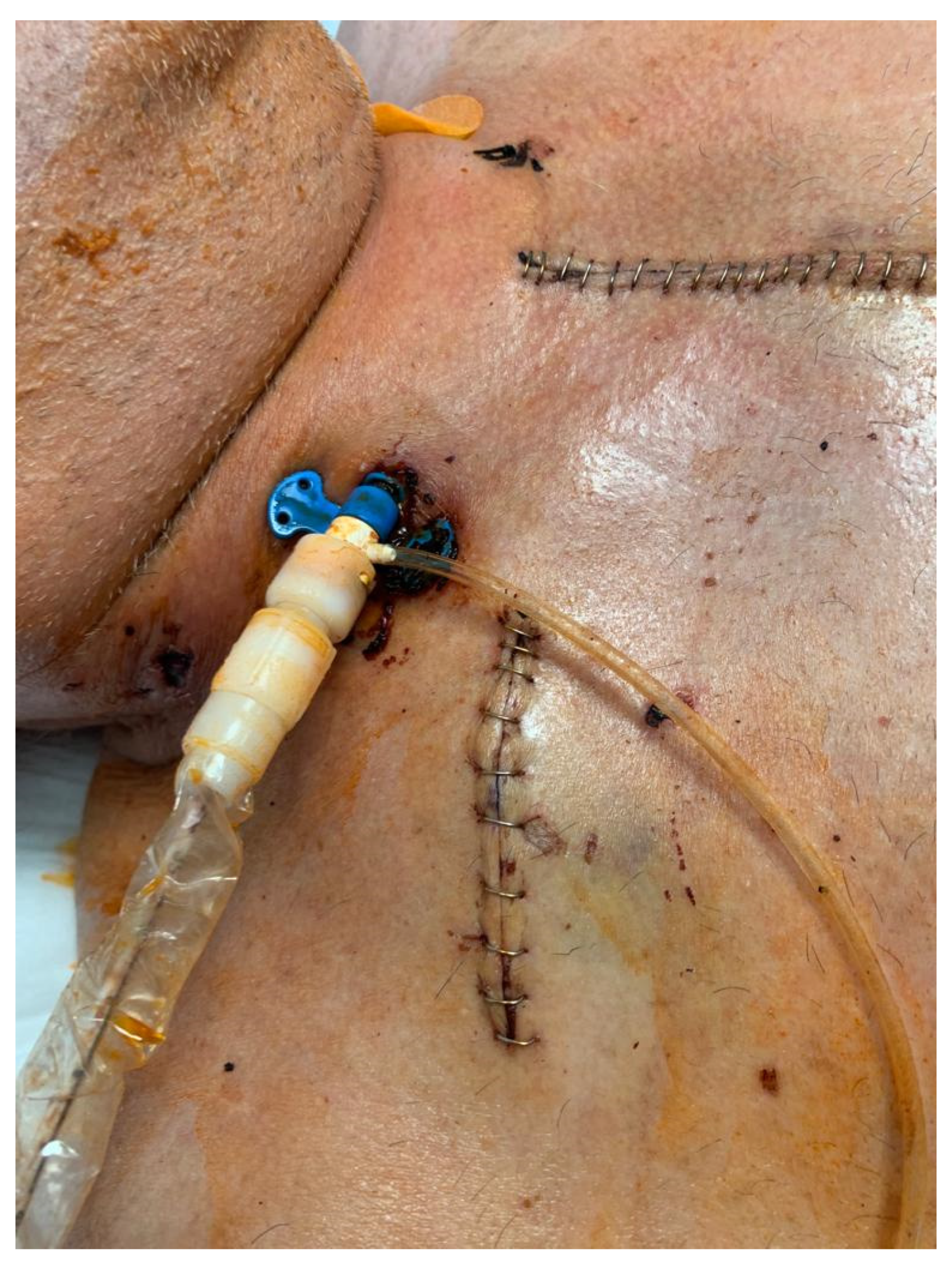
2. Technique
- -
- Reduction of the surgical trauma is pivotal in patients on ECMO to reduce bleeding, assuming that anticoagulation and ECMO-associated coagulopathy are systematically considered. Many patients might also receive DAPT because of the ischemic etiology of cardiogenic shock and recent PCI.
- -
- Avoidance of opening the pericardium might warrant better preservation of right ventricular function, allowing safe de-escalation to isolated left ventricular support from the ECPella configuration.
- -
- Upper body strategies are pursued due to their benefits for patient management: feasibility in severe peripheral artery disease, easy mobilization on support, and early rehabilitation. Indeed, as soon as the hemodynamic status improves, the patient is able to get out of bed, rest in an armchair, walk around the room, undergo phydiotherapy, and start oral feeding.
- -
- Bedside weaning without reopening of the surgical route is an option.
- -
- Proximity to the aortic valve overcomes the technical issues encountered via the axillary approach (calcification, tortuosity under the clavicle, small vessel size).
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VA-ECMO | venoarterial extracorporeal membrane oxygenation |
| RV | right ventricle |
| INTERMACS | interagency registry for mechanically assisted circulatory support |
| LVAD | left ventricular assist device |
| LIMA | left internal mammary artery |
| LAD | left anterior descending artery |
| DAPT | dual antiplatelet therapy |
| PCI | percutaneous coronary intervention |
| RHF | right heart failure |
| LIS | less invasive surgery |
| CPB | cardiopulmonary bypass |
| TAVI | transcatheter aortic valve implantation |
References
- Pahuja, M.; Hernandez-Monfort, J.; Whitehead, E.H.; Kawabori, M.; Kapur, N.K. Device profile of the Impella 5.0 and 5.5 system for mechanical circulatory support for patients with cardiogenic shock: Overview of its safety and efficacy. Expert Rev. Med. Devices 2022, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, L.F.; Pappalardo, F.; Lubos, E.; Grahn, H.; Rybczinski, M.; Barten, M.J.; Legros, T.; Bertoglio, L.; Schrage, B.; Westermann, D.; et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J. Crit. Care 2020, 57, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Jablonski, J.; Kras, A.; Krasney, S.; Kapur, N.K. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int. J. Artif. Organs 2018, 41, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Katsarou, M.; Scandroglio, M.; Bertoldi, L.; Chiesa, R.; Pappalardo, F. Surgical transaxillary placement of the Impella 5.0 ventricular assist device. J. Card. Surg. 2019, 34, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, A.; Fahr, F.; Khan, A.; Bireta, C.; Nozdrzykowski, M.; Feder, S.; Deshmukh, N.; Jubeh, M.; Eifert, S.; Jawad, K.; et al. Minimally invasive ventricular assist device implantation. J. Thorac. Dis. 2021, 13, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Hudziak, D.; Hajder, A.; Gocol, R.; Malinowski, M.; Kazmierski, M.; Morkisz, L.; Ciosek, J.; Wanha, W.; Jarosinski, G.; Parma, R.; et al. Long-Term Clinical Outcomes and Carotid Ultrasound Follow-Up of Transcarotid TAVI. Prospective Single-Center Registry. J. Clin. Med. 2021, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, I.; Nguyen, S.N.; Barry, O.; Bacha, E.A.; Goldstone, A.B. Trans-innominate Impella 5.5 insertion as a bridge to transplantation in a pediatric patient in refractory cardiogenic shock. JTCVS Tech. 2022, 14, 201–203. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolin, S.; Maj, G.; Cavozza, C.; Cardinale, A.; Pullara, A.; Audo, A.; Pappalardo, F. Impella 5.0/5.5 Implantation via Innominate Artery: Further Expanding the Opportunities for Temporary Mechanical Circulatory Support. J. Clin. Med. 2022, 11, 5917. https://doi.org/10.3390/jcm11195917
Bertolin S, Maj G, Cavozza C, Cardinale A, Pullara A, Audo A, Pappalardo F. Impella 5.0/5.5 Implantation via Innominate Artery: Further Expanding the Opportunities for Temporary Mechanical Circulatory Support. Journal of Clinical Medicine. 2022; 11(19):5917. https://doi.org/10.3390/jcm11195917
Chicago/Turabian StyleBertolin, Stephanie, Giulia Maj, Corrado Cavozza, Astrid Cardinale, Alberto Pullara, Andrea Audo, and Federico Pappalardo. 2022. "Impella 5.0/5.5 Implantation via Innominate Artery: Further Expanding the Opportunities for Temporary Mechanical Circulatory Support" Journal of Clinical Medicine 11, no. 19: 5917. https://doi.org/10.3390/jcm11195917
APA StyleBertolin, S., Maj, G., Cavozza, C., Cardinale, A., Pullara, A., Audo, A., & Pappalardo, F. (2022). Impella 5.0/5.5 Implantation via Innominate Artery: Further Expanding the Opportunities for Temporary Mechanical Circulatory Support. Journal of Clinical Medicine, 11(19), 5917. https://doi.org/10.3390/jcm11195917