The Impact of New Treatments on Short- and MID-Term Outcomes in Bilateral Lung Transplant: A Propensity Score Study
Abstract
1. Introduction
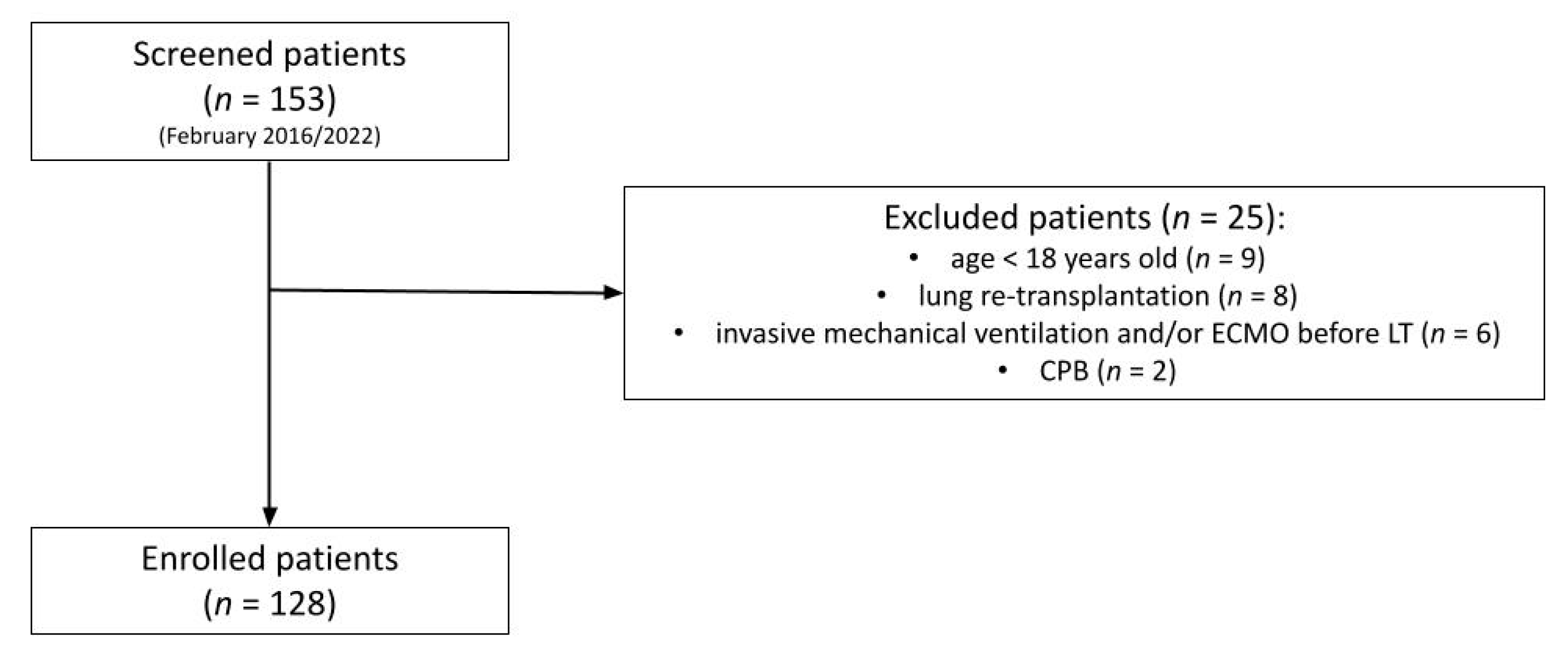
2. Materials & Methods
2.1. Study Population
2.2. Study Outcomes
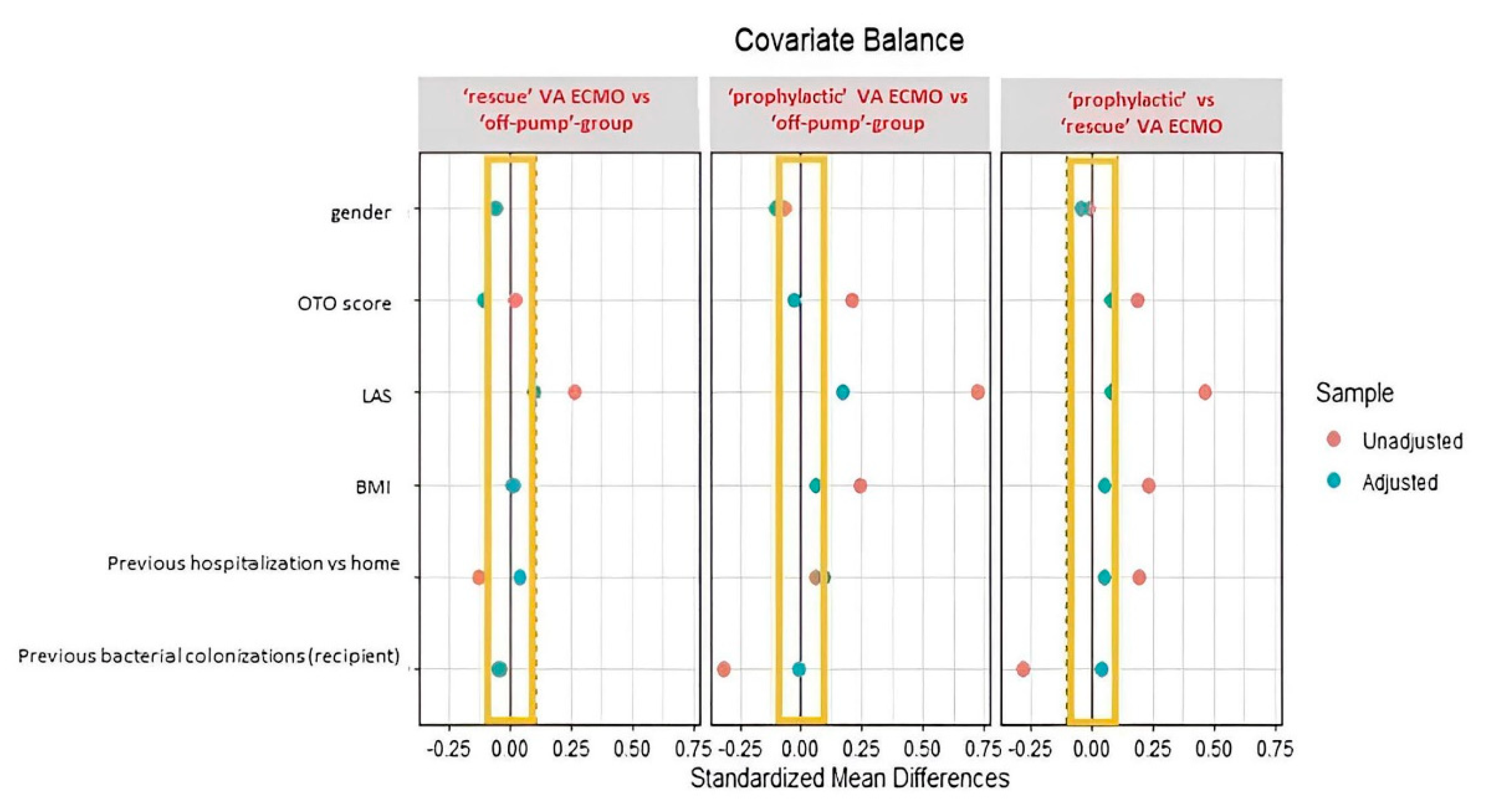
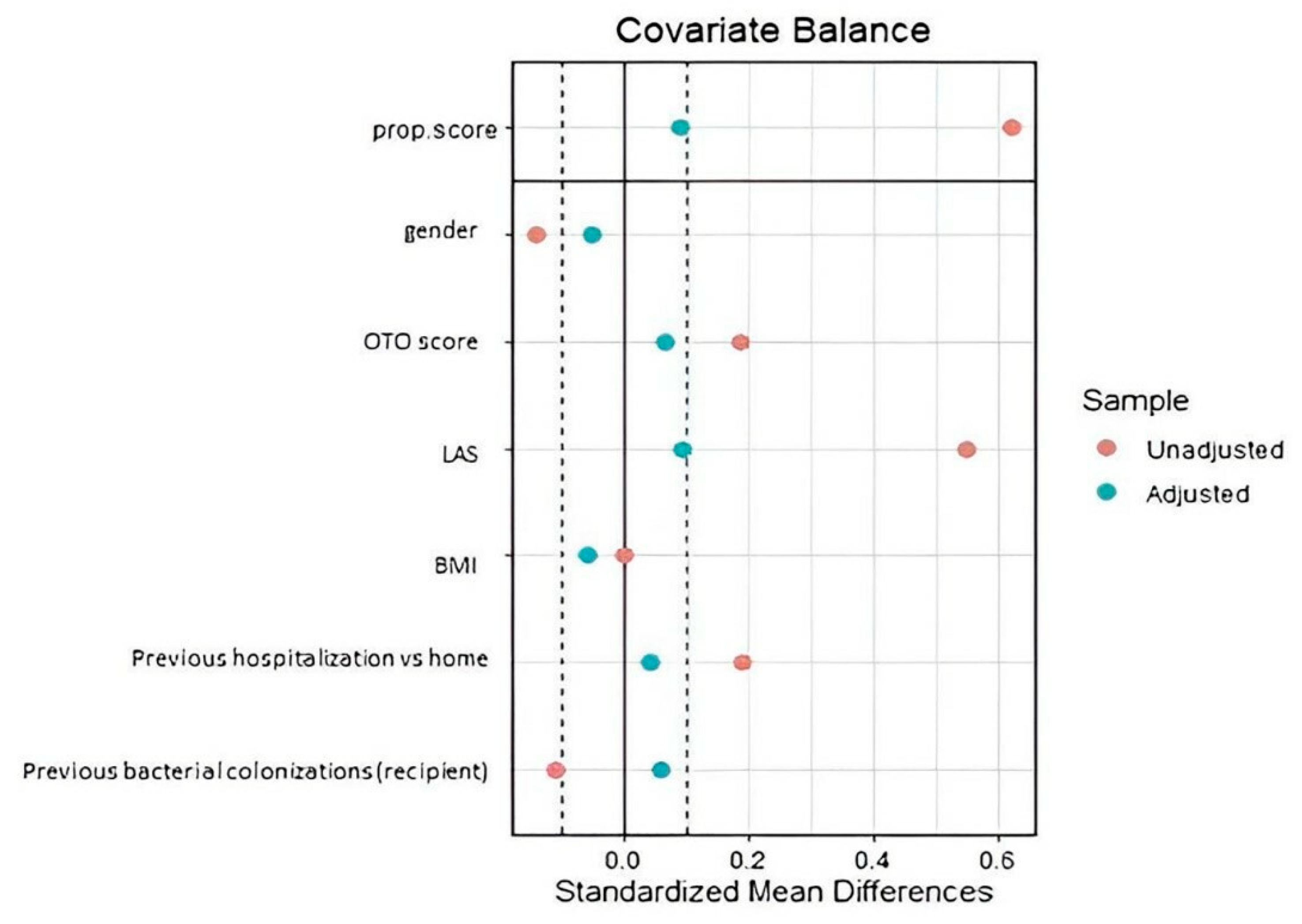
2.3. Statistical Analysis
3. Results
3.1. ‘ Off-Pump’-Group versus ‘Prophylactic’ or ‘Rescue’ VA ECMO
3.2. Tacrolimus versus Cyclosporine-Treated Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AME | average marginal effect |
| BMI | body mass index |
| CI | confidence interval |
| CPB | cardio-pulmonary bypass |
| ECMO | extracorporeal membrane oxygenation |
| ESBL | extended spectrum beta-lactamase |
| GN | Gram-negative |
| HR | hazard ratio |
| H | hospital |
| ICU | intensive care unit |
| IQR | interquartile ranges |
| io | intraoperative |
| IMV | invasive mechanical ventilation |
| LOS | length of stay |
| LAS | lung-allocation score |
| LT | lung transplant |
| MDR | multidrug-resistant |
| PGD | primary graft dysfunction |
| POD | post-operative day |
| po | postoperative |
| RRT | renal replacement therapy |
| VA ECMO | veno-arterial extracorporeal membrane oxygenation |
References
- Di Nardo, M.; Tikkanen, J.; Husain, S.; Singer, L.G.; Cypel, M.; Ferguson, N.D.; Keshavjee, S.; Del Sorbo, L. Posoperative Management of Lung Transplant Recipients in the Intensive Care Unit. Anesthesiology 2022, 136, 482–499. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Ruszel, N.; Kiełbowski, K.; Piotrowska, M.; Kubisa, M.; Grodzki, T.; Wójcik, J.; Kubisa, B. Central, peripheral ECMO or CPB? Comparsion between circulatory support methods used during lung transplantation. J. Cardiothorac. Surg. 2021, 16, 341. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]
- Gajkowski, E.F.; Herrera, G.; Hatton, L.; Antonini, M.V.; Vercaemst, L.; Cooley, E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO J. 2022, 68, 133–152. [Google Scholar] [CrossRef]
- Fessler, J.; Sage, E.; Roux, A.; Feliot, E.; Gayat, E.; Pirracchio, R.; Parquin, F.; Cerf, C.; Fischler, M.; Le Guen, M. Is Extracorporeal Membrane Oxygenation Withdrawal a Safe Option after Double-Lung Transplantation? Ann. Thorac. Surg. 2020, 110, 1167–1174. [Google Scholar] [CrossRef]
- Andreasson, A.; Hoetzenecker, K. Commentary: Why a routine venoarterial extracorporeal membrane oxygenation support strategy is a good idea in lung transplantation. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W.; Kifjak, D.; Baron, D.; Hager, H.; et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1. [Google Scholar] [CrossRef]
- Song, J.H.; Park, J.E.; Lee, J.G.; Lee, C.Y.; Nam, K.S.; Suh, J.W.; Kim, A.; Lee, S.H.; Joo, H.C.; Youn, Y.N.; et al. Outcomes of perioperative extracorporeal membrane oxygenation use in patients undergoing lung transplantation. J. Thorac. Dis. 2017, 9, 5075–5084. [Google Scholar] [CrossRef]
- Marasco, S.F.; Vale, M.; Preovolos, A.; Pellegrino, V.; Lee, G.; Snell, G.; Williams, T. Institution of extracorporeal membrane oxygenation late after lung transplantation-A futile exercise? Clin. Transplant. 2012, 26, E71–E77. [Google Scholar] [CrossRef]
- Faccioli, E.; Terzi, S.; Pangoni, A.; Lomangino, I.; Rossi, S.; Lloret, A.; Cannone, G.; Marino, C.; Catelli, C.; Dell’Amore, A. Extracorporeal membrane oxygenation in lung transplantation: Indications, techniques and results. World J. Transplant. 2021, 11, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Gratz, J.; Pausch, A.; Schaden, E.; Baierl, A.; Jaksch, P.; Erhart, F.; Hoetzenecker, K.; Wiegele, M. Low molecular weight heparin versus unfractioned heparin for anticoagulation during perioperative extracorporeal membrane oxygenation: A single center experience in 102 lung transplant patients. Artif. Organs 2020, 44, 638–646. [Google Scholar] [CrossRef]
- Chung, P.A.; Dilling, D.F. Immunosuppressive strategies in lung transplantation. Ann. Transl. Med. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.B.; Lyster, H.; Lindenfeld, J.; Doligalski, C.; Baran, D.; Yost, C.; Shullo, M.; Schweiger, M.; Weill, D.; Stuckey, L.; et al. Report from the 2018 consensus conference on immunomodulating agents in thoracic transplantation: Access, formulations, generics, therapeutic drug monitoring, and special populations. J. Heart Lung Transplant. 2020, 39, 1050–1069. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Jaksch, P.; Klepetko, W.; Hoetzenecker, K. Immunosuppression after lung transplantation: The search for the holy grail continues. J. Thorac. Dis. 2017, 9, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Aguado, J.; Silva, J.; Fernández-Ruiz, M.; Cordero, E.; Fortún, J.; Gudiol, C.; Martínez-Martínez, L.; Vidal, E.; Almenar, L.; Almirante, B.; et al. Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant. Rev. 2017, 32, 36–57. [Google Scholar] [CrossRef]
- Marczin, N.; de Waal, E.E.; Hopkins, P.M.; Mulligan, M.S.; Simon, A.; Shaw, A.D.; Van Raemdonck, D.; Neyrinck, A.; Gries, C.J.; Algotsson, L.; et al. International consensus recommendations for anesthetic and intensive care management of lung transplantation. An EACTAIC, SCA, ISHLT, ESOT, ESTS, and AST approved document. J. Heart Lung Transplant. 2021, 40, 1327–1348. [Google Scholar] [CrossRef]
- Dell’Amore, A.; Campisi, A.; Congiu, S.; Mazzarra, S.; Pastore, S.; Dolci, G.; Baiocchi, M.; Frascaroli, G. Extracorporeal life support during and after bilateral sequential lung transplantation in patients with pulmonary artery hypertension. Artif. Organs 2019, 44, 628–637. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Frommlet, F.; Schweiger, T.; Bata, O.; Jaksch, P.; Ahmadi, N.; Muraközy, G.; et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2018, 155, 2193–2206.e3. [Google Scholar] [CrossRef]
- Saldanha, I.J.; Akinyede, O.; Robinson, K.A. Immunosuppressive drug therapy for preventing rejection following lung transplantation in cystic fibrosis. Cochrane Database Syst. Rev. 2018, 6, CD009421. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Rahimi, N.; Kifjak, D.; Muckenhuber, M.; Watzenböck, M.; Benazzo, A.; Jaksch, P.; Knapp, S.; Klepetko, W.; Hoetzenecker, K.; et al. Comparison of donor scores in bilateral lung transplantation—A large single-center analysis. Am. J. Transplant. 2020, 21, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Miller, R.; Tumin, D.; Stewart, W.C.; Tobias, J.D.; Hayes, D. Lung Allocation Score Thresholds Prioritize Survival After Lung Transplantation. Chest 2019, 156, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Huddleston, S.; Hartwig, M.; Bottiger, B.; Daoud, D.; Wei, Q.; Zhang, Q.; Ius, F.; Warnecke, G.; Villavicencio, M.A.; et al. Effect of mode of intraoperative support on primary graft dysfunction after lung transplant. J. Thorac. Cardiovasc. Surg. 2022. [Google Scholar] [CrossRef]
- Bolliger, M.; Kroehnert, J.-A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Avtaar Singh, S.S.; Banner, N.R.; Rushton, S.; Simon, A.R.; Berry, C.; Al-Attar, N. ISHLT Primary Graft Dysfunction Incidence, Risk Factors, and Outcome: A UK National Study. Transplantation 2019, 103, 336–343. [Google Scholar] [CrossRef]
- Imai, K.; Ratkovic, M. Covariate balancing propensity score. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2013, 76, 243–263. [Google Scholar] [CrossRef]
- Team: R: A language and environment for statistical...-Google Scholar [Internet]. Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2018&author=R+Core+Team&title=R%3A+A+Language+and+Environment+for+Statistical+Computing (accessed on 1 January 2022).
- Verhelst, N.D. Exponential family models for continuous responses. In Theoretical and Practical Advances in Computer-Based Educational Measurement; Springer: Cham, Switzerland, 2019; pp. 135–160. [Google Scholar]
- Weighting for Covariate Balance in Observational Studies [Internet]. Available online: https://ngreifer.github.io/WeightIt/ (accessed on 13 May 2022).
- Marginal Effects for Model Objects [Internet]. Available online: https://thomasleeper.com/margins/ (accessed on 13 May 2022).
- Ius, F.; Aburahma, K.; Boethig, D.; Salman, J.; Sommer, W.; Draeger, H.; Poyanmehr, R.; Avsar, M.; Siemeni, T.; Bobylev, D.; et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J. Heart Lung Transplant. 2020, 39, 915–925. [Google Scholar] [CrossRef]
- Machuca, T.N.; Collaud, S.; Mercier, O.; Cheung, M.; Cunningham, V.; Kim, S.J.; Azad, S.; Singer, L.; Yasufuku, K.; de Perrot, M.; et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1152–1157. [Google Scholar] [CrossRef]
- Pettenuzzo, T.; Faggi, G.; Di Gregorio, G.; Schiavon, M.; Marulli, G.; Gregori, D.; Rea, F.; Ori, C.; Feltracco, P. Blood Products Transfusion and Mid-Term Outcomes of Lung Transplanted Patients Under Extracorporeal Membrane Oxygenation Support. Prog. Transplant. 2018, 28, 314–321. [Google Scholar] [CrossRef]
- Treede, H.; Glanville, A.R.; Klepetko, W.; Aboyoun, C.; Vettorazzi, E.; Lama, R.; Bravo, C.; Knoop, C.; Aubert, J.-D.; Reichenspurner, H. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: Results of a prospective, randomized international trial in lung transplantation. J. Heart Lung Transplant. 2012, 31, 797–804. [Google Scholar] [CrossRef]
- Hachem, R.R.; Yusen, R.D.; Chakinala, M.M.; Meyers, B.F.; Lynch, J.P.; Aloush, A.A.; Patterson, G.A.; Trulock, E.P. A Randomized Controlled Trial of Tacrolimus Versus Cyclosporine After Lung Transplantation. J. Heart Lung Transplant. 2007, 26, 1012–1018. [Google Scholar] [CrossRef]
- Jing, L.; Chen, W.; Guo, L.; Zhao, L.; Liang, C.; Chen, J.; Wang, C. Acute kidney injury after lung transplantation: A narrative review. Ann. Transl. Med. 2021, 9, 717. [Google Scholar] [CrossRef]
- Pouch, S.M.; Patel, G.; the AST Infectious Diseases Community of Practice. Multidrug-resistant Gram-negative bacterial infections in solid organ transplant recipients—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13594. [Google Scholar] [CrossRef]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef]
| ‘Off-Pump’ N = 47 (37) | ‘Prophylactic’ VA ECMO N = 51 (40) | ‘Rescue’ VA ECMO N = 30 (23) | p-Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 50 [43, 60] | 56 [48, 61] | 50 [40, 58] | 0.201 |
| M, n (%) | 31 (66) | 32 (63) | 19 (63) | 0.999 |
| F, n (%) | 16 (34) | 19 (37) | 11 (37) | |
| BMI, kg/m2 | 22 [20, 27] | 24 [20, 28] | 23 [21, 25] | 0.550 |
| Corticosteroids, n (%) | 25 (53) | 24 (47) | 23 (77) | 0.030 |
| O2 therapy, n (%) | 41 (87) | 44 (86) | 30 (100) | 0.108 |
| Diabetes, n (%) | 6 (13) | 12 (24) | 5 (17) | 0.421 |
| Chronic renal disease, n (%) | 1 (2) | 0 (0) | 1 (3) | 0.474 |
| Oto score | 3 (1–5) | 3 [2, 5] | 3 [1, 5] | 0.400 |
| LAS | 34 [33, 38] | 37 [34, 44] | 37 [34, 40] | 0.003 |
| Underlying diseases * | ||||
| Septic, n (%) | 14 (30) | 9 (18) | 6 (20) | 0.031 |
| Interstitial, n (%) | 17 (36) | 33 (65) | 15 (50) | |
| Obstructive, n (%) | 10 (21) | 9 (18) | 4 (13) | |
| Others, n (%) | 6 (13) | 0 (0) | 5 (17) | |
| Previous colonization | ||||
| Recipient-related, n (%) | 18 (38) | 12 (24) | 11 (37) | 0.200 |
| None, n (%) | 29 (62) | 39 (76) | 19 (63) | |
| Provenience | ||||
| Hospital, n (%) | 3 (6) | 4 (8) | 1 (3) | 0.900 |
| Home, n (%) | 44 (94) | 47 (92) | 29 (97) |
| ‘Off-Pump’ N = 47 (37) | ‘Prophylactic’ VA ECMO N = 51 (40) | ‘Rescue’ VA ECMO N = 30 (23) | p-Value | ||
|---|---|---|---|---|---|
| Surgical characteristics | |||||
| Time of LT, minutes | 420 [370, 470] | 413 [371, 450] | 490 [450, 533] | 0.001 | |
| Time of graft ischemia, minutes | 565 [460, 630] | 585 [473, 678] | 573 [480, 715] | 0.662 | |
| Io fluid support, mL | 4200 [3400, 5175] | 4250 [2925, 5640] | 5600 [4050, 7850] | 0.010 | |
| ‘prolonged’ ECMO, n (%) | 0 (0) | 11 (22) | 12 (40) | 0.001 | |
| During hospitalization | |||||
| Surgical revisions, n (%) | 6 (13) | 10 (20) | 11 (37) | 0.041 | |
| Surgical bleeding, n (%) | 2 (4) | 5 (10) | 6 (20) | 0.083 | |
| Thromboembolic/ischemic events, n (%) | 2 (4) | 1 (2) | 2 (7) | 0.570 | |
| Invasive mechanical ventilation, hours | 22 [18, 36] | 47 [24, 154] | 60 [36, 434] | 0.001 | |
| Clavien-Dindo score | 21 [0, 46] | 0 [0, 42] | 47 [5, 61] | 0.001 | |
| Bacteria isolation, n (%) | 23 (49) | 13 (25) | 11 (37) | 0.055 | |
| 30-day acute cellular rejection *, n (%) | 14 (31) | 7 (16) | 4 (17) | 0.083 | |
| Primary outcome | OR a, p-value | OR b, p-value | |||
| PGD at 72 h (ref. PGD ≥ 2) | 1 [0, 2] 14 (37) | 0 [0, 2] 16 (33) | 2 [1, 3] 19 (68) | 0.69 [0.39, 1.24], 0.210 | 3.44 [1.94, 6.10], 0.001 |
| Secondary outcomes | AME a, p-value | AME b, p-value | |||
| Perioperative blood units **, n | 2 [1, 3] | 4 [2, 6] | 8 [4, 14] | 1.08 [−1.72, 3.89], 1 | 8.12 [2.22–14.03], 0.001 |
| ICU LOS, days | 7 [5, 13] | 8 [5, 14] | 17 [9, 32] | −3.80 [−12.18, 4.59], 1 | 9.07 [−3.22, 21.35], 0.414 |
| H LOS, days (%) | 32 [28, 44] | 33 [29, 43] | 38 [31, 48] | −6.63 [−22.47, 9.20], 1 | 4.08 [−13.34, 21.49], 1 |
| OR a, p-value | OR b, p-value | ||||
| Re-intubation and/or tracheostomy, n (%) | 11 (23) | 13 (25) | 13 (43) | 0.63 [0.25, 1.55], 1 | 1.92 [0.87, 4.37], 0.235 |
| Renal dysfunction, n (%) | 13 (28) | 6 (12) | 15 (30) | 0.19 [0.06, 0.53], 1 | 2.05 [0.95, 4.52], 0.098 |
| H mortality, n (%) | 4 (9) | 4 (8) | 7 (23) | 0.33 [0.06, 1.43], 0.472 | 2.46 [0.88, 7.70], 0.189 |
| Overall N = 128 (100) | Tacrolimus N = 42 (33) | Cyclosporine N = 86 (67) | p-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 53 [43, 60] | 55 [42, 61] | 52 [44, 60] | 0.401 |
| M, n (%) | 82 (64) | 25 (60) | 57 (66) | 0.502 |
| F, n (%) | 46 (36) | 17 (40) | 29 (34) | |
| BMI, kg/m2 | 23 [20, 27] | 24 [20, 27] | 23 [21, 27] | 0.999 |
| Corticosteroids, n (%) | 72 (56) | 18 (43) | 54 (63) | 0.040 |
| O2 therapy, n (%) | 115 (90) | 36 (86) | 79 (92) | 0.350 |
| Diabetes, n (%) | 23 (18) | 10 (24) | 13 (15) | 0.230 |
| Chronic renal disease, n (%) | 2 (2) | 0 (0) | 2 (2) | 0.999 |
| Oto score | 3 [1, 5] | 3 [2, 5] | 3 [1, 5] | 0.201 |
| LAS | 36 [33, 40] | 38 [34, 45] | 35 [33, 39] | <0.001 |
| Underlying diseases * | ||||
| Septic, n (%) | 29 (23) | 9 (21) | 20 (23) | 0.010 |
| Interstitial, n (%) | 65 (51) | 26 (62) | 39 (45) | |
| Obstructive, n (%) | 23 (18) | 7 (17) | 16 (19) | |
| Others, n (%) | 11 (9) | 0 (0) | 11 (13) | |
| Previous colonization | ||||
| Recipient-related, n (%) | 41 (32) | 12 (29) | 29 (34) | 0.600 |
| None, n (%) | 87 (68) | 30 (71) | 57 (66) | |
| Provenience | ||||
| Hospital, n (%) | 8 (6) | 4 (10) | 4 (5) | |
| Home, n (%) | 120 (94) | 38 (90) | 82 (95) | 0.400 |
| Overall N = 128 (100) | Tacrolimus N = 42 (33) | Cyclosporine N = 86 (67) | p-Value | |
|---|---|---|---|---|
| Surgical characteristics | ||||
| Time of LT, minutes | 435 [376, 484] | 430 [375, 490] | 448 [405, 498] | 0.010 |
| Time of graft ischemia, minutes | 568 [480, 655] | 580 [510, 655] | 568 [480, 660] | 0.980 |
| Surgical revisions, n (%) | 27 (21) | 5 (12) | 22 (26) | 0.110 |
| Surgical bleeding, n (%) | 13 (10) | 3 (7) | 10 (12) | 0.541 |
| Primary outcomes | OR a, p-Value | |||
| 30-day acute cellular rejection *, n (%) | 25 (22) | 3 (8) | 22 (29) | 0.21 [0.09, 0.48], 0.010 |
| Secondary outcomes | OR a, p-Value | |||
| Re-tracheal intubation and/or tracheostomy, n (%) | 37 (29) | 8 (19) | 29 (34) | 0.33 [0.14, 0.73], 0.002 |
| Renal dysfunction, n (%) | 34 (27) | 3 (7) | 31 (36) | 0.10 [0.03, 0.28], 0.001 |
| Bacteria isolation, n (%) | 47 (37) | 9 (21) | 38 (44) | 0.41 [0.19, 0.85], 0.009 |
| H mortality, n (%) | 15 (12) | 1 (2) | 14 (16) | 0.04 [0.01, 0.28], 0.008 |
| AME b, p-Value | ||||
| ICU LOS, days | 9 [6, 18] | 7 [5, 14] | 10 [6, 22] | −8.07 [−14.56, −1.57], 0.006 |
| H LOS, days | 33 [28, 46] | 33 [30, 44] | 33 [28, 46] | −7.14 [−18.11, 4.30], 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscolo, A.; Dell’Amore, A.; Pettenuzzo, T.; Sella, N.; De Cassai, A.; Pistollato, E.; Cacco, N.; Manzan, A.; De Carolis, A.; Geraldini, F.; et al. The Impact of New Treatments on Short- and MID-Term Outcomes in Bilateral Lung Transplant: A Propensity Score Study. J. Clin. Med. 2022, 11, 5859. https://doi.org/10.3390/jcm11195859
Boscolo A, Dell’Amore A, Pettenuzzo T, Sella N, De Cassai A, Pistollato E, Cacco N, Manzan A, De Carolis A, Geraldini F, et al. The Impact of New Treatments on Short- and MID-Term Outcomes in Bilateral Lung Transplant: A Propensity Score Study. Journal of Clinical Medicine. 2022; 11(19):5859. https://doi.org/10.3390/jcm11195859
Chicago/Turabian StyleBoscolo, Annalisa, Andrea Dell’Amore, Tommaso Pettenuzzo, Nicolò Sella, Alessandro De Cassai, Elisa Pistollato, Nicola Cacco, Andrea Manzan, Agnese De Carolis, Federico Geraldini, and et al. 2022. "The Impact of New Treatments on Short- and MID-Term Outcomes in Bilateral Lung Transplant: A Propensity Score Study" Journal of Clinical Medicine 11, no. 19: 5859. https://doi.org/10.3390/jcm11195859
APA StyleBoscolo, A., Dell’Amore, A., Pettenuzzo, T., Sella, N., De Cassai, A., Pistollato, E., Cacco, N., Manzan, A., De Carolis, A., Geraldini, F., Lorenzoni, G., Pezzuto, F., Zambello, G., Schiavon, M., Calabrese, F., Gregori, D., Cozzi, E., Rea, F., & Navalesi, P. (2022). The Impact of New Treatments on Short- and MID-Term Outcomes in Bilateral Lung Transplant: A Propensity Score Study. Journal of Clinical Medicine, 11(19), 5859. https://doi.org/10.3390/jcm11195859










