Clinicopathological Correlation of Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Study
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Clinical Assessment
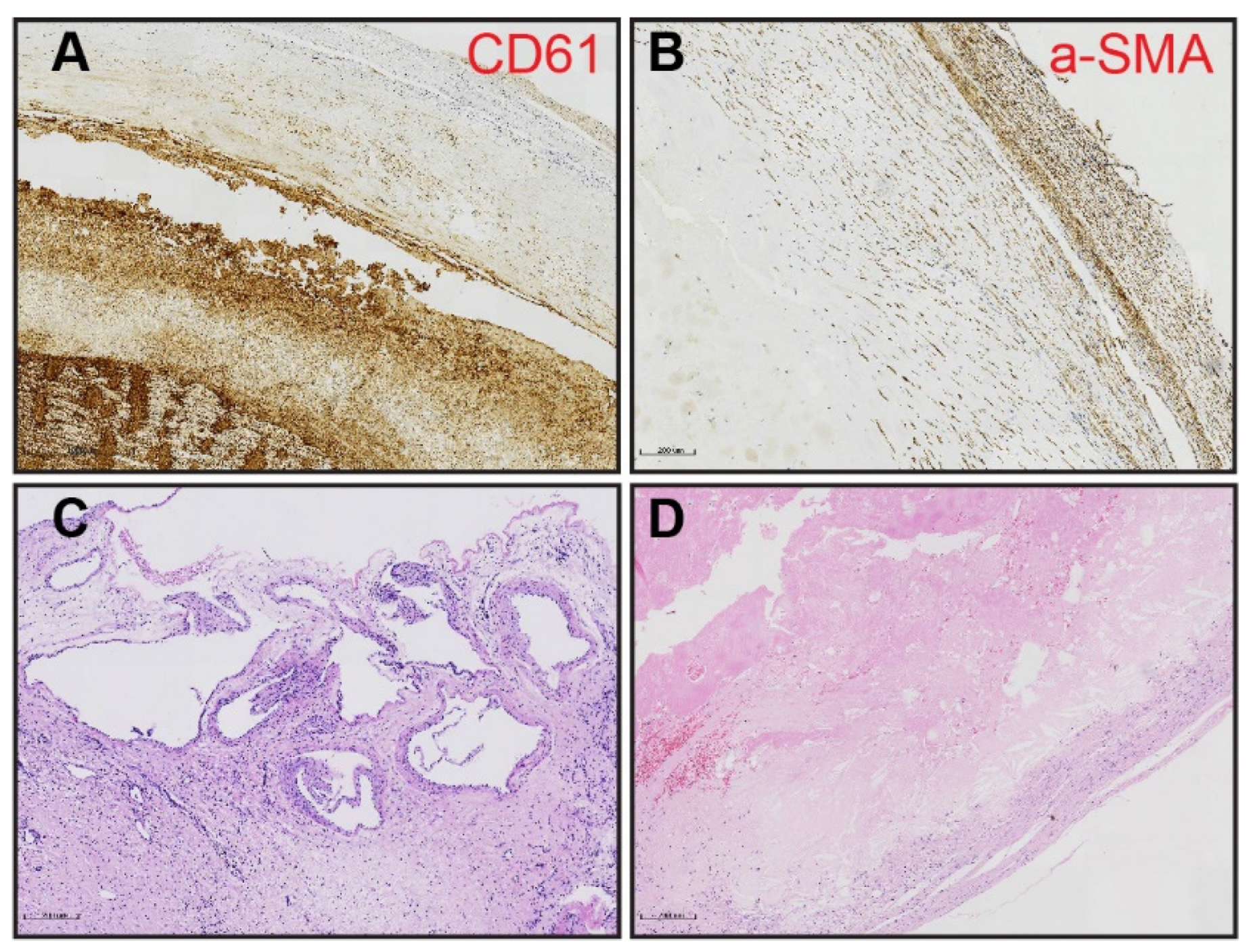
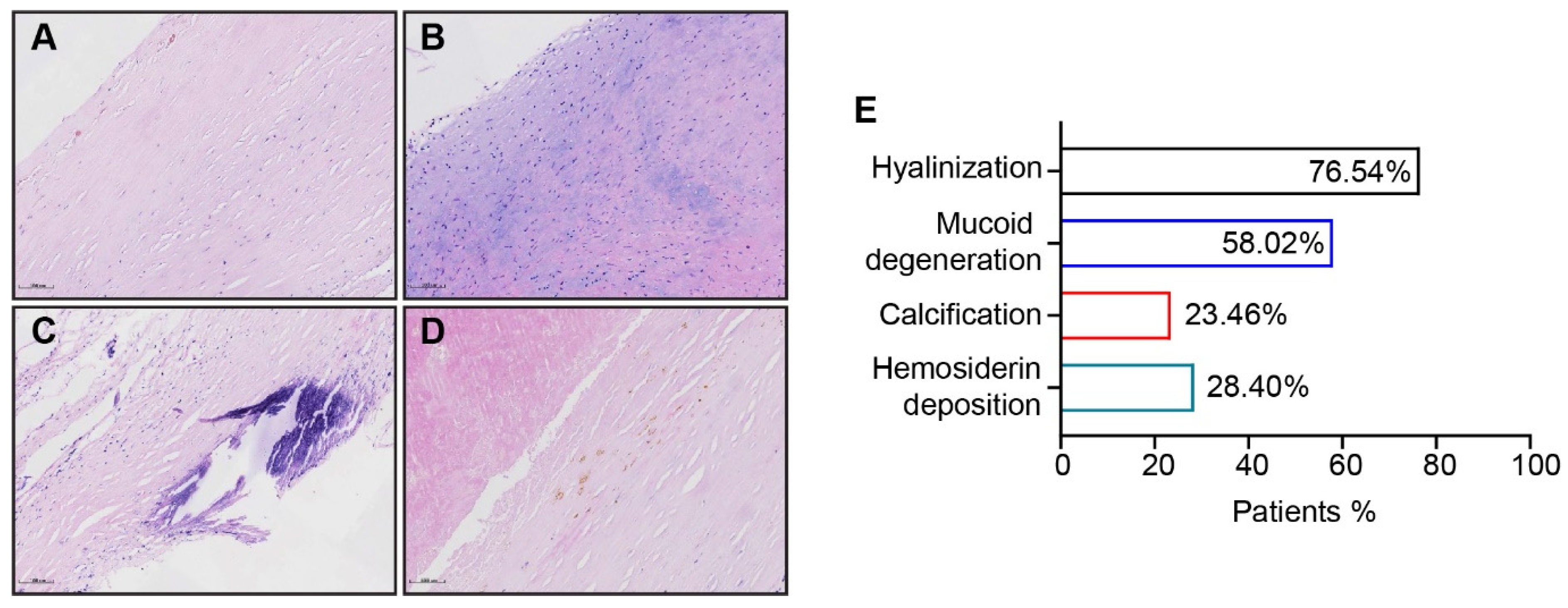
2.3. Morphometric Analysis of PEA Specimens
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics and Surgical Outcome
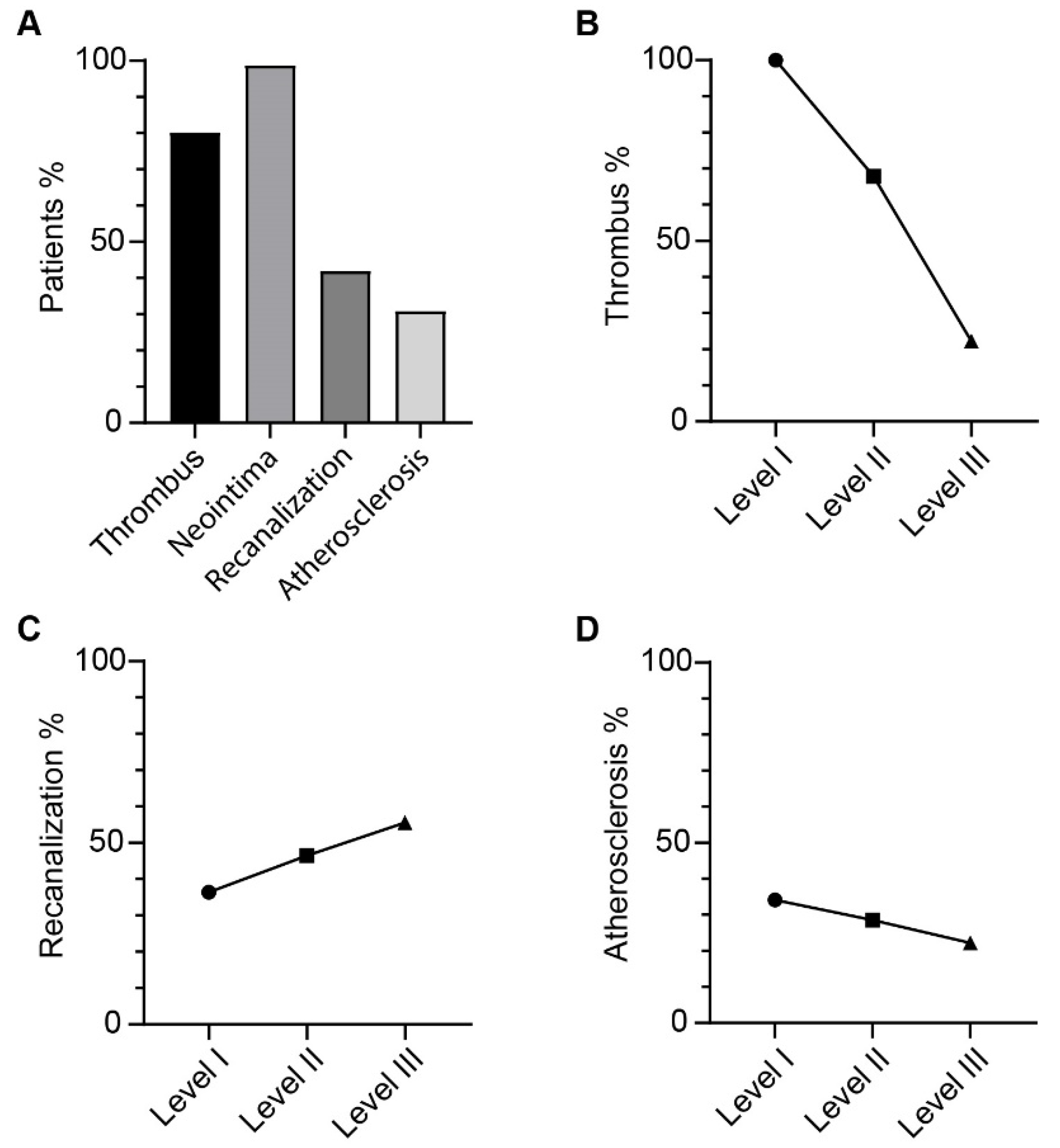
3.2. Morphometric Analysis of Thromboembolic Disease
3.3. Atherosclerotic Lesion Formation Corresponds with Main Pulmonary Artery Dilation in CTEPH Patients
3.4. Patients with Complex Lesions have Greater Haemodynamic Improvement after PEA
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nonstandard Abbreviations
| CTEPH | chronic thromboembolic pulmonary hypertension |
| PEA | pulmonary endarterectomy |
| BPA | balloon pulmonary angioplasty |
| PH | pulmonary hypertension |
| PA/AA | ratio of pulmonary artery diameter to ascending aorta diameter |
| mPAP | mean pulmonary arterial pressure |
| PE | pulmonary embolism |
| DVT | deep vein thrombosis |
| PVR | pulmonary vascular resistance |
| mPCWP | mean pulmonary capillary wedge pressure |
| CO | cardiac output |
| ECM | extracellular matrix |
References
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Kerr, K.; Fedullo, P. Chronic Thromboembolic Pulmonary Hypertension. Epidemiology and Risk Factors. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 3), S201–S206. [Google Scholar] [CrossRef]
- Jenkins, D.; Madani, M.; Fadel, E.; D’Armini, A.M.; Mayer, E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160111. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Mayer, E.; Simonneau, G.; Rubin, L.J. Chronic thromboembolic pulmonary hypertension. Circulation 2006, 113, 2011–2020. [Google Scholar] [CrossRef]
- Thistlethwaite, P.A.; Mo, M.; Madani, M.M.; Deutsch, R.; Blanchard, D.; Kapelanski, D.P.; Jamieson, S.W. Classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2002, 124, 1203–1211. [Google Scholar] [CrossRef]
- Bernard, J.; Yi, E.S. Pulmonary thromboendarterectomy: A clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum. Pathol. 2007, 38, 871–877. [Google Scholar] [CrossRef]
- Quarck, R.; Wynants, M.; Verbeken, E.; Meyns, B.; Delcroix, M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2015, 46, 431–443. [Google Scholar] [CrossRef]
- Hosokawa, K.; Ishibashi-Ueda, H.; Kishi, T.; Nakanishi, N.; Kyotani, S.; Ogino, H. Histopathological multiple recanalized lesion is critical element of outcome after pulmonary thromboendarterectomy. Int. Heart J. 2011, 52, 377–381. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: Results from the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef]
- Guth, S.; D’Armini, A.M.; Delcroix, M.; Nakayama, K.; Fadel, E.; Hoole, S.P.; Jenkins, D.P.; Kiely, D.G.; Kim, N.H.; Lang, I.M. Current strategies for managing chronic thromboembolic pulmonary hypertension: Results of the worldwide prospective CTEPH Registry. ERJ Open Res. 2021, 7, 00850–02020. [Google Scholar]
- Lippi, G.; Favaloro, E.J. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin. Thromb. Hemost. 2018, 44, 239–248. [Google Scholar]
- Southgate, L.; Machado, R.D.; Gräf, S.; Morrell, N.W. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol. 2020, 17, 85–95. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Sanal, S.; Aronow, W.S.; Ravipati, G.; Maguire, G.P.; Belkin, R.N.; Lehrman, S.G. Prediction of moderate or severe pulmonary hypertension by main pulmonary artery diameter and main pulmonary artery diameter/ascending aorta diameter in pulmonary embolism. Cardiol. Rev. 2006, 14, 213–214. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Iwano, S.; Okumura, N.; Adachi, S.; Abe, S.; Kondo, T.; Kato, K.; Naganawa, S. Assessment of Severity in Chronic Thromboembolic Pulmonary Hypertension by Quantitative Parameters of Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2020, 44, 578–585. [Google Scholar]
- Kivrak, T.; Bolayir, H.A.; Kanar, B.G.; Akaslan, D.; Kepez, A.; Mutlu, B.; Yildizeli, B. Prevelance of pulmonary atherosclerosis in patients with chronic thromboembolic pulmonary hypertension. J. Cardiovas. Thoracic. Surg. 2017, 2, 15–79. [Google Scholar] [CrossRef]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.; Prins, M.H.; Girolami, A. An association between atherosclerosis and venous thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Deng, L.; Quan, R.; Yang, Y.; Yang, Z.; Tian, H.; Li, S.; Shen, J.; Ji, Y.; Zhang, G.; Zhang, C.; et al. Characteristics and long-term survival of patients with chronic thromboembolic pulmonary hypertension in China. Respirology 2021, 26, 196–203. [Google Scholar] [CrossRef]
- Corsico, A.G.; D’Armini, A.M.; Cerveri, I.; Klersy, C.; Ansaldo, E.; Niniano, R.; Gatto, E.; Monterosso, C.; Morsolini, M.; Nicolardi, S.; et al. Long-term outcome after pulmonary endarterectomy. Am. J. Respir. Crit. Care Med. 2008, 178, 419–424. [Google Scholar] [CrossRef]


| Parameters | Case Number |
|---|---|
| Subjects, n | 81 |
| age at PEA years | 53 (41–61) |
| Sex male | 54 (66.67%) |
| BMI (kg·m2) | 24.38 ± 3.53 |
| Disease duration (year) | 2.17 (0.92–5) |
| History of acute PE/DVT | 59 (72.84%) |
| Thrombophilia | 8 (9.88%) |
| WHO functional class I~II WHO functional class III~IV | 28 (34.57%) 51 (62.96%) |
| 6MWD (m) | 379 ± 100 |
| SvO2% | 68.38 ± 11.25 |
| use of PH targeting drugs | 44 (54.32%) |
| pre-/post-operative mPAP (mmHg) | 45 ± 11/26 ± 7 |
| pre-/post-operative PVR (dynes·sec−1·cm−5) | 937 ± 414/470 ± 172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Liu, J.; Wang, B.; Zhang, H.; Zhao, L.; Su, Y.; Xie, W.; Huang, Q.; Zhen, Y.; Lin, F.; et al. Clinicopathological Correlation of Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Study. J. Clin. Med. 2022, 11, 6659. https://doi.org/10.3390/jcm11226659
Chang Z, Liu J, Wang B, Zhang H, Zhao L, Su Y, Xie W, Huang Q, Zhen Y, Lin F, et al. Clinicopathological Correlation of Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Study. Journal of Clinical Medicine. 2022; 11(22):6659. https://doi.org/10.3390/jcm11226659
Chicago/Turabian StyleChang, Ziyi, Jixiang Liu, Bei Wang, Honglei Zhang, Ling Zhao, Yunchao Su, Wanmu Xie, Qiang Huang, Yanan Zhen, Fan Lin, and et al. 2022. "Clinicopathological Correlation of Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Study" Journal of Clinical Medicine 11, no. 22: 6659. https://doi.org/10.3390/jcm11226659
APA StyleChang, Z., Liu, J., Wang, B., Zhang, H., Zhao, L., Su, Y., Xie, W., Huang, Q., Zhen, Y., Lin, F., Liu, M., Gao, Q., Pang, W., Zhang, Z., Tian, H., Li, Y., Yang, P., Zhai, Z., & Zhong, D. (2022). Clinicopathological Correlation of Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Study. Journal of Clinical Medicine, 11(22), 6659. https://doi.org/10.3390/jcm11226659





