1. Introduction
Osteochondritis dissecans (OCD) is a condition describing the focal separation of an articular cartilage and subchondral bone from the remaining articular surface. It affects boys 2–4 times more than girls and is most common between 10 and 20 years of age [
1,
2]. The prevalence ranges from 9.5 to 29 cases per 100,000; however, it could be underestimated because some cases are asymptomatic and are found incidentally [
1,
3]. Typical locations include the knee, talus, and humeral capitellum. OCD mostly affects the knee with approximately 85% of lesions occurring in the medial femoral condyle [
4]. The etiology remains unclear and poorly understood; genetic, traumatic, and vascular causes have all been considered [
4]. OCD can be classified as “juvenile”, when it is diagnosed before physeal closure, and as “adult” in skeletally mature patients. Symptoms can be minor and nonspecific, or more disturbing, such as severe pain, swelling or locking, especially in patients with free loose bodies, [
5].
Correct diagnosis of OCD requires a combination of precise physical examination and imaging studies (MRI stands as the gold standard). Some cases require additional invasive diagnostics during arthroscopic joint inspection. Treatment methods depend on the patient’s age and the size, location and stability of the lesion; methods include trial of non-weightbearing for 3–6 months, cast immobilization, bone marrow stimulation techniques such as transarticular or retroarticular drilling, lesion fixation (open or using arthroscopic techniques) and, least often, excision of fragments [
5,
6,
7]. Multiple implants are used for OCD fixation, including K-wires, cannulated screws, Herbert screws, bone pegs and bioabsorbable pegs and screws. Bioabsorbable fixation devices have been used for years to treat small fractures, osteochondral and chondral lesions [
7,
8,
9]. Recently, a lot of effort has been directed at using more biologic methods of fixation. Therefore, bioabsorbable devices have gained in popularity especially for treating unstable OCD lesions [
10].
There is a certain inaccuracy in the prognosis for OCD. Based on the literature, it is thought that juvenile OCD has a better prognosis than adult OCD. Stable lesions in skeletally immature patients have an excellent prognosis when treated nonoperatively. Surgical treatment is indicated for unstable lesions and after nonoperative treatment failure.
On the contrary, according to many authors, juvenile and adult OCD are very similar regarding the prognosis but discovered at different points of skeletal maturity [
6,
7,
8,
9]. The main limitation of the literature studies of bioabsorbable fixation of OCD in the pediatric population is that they are limited to small series. The aim of this study was to review the literature and evaluate the OCD cases treated with bioabsorbable implants. An additional purpose was to interpret whether the surgical treatment of stable and unstable OCD in children repaired via a bioabsorbable fixation device provides healing and a good clinical outcome.
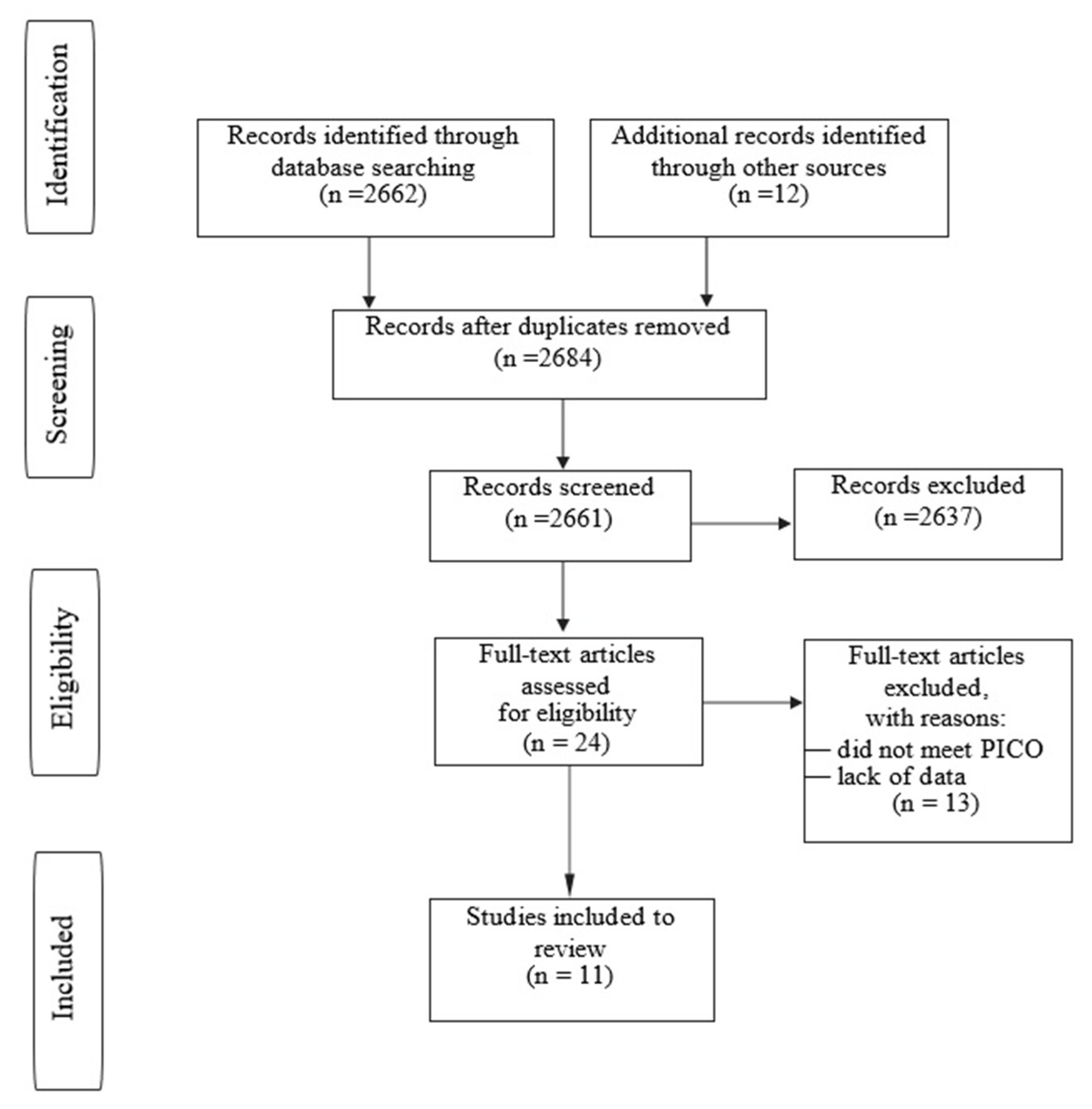
3. Results
The study group included a total of 164 OCD lesions in 158 patients. There were 113 boys (71.5%) and 45 girls (28.5%). The mean age of patients was 13.69 years, and the mean follow-up was 32.43 months. The study included 89 immature patients with open physes and 39 patients with closed physes. There were 30 patients from the Adachi et al. [
14] group; although the study concerned “juvenile OCD”, we couldn’t find the data on whether all of these patients were immature with open physes.
The most common OCD location was MFC—95 cases, then LFC—31 cases, humeral capitellum—22 cases, patella—10 cases, tallus—3 cases and patellar groove—2 cases. Of the 93 patients with available data, the mean size of OCD was 289.13 mm
2. OCD dimensions of the remaining patients are summarized in
Table 2.
The number of bioabsorbable implants (n) used for OCD stabilization depended on lesion size and surgeon experience. The distribution was checked with the Shapiro–Wilk test; it was statistically significant (W = 0.981; p = 0.972) and consistent with the normal distribution. The median was 3.00; therefore notwithstanding the mean, we could conclude that 50% of the implants were at least n > 3.
The average number of bioabsorbable implants used for OCD stabilization was 3 (1–11) depending on lesion size and surgeon experience. In 8 studies, the authors used bioabsorbable pins for OCD stabilization (136 lesions), in 2 studies bioabsorbable screws (27 lesions) and, in last case, one bioabsorbable pin plus one screw. Details are shown in
Table 3.
The analysis included 32 OCD lesions that were stable on MRI (Hefti grade II) that qualified for surgery after conservative treatment failure (after 3 months for the Tabador et al. [
15] group and 6 months for the Komnos et al. [
16] group) and 132 OCD lesions that were unstable on MRI scans. There were 22 cases of detached OCD in the study group. A compilation of OCD grades according to the MRI classification with grades according to the intraoperative classification (if available) is presented in
Table 2. We emphasize that only patients with reduction and ostechondral stabilization with bioabsorbable implants were analyzed; all additional procedures affecting the treatment outcome were excluded from the review. In only one case we found a mechanical implant complication, a broken biodegradable magnesium-based pin. In eight cases, there was a prolonged joint effusion after surgery due to synovitis (knee—four cases; elbow—four cases). Considering the total number of knee joints, synovitis after surgery occurred in 2.8% of cases. For the elbow joint, synovitis occurred in 18.2% of cases. No patient had the implant dislodged or showed destabilization causing secondary damage to adjacent cartilage. In total, 94.86% of postoperative cases showed complete healing or local improvement on follow-up CT or MRI scans; moreover, the great majority of patients achieved a good clinical effect. Treatment results, including healing rates, are presented in the
Table 4. Out of 164 OCD lesions, 10 did not heal (6.09%). Failure cases are presented in the
Table 5.
Table 2.
The study group overview (concerns were marked—*; not available—NA).
Table 2.
The study group overview (concerns were marked—*; not available—NA).
| Author | Study Period | Study Group | Male/Female | Open/Closed Physes | Age [Year] | Follow-Up [Month] | OCD Stage MRI Class | OCD Stage Arthroscopic Class |
|---|
| Ronga et al.[17] | NA | 1 | 1/0 | 1/0 | 11 | 24 | 1 grade IV ICRS | NA |
| Tabaddor et al.[15] | 2000–2006 | 24 | 14/10 | 6/18 | 14.4 (11–16) | 39.6 (19–74) | 11 grade II; 12 grade III; 1 grade V Hefti | 13 grade II; 10 grade III; 1 grade IV Ewing and Voto |
| Takeba et al.[18] | NA | 4 | 4/0 | 2/2 | 14.5 (12–16) | 6 (3–7) | 4 grade III ICRS | NA |
| Camathias et al.[19] | 2005–2009 | 13 patients/16 OCD | 12/4 | 13/0 | 12.3 (11–15) | 27 (10–53) | 2 grade II; 3 grade III; 11 grade IVa Hughes | 4 grade I; 7 grade II; 5 grade III Guhl |
| Adachi et al.[14] | 2002–2010 | 30 patients/33 OCD | 23/7 | 30/0 NA * (juvenile?) | 14.4 (11–17) | 39.6 (25.2–75.6) | 17 grade III; 16 grade IV Nelson’s | NA |
| Galagali et al.[20] | NA | 1 | 0/1 | 1/0 | 14 | 10 | 1 grade IV ICRS | 1 grade IV Ewing and Voto |
| Chun et al.[21] | 2007–2014 | 11 | 10/1 | 2/9 | 16.3 (11–19) | 51 (12–91) | 4 grade II; 7 grade III Dipaola | 5 grade II; 6 grade III Guhl |
| Jungesblut et al.[22] | 2018–2021 | 9 out of 19 patients | 3/6 | 4/5 | 14.22 (12–16) | 11.44 (6–20) | 9 grade III ICRS | NA |
| Komnos et al.[16] | 2004–2016 | 40 * (up to 16 yo) | 28/12 | 40/0 | 13.1 (11–16) | 79.2 (36–156) | 21 grade II; 19 grade III Hefti | 21 grade II; 19 grade III Guhl |
| Zeilinger et al.[23] | 2014–2016 | 7 | 3/4 | 7/0 | 12.1 (10–16) | 29.9 (7–49) | 3 grade 2; 3 grade III; 1 grade IV Dipaola | 3 grade I; 2 grade II; 1 graed III; 1 grade IV Guhl |
| Uchida et al.[24] | 2006–2009 | 18 | 18/0 | 13/5 | 14.28 (12–16) | 39 (36–50) | 7 grade II; 9 grade III; 2 grade IV De Smet | 5 grade II; 11 grade III; 2 grade IV ICRS |
Table 3.
The study group overview (continuation). Not available—NA.
Table 3.
The study group overview (continuation). Not available—NA.
| Author | MFC | LFC | Patella | Patellar Groove | Humeral Capitellum | Tallus | OCD Size Overall | Number of Implants | Pin Type |
|---|
| Ronga et al.[17] | - | - | - | 1 entire | - | - | 550 mm2 | 3 | poly-lactic acid pins (SmartNail, Bionix Implants, Tampere, Finland) |
| Tabaddor et al.[15] | 14 | 5 | 5 | - | - | - | 257 mm2 (40–900) | 2.3 (1–7) | poly-96L/4D-lactide copolymer pins (SmartNail, ConMed, Linvatec, Finland) |
| Takeba et al.[18] | - | - | - | - | 4 | - | 62.75 mm2 (40–96) | 4 (3–5) | poly-L-lactide absorbable pins (GBFDÒ, Stryker, Japan) |
| Camathias et al.[19] | 14 | 2 | - | - | - | - | 244 mm2 (50–961) | 2 (1–3) | poly-96L/4D-lactide copolymer (SmartScrews, ConMed Linvatec, Finland) |
| Adachi et al.[14] | 16 | 11 | 4 | 2 | - | - | 427.9 mm2 ± 197.2 | 3.4 (1–9) | bioabsorbable pins (NEOFIX, Gunze, Kyoto, Japan) |
| Galagali et al.[20] | - | 1 | - | - | - | - | 160 mm2 | 1 pin; 1 screw | NA |
| Chun et al.[21] | 7 | 4 | - | - | - | - | 319 mm2 (120–500) | at least 2/NA | poly-L-lactic acid screws (Arthrex, Naples, FL, USA) |
| Jungesblut et al.[22] | 6 | - | - | - | - | 3 | 292.44 mm2 (60–532) | 3.66 (2–6) | magnesium-based pins (MAGNEZIX Pins, Hannover, Germany) |
| Komnos et al.[16] | 33 | 7 | - | - | - | - | grade II 18.5 (14–26 mm) grade III 20.3 (14–28 mm) | 2.3 (1–4) | poly L-Lactide pins (SmartNail, ConMed, Linvatec, NY, USA) |
| Zeilinger et al.[23] | 5 | 1 | 1 | - | - | - | 3800 mm3 (200–20,200) | 4.7 (3–9) | hydroxyapatite/poly-L-lactic acid pin (u-HA/PLLA, Osteotrans®, Takiron Co Ltd., Osaka, Japan) |
| Uchida et al.[24] | - | - | - | - | 18 | - | NA | 3.1 (1–5) | HA/PLLA thread pins (Super Fixorb 30-thread pin; Takiron Co., Ltd., Osaka, Japan) |
Table 4.
Treatment results overview (concerns were marked—*; not available—NA).
Table 4.
Treatment results overview (concerns were marked—*; not available—NA).
| Author | Clinical Outcome | MRI Findings | Recovery Rate | General Complication | Implants Complication | Synovitis |
|---|
| Ronga et al.[17] | improvement from 90 to 95 Lysholm score | complete healing on MRI | 100% | none | 0 | 0 |
| Tabaddor et al.[15] | improvement from 7.3 to 7.9 Tegner activity score * (2 patients lower score) | interval or complete healing in 15 out of 17 MRI * 17 out of 24 had MRI | 87.5% | 2 out of 24 non-healed needed restabilisation (1 more non-healed with no symptoms-no reoperation) | 0 | 2/24 |
| Takeba et al.[18] | NA; good short-term results | 3 out of 4 healing on CT; 1 improvement on CT | 100% | none | 0 | 0 |
| Camathias et al.[19] | improvement from 1.43 to 3.44 Hughston score | NA | 100% | 2 out of 16 (transient peroneal nerve neurapraxia due to concomitant meniscal repair; saphenous nerve neurapraxia) | 0 | 2/16 |
| Adachi et al.[14] | improvement from 80 to 96 Lysholm score | 32 out of 33 complete healing on MRI | 96.96% | none | 0 | 0 |
| Galagali et al.[20] | NA; 4 Hughston score | compleat healing on CT | 100% | none | 0 | 0 |
| Chun et al.[21] | improvement from 32.6 to 82.8 Lysholm score | 11 out of 11 improvements on MRI (imrovement from 2.6 to 1.27 Dipaola score) | 100% | none | 0 | |
| Jungesblut et al.[22] | NA | 8 out of 9 complete healing on MRI (1 non-healed) | 88.88% | 1 needed second surgery | 1 pin broken | 0 |
| Komnos et al.[16] | improvement from 70.4 to 95.1 Lysholm score | 36 out of 40 complete healing on MRI (4 non-healed) | 90% | 4 needed second surgery | 0 | 0 |
| Zeilinger et al.[23] | improvement from 13 to 15 Ogilvie-Harris scale | 6 out of 7 complete healing on MRI (imrovement from 3 to 1 Dipaola score) | 85.7% | 1 out of 7 non-healed needed restabilisation (OCD 20.2 cm3) | 0 | 0 |
| Uchida et al.[24] | improvement from 68 to 98.06 Mayo Elbow Performance Index | 15 out of 18 healing with mean MOCART score of 87 | 94.44% | 1 out of 18 needed second surgery (loose body) | 0 | 4/18 |
Table 5.
Failure cases overview. Not available—NA.
Table 5.
Failure cases overview. Not available—NA.
| No | Sex | Physes Status | Age [Year] | OCD Stage/MRI | OCD Stage/Arthroscopy | OCD Location | OCD Size | Number of Implants | Revision Surgery |
|---|
| 1. | female | partially closed | 14 | Hefti II | Ewing II | MFC | 16 × 12 mm | 1 | yes/further fixation |
| 2. | male | partially closed | 15 | Hefti II | Ewing III | LFC | 30 × 30 mm | 2 | no/pain free |
| 3. | female | closed | 17 | Hefti V | Ewing IV | MFC | 15 × 15 mm | 2 | yes/further fixation |
| 4. | female | open | 12 | ICRS III | NA | MFC | 19 × 28 mm | 4 | yes/repeated OCD drilling |
| 5. | male | open | 11.6 | Hefti II | NA | MFC | 19 mm | 2 | yes/autologus bone grafting + HA scaffold |
| 6. | male | open | 12.1 | Hefti III | NA | MFC | 20 mm | 3 | yes/autologus chondrocyte implantation |
| 7. | female | open | 13 | Hefti III | NA | LFC | 24 mm | 3 | yes/autologus chondrocyte implantation |
| 8. | female | open | 13 | Hefti III | NA | MFC | 24 mm | 3 | yes/autologus chondrocyte implantation |
| 9. | NA | closed | 16 | Dipaola IV | Guhl III | MFC | 43 × 47 × 10 mm | 4 | yes/refixation with metal screws |
| 10. | male | open | 14 | IV ICRS | NA | humeral capitellum | 10 × 8 × 4.5 mm | 3 | yes/lesion removal |
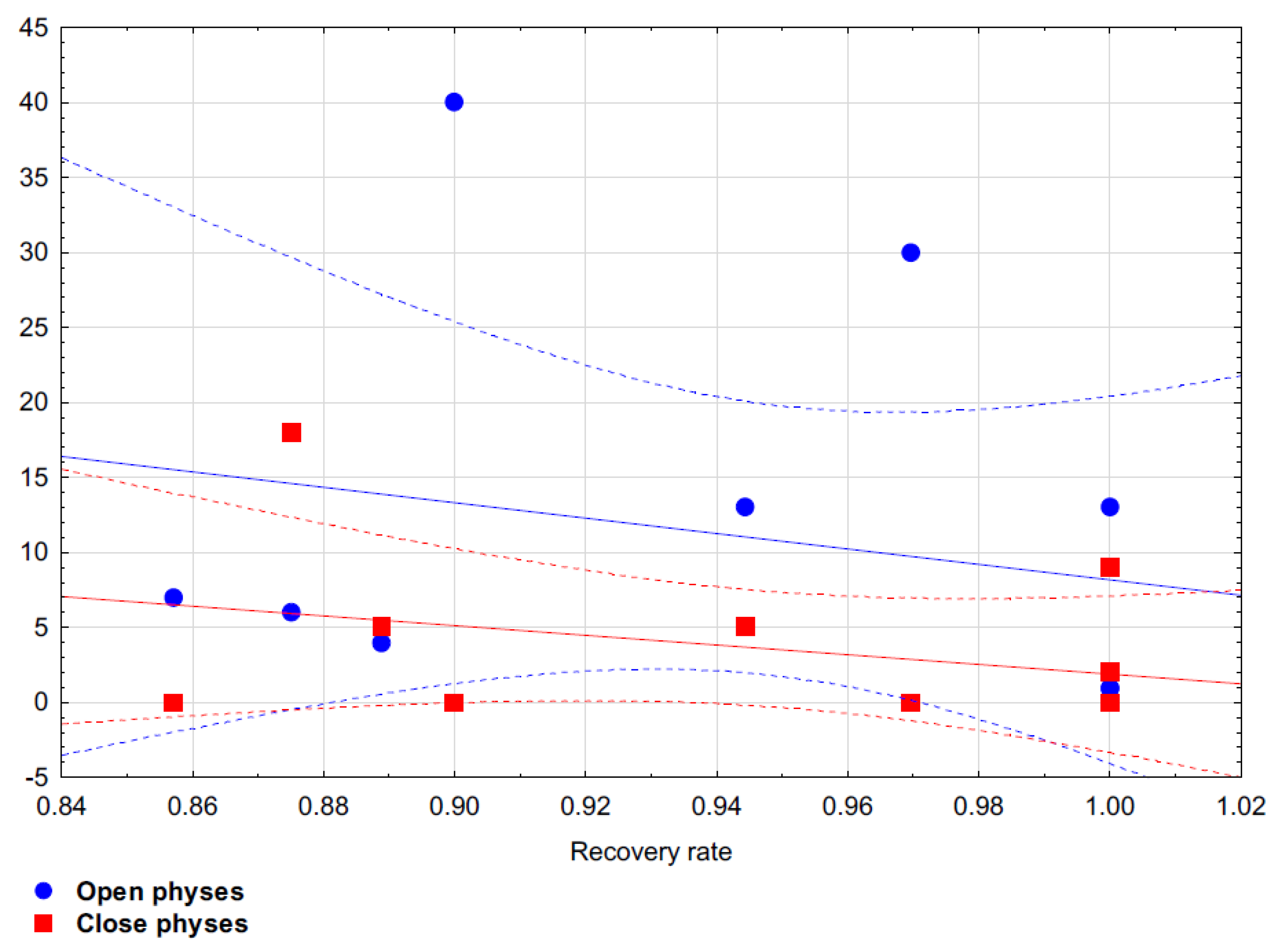
After applying the principal components method with Varimax factor rotation, two factors were identified. All analyzed variables explained 63.96% of the total variance. It was shown in the first construct that the recovery rate was better if there were fewer patients with closed physes in the study (
Table 6). On the other hand, the second construct showed an association between fewer implants used for stabilization and fewer patients with open physes (
Table 6). Based on Spearman’s rank correlation coefficient it has been proven that there is no statistical correlation between OCD size and recovery rate (Rho = −0.191;
p = 0.651) and there is no statistical correlation between number of implants used and recovery rate (Rho = −0.479;
p = 0.136). Spearman’s rank correlation coefficient has shown that there is statistical correlation between the ratio of open to closed physes and recovery rate: Rho = −0.470;
p = 0.144 for patients with open physes and Rho = −0.209;
p = 0.538 for patients with closed physes.
Figure 4 shows the dispersion between the analyzed variables.
4. Discussion
The crucial finding of this review is that the surgical treatment of stable and unstable OCD in children with the use of bioabsorbable implants is a good method that facilitates a high rate of healing and a good clinical outcome. For patients with nonoperative treatment failure on stable OCD lesions and patients with unstable lesions the healing rate was 94.86%. Our review is in line with a recent, large case series, of 47 adolescent patients treated with bioabsorbable implants by Bradley at al., which reports 87.2% of patients returning to full activities or permitted to return to full activities [
25]. That study was excluded from our review because it contained OCD cases and osteochondral fractures with no clear demarcation between them. There is no gold standard on treating OCD lesions, furthermore, the definition of OCD stability is not precisely defined. Moreover, the natural history of untreated OCD, like the prognosis of treated OCD, is also poorly defined. However, it is confirmed that, if lesions persist despite an adequate nonoperative treatment they have the potential to detach [
5]. The period of time destined for nonoperative management was variable and ranged from 3 to 6 months for the included studies. The heterogeneity of the length of time for clinical improvement before proceeding to surgery constitutes a limitation of this review. Magnetic resonance imaging is currently considered the gold standard for the evaluation of OCD. However, inconsistency of preoperative OCD classifications within a variety of MRI classification systems is another limitation. This heterogeneity, together with the lack of standardization in OCD classification and the eligibility for surgical treatment makes comparative assessment challenging and mainly limited to a descriptive assessment. Many techniques for the treatment of OCD are described. Antegrade or retrograde OCD drilling has been well documented in the literature, but as standalone procedures they are reserved for stable lesions. Kocher et al. reported 100% efficacy of retrograde transarticular drilling in immature patients [
9]. Furthermore, Anderson et al. reported that antegrade drilling gave an 83% healing rate in skeletally immature patients [
26]. While reviewing the literature, there were also reports that drilling alone had no influence on the OCD outcome [
2]. Such dissonance about treatment results may raise doubts in the system of decision making for appropriate treatment method.
Unstable lesions require the internal fixation of the osteochondral fragment in order to improve the healing [
2]. Many kinds of metal implant are used for OCD stabilization, including cannulated screws, Herbert screws and metal staples [
27,
28,
29,
30,
31].
There are many reports of both skeletally mature and immature patients with very good OCD treatment results (more than 90% healing rate) using metal implants [
28,
29,
30]. A great advantage of metal devices is the possibility of early postsurgical mobilization with implementation of the rehabilitation program. Metal implants are associated with MRI interference, which makes noninvasive assessment of the healing process difficult. In our opinion, this is the main factor which should limit the use of metal implants in the OCD treatment. Although they provide good stability, there are also some mechanical complications associated with these implants, such as loosening or damage requiring additional surgical procedures [
28]. That is why the use of bioabsorbable implants in the treatment of OCD becomes so important and has recently come into favor. The main advantages include gradual load transfer to bone during the implant resorption and no need for subsequent implant removal. There are also some concerns about less rigid fixation with bioabsorbable pins which could eventually lead to nonunion [
32]. Based on studies designed for adult patients, bioabsorbable pins and screws provide both OCD healing and symptom relief [
7,
33,
34,
35].
With reference to inaccuracy in the prognosis for OCD treatment, based on the data analysis in our study, we conclude that treatment of juvenile OCD is associated with better outcome (Rho = −0.209;
p = 0.538) and requires fewer implants for fixation. Unfortunately, there are no studies directly comparing different kinds of bioabsorbable implants. Weckstrom et al. compared outcomes in young adults treated with bioabsorbable nails (73% healing) with pins (35% healing) [
36]. This confirms how important compression is, and not just the stabilization of the OCD lesion. All implants used in the reviewed studies allowed for compression, which probably corresponds with high healing rate. On the other hand, use of bioabsorbable implants is not without drawbacks. The most frequently reported one is synovitis, which is related to the host response to the polymer’s biodegradation [
29,
37,
38]. The synovitis is more often associated with polyglycolic acid (PGA) implants as a result of the rapid degradation of the biomaterial, in contrast to polylactic acid (PLA) implants that degrade slowly [
39]. We noticed eight cases of synovitis, four in the knee and four in the elbow.
Confirmed knee synovitis has been associated with SmartNail (ConMed, Tampere, Finland) which is a copolymer comprised of both PGA and PLA. The goal of this combination is to reduce the complications associated with the inflammatory response related to PGA’s rapid degradation and the complications associated with the slow degradation of PLA.
Uchida et al. reported joint effusion in 4 out of 18 patients (24%) which was significantly higher for the elbow compared to other locations [
24]. They used SuperFixorb 30-thread pins (Takiron Co., Ltd.) which are the composite of hydroxyapatite (HA) and poly-L-lactide acid (PLLA).
This combination is believed to ensure much greater initial mechanical strength than PLLA alone and has osteobinding and osteoconductive potential [
40]. In our opinion, high effusion rate in the elbow may constitute a limitation of the use of bioabsorbable material in the treatment of humeral capitellum lesions. Use of a low number of bioabsorbable implants, ensuring the OCD stabilization, or use of metal implants should be considered. In addition, implant destabilization with back-out, implant breakage and OCD nonunion has been described [
37,
41,
42,
43,
44,
45,
46,
47]. Some authors emphasize the possibility of uneven resorption of the implant which can lead to back-out of articular implant part, resulting in intra-articular loose bodies and chondral abrasions [
33,
48,
49,
50]. In our review, we observed only one mechanical complication in the form of a pin breakage. Camathias et al. reported, altogether, 14 broken screws (SmartScrew; ConMed Linvatec) of a total of 61 implants in 24 patients (breakage rate −23%) based on MRI follow-up. Interestingly, not all patients were symptomatic and only four patients underwent surgical revision for implant failure during the follow-up [
43]. In our review, we included the earlier work of this author from 2011, because the paper describing complications published in 2015 lacked data relevant for analysis, which distorts the frequency of bioabsorbable implants complications.
The treatment of OCD lesions should be relatively straightforward and should be taken at the right time, preferably before detachment of a loose body, in order to avoid specialist techniques like autologous chondrocyte implantation (ACI) or osteochondral grafts. Further analysis is needed to understand the impact of lesion size on outcomes after bioabsorbable fixation. A head-to-head study comparing metal to bioabsorbable fixation implants may bring some important findings.