Experimental Phaeohyphomycosis of Curvularia lunata
Abstract
1. Introduction
2. Methods
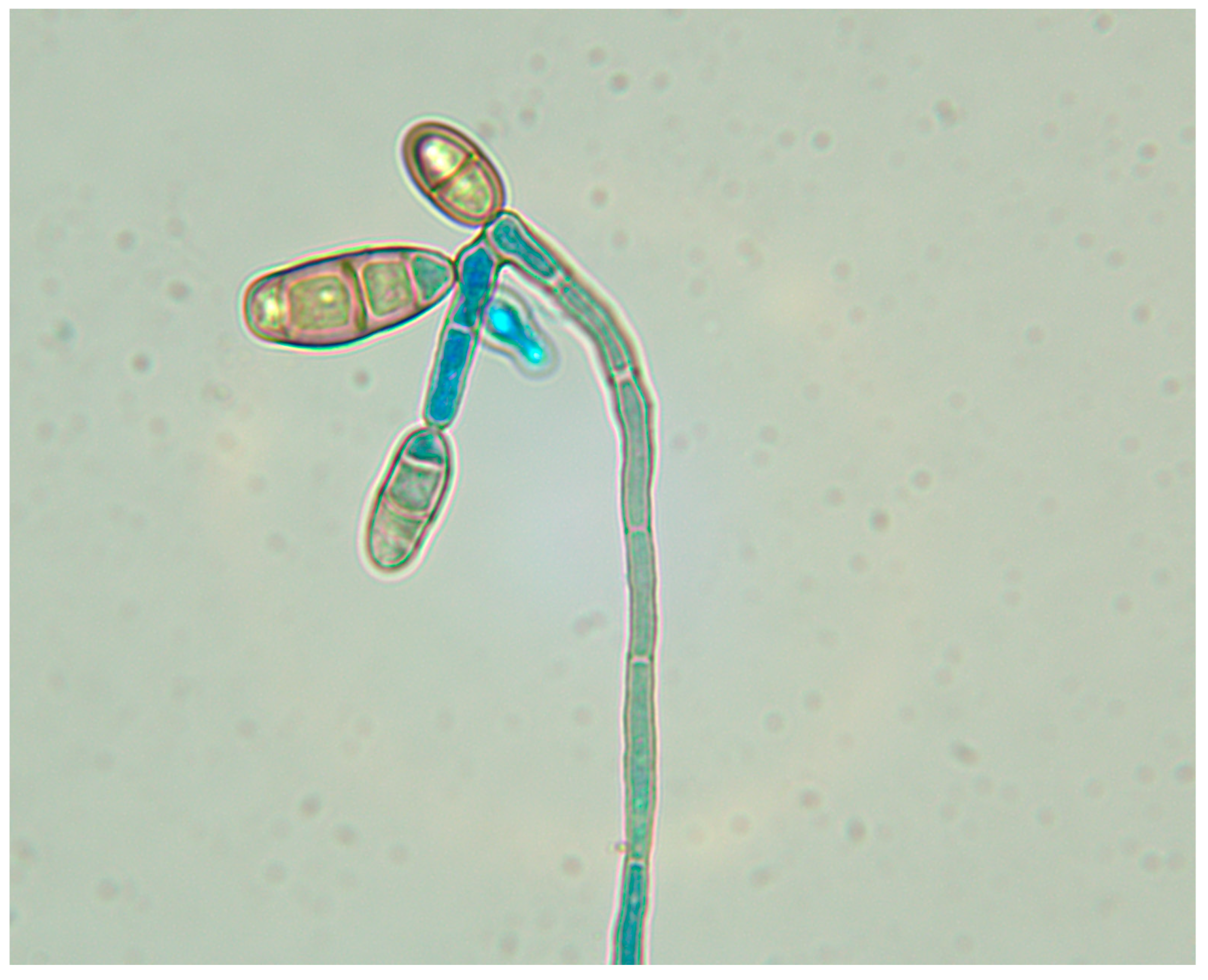
2.1. Curvularia Lunata Isolates
2.2. Animals
2.3. Preparation of Inoculum
2.4. Inoculation
2.4.1. Transdermal Inoculation
2.4.2. Subcutaneous Inoculation
2.4.3. Intraperitonially Inoculation
2.5. Clinical Evaluation
2.6. In Vitro Antifungal Susceptibility
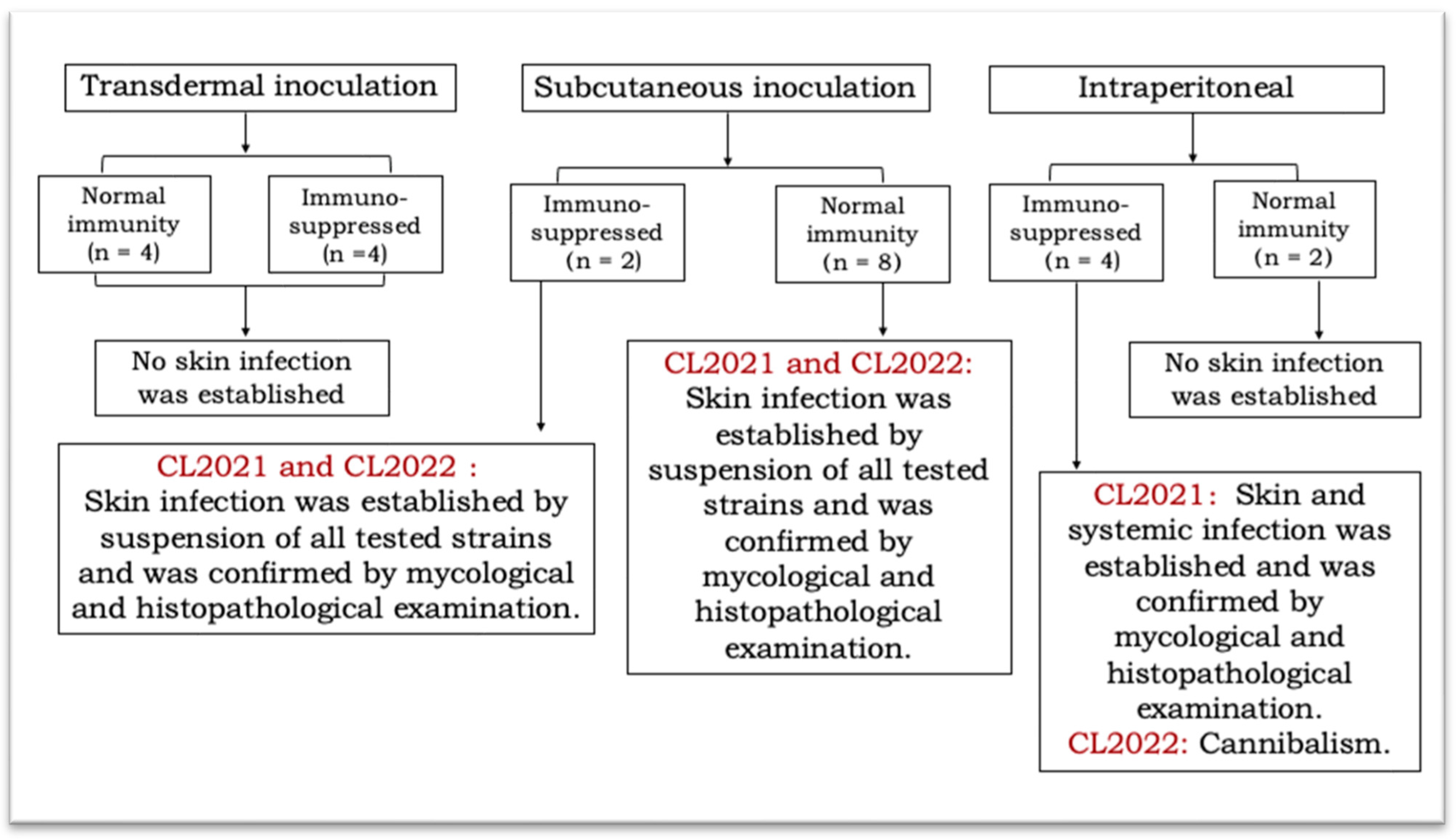
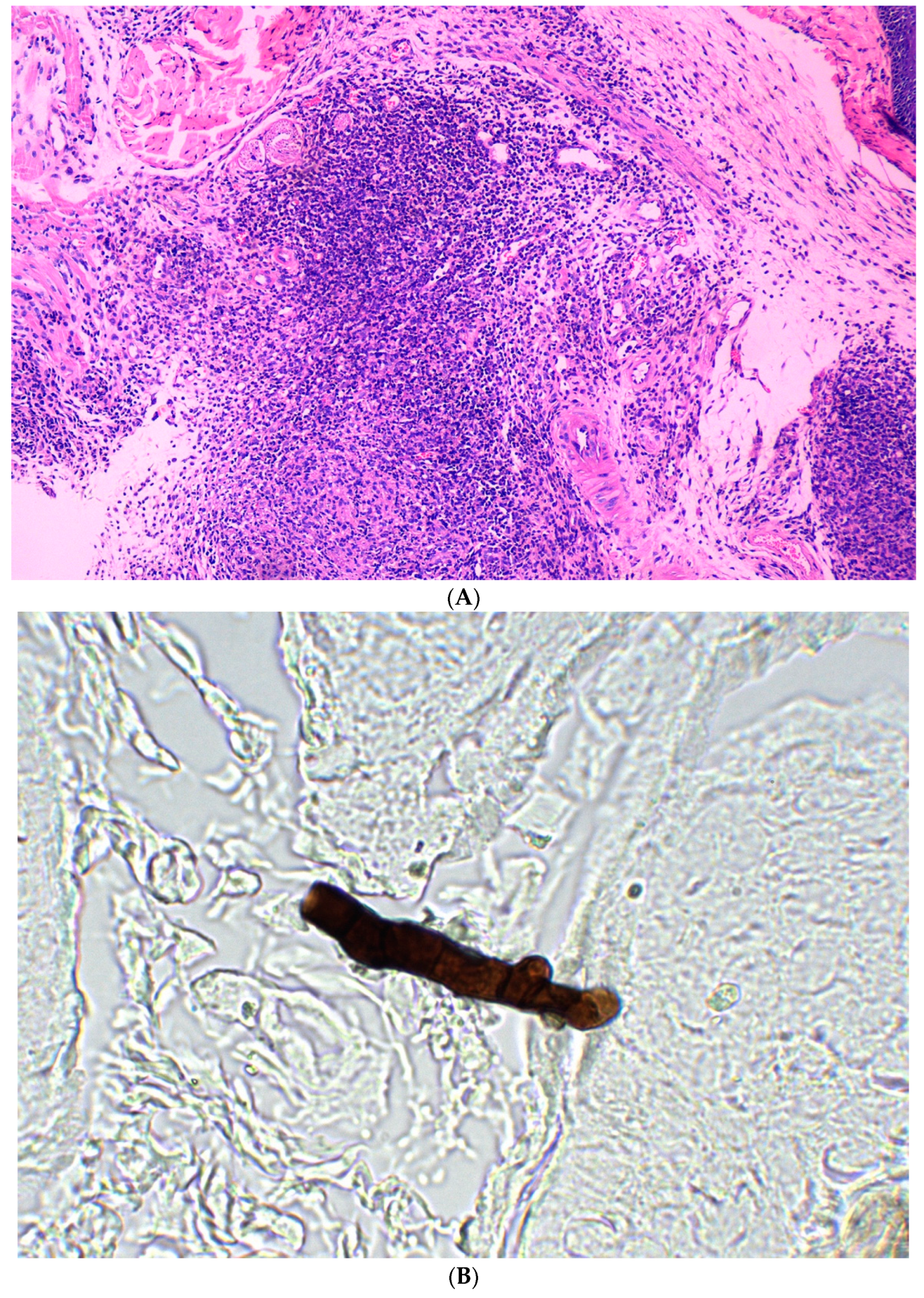
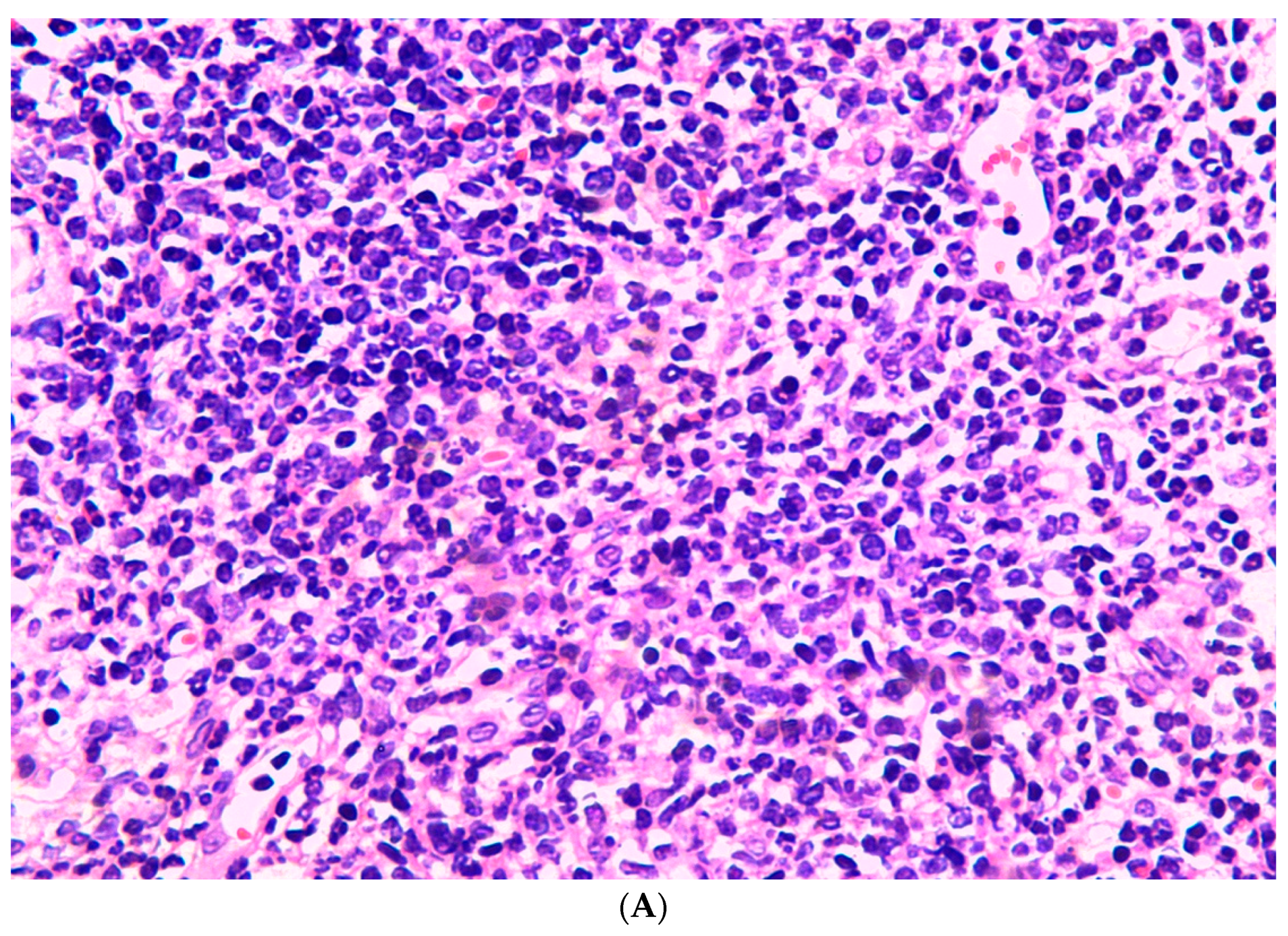
3. Results
3.1. Transdermal Inoculation
3.2. Subcutaneous Inoculation
3.3. Intraperitoneal Inoculation
3.4. In Vitro Antifungal Susceptibility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinaldi, M.G. Phaeohyphomycosis. Dermatol. Clin. 1996, 14, 147–153. [Google Scholar] [CrossRef]
- Revankar, S.G.; Sutton, D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef] [PubMed]
- Arcobello, J.T.; Revankar, S.G. Phaeohyphomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Shoff, C.J.; Perfect, J.R. Uncommon Yeasts and Molds Causing Human Disease. Encycl. Mycol. 2021, 1, 813–834. [Google Scholar]
- Wilhelmus, K.R.; Jones, D.B. Curvularia keratitis. Trans. Am. Ophthalmol. Soc. 2001, 99, 111–130; Discussion 130–112. [Google Scholar]
- Kumar, A.; Khurana, A. Bilateral Curvularia Keratitis. J. Ophthalmic Vis. Res. 2020, 15, 574–575. [Google Scholar] [CrossRef]
- Pathengay, A.; Shah, G.Y.; Das, T.; Sharma, S. Curvularia lunata endophthalmitis presenting with a posterior capsular plaque. Indian J. Ophthalmol. 2006, 54, 65–66. [Google Scholar] [CrossRef]
- Bartynski, J.M.; McCaffrey, T.V.; Frigas, E. Allergic fungal sinusitis secondary to dermatiaceous fungi—Curvularia lunata and Alternaria. Otolaryngology 1990, 103, 32–39. [Google Scholar] [CrossRef]
- Kamalam, A.; Ajithadass, K.; Sentamilselvi, G.; Thambiah, A.S. Paronychia and black discoloration of a thumb nail caused by Curvularia lunata. Mycopathologia 1992, 118, 83–84. [Google Scholar] [CrossRef]
- Garg, A.; Sujatha, S.; Garg, J.; Parija, S.C.; Thappa, D.M. Eumycetoma due to Curvularia lunata. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 515–516. [Google Scholar] [CrossRef]
- Bryan, C.S.; Smith, C.W.; Berg, D.E.; Karp, R.B. Curvularia lunata endocarditis treated with terbinafine: Case report. Clin. Infect. Dis. 1993, 16, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.C.; de Nanassy, J.; Summerbell, R.C.; Matlow, A.G.; Richardson, S.E. Fungal sternal wound infection due to Curvularia lunata in a neonate with congenital heart disease: Case report and review. Clin. Infect. Dis. 1994, 19, 735–740. [Google Scholar] [CrossRef] [PubMed]
- McAleer, R.; Kroenert, D.B.; Elder, J.L.; Froudist, J.H. Allergic bronchopulmonary disease caused by Curvularia lunata and Drechslera hawaiiensis. Thorax 1981, 36, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; del Rio, C.; Williams, P.; McGowan, J.E., Jr. Fungal peritonitis caused by Curvularia lunata in a patient undergoing peritoneal dialysis. Am. J. Med. Sci. 1989, 298, 320–323. [Google Scholar] [CrossRef]
- Rohwedder, J.J.; Simmons, J.L.; Colfer, H.; Gatmaitan, B. Disseminated Curvularia lunata infection in a football player. Arch. Intern. Med. 1979, 139, 940–941. [Google Scholar] [CrossRef]
- Tessari, G.; Forni, A.; Ferretto, R.; Solbiati, M.; Faggian, G.; Mazzucco, A.; Barba, A. Lethal systemic dissemination from a cutaneous infection due to Curvularia lunata in a heart transplant recipient. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 440–442. [Google Scholar] [CrossRef]
- Rasheeduddin, M.V.; Visalakshi, P. Cutaneous phaeohyphomycosis of foot web by Curvularia lunata. Glob. J. Med. Clin. Case Rep. 2017, 4, 74–75. [Google Scholar] [CrossRef]
- Vineetha, M.; Palakkal, S.; Sobhanakumari, K.; Celine, M.I.; Letha, V. Verrucous Onychomycosis Caused by Curvularia in a Patient with Congenital Pterygium. Indian J. Dermatol. 2016, 61, 701. [Google Scholar] [CrossRef]
- Vásquez-del-Mercado, E.; Lammoglia, L.; Arenas, R. Subcutaneous phaeohyphomycosis due to Curvularia lunata in a renal transplant patient. Rev. Iberoam. Micol. 2013, 30, 116–118. [Google Scholar] [CrossRef]
- Al-Odaini, N.; Wei, J.Y.; Zheng, Y.Q.; Zheng, D.Y.; Khader, J.A.; Cao, C.W. A Special Tinea Nigra Caused by Curvularia lunata: Case Report and Literature Review. Mycopathologia 2022, 187, 291–298. [Google Scholar] [CrossRef]
- Whitcomb, M.P.; Jeffries, C.; Weise, R.W. Curvularia lunata in experimental phaeohyphomycosis. Mycopathologia 1981, 75, 81–88. [Google Scholar] [CrossRef] [PubMed]
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Flimentous Fungi; Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Pagnussat, V.; da Rosa Monte Machado, G.; Scarton, J.; Meneghello Fuentefria, A. In vitro Antifungal Susceptibility of Agents for Superficial Phaeohyphomycosis. J. Port. Soc. Dermatol. Venereol. 2020, 78, 135–139. [Google Scholar] [CrossRef]
- Koc, A.N.; Silici, S.; Ayangil, D.; Ferahbas, A.; Cankaya, S. Comparison of in vitro activities of antifungal drugs and ethanolic extract of propolis against Trichophyton rubrum and T. mentagrophytes by using a microdilution assay. Mycoses 2005, 48, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Bonduel, M.; Santos, P.; Turienzo, C.F.; Chantada, G.; Paganini, H. Atypical skin lesions caused by Curvularia sp. and Pseudallescheria boydii in two patients after allogeneic bone marrow transplantation. Bone Marrow Transpl. 2001, 27, 1311–1313. [Google Scholar] [CrossRef]
- Grieshop, T.J.; Yarbrough, D.; Farrar, W.E. Phaeohyphomycosis due to Curvularia lunata involving skin and subcutaneous tissue after an explosion at a chemical plant. Am. J. Med. Sci. 1993, 305, 387–389. [Google Scholar] [CrossRef]
- Balla, A.; Pierson, J.; Hugh, J.; Wojewoda, C.; Gibson, P.; Greene, L. Disseminated cutaneous Curvularia infection in an immunocompromised host; diagnostic challenges and experience with voriconazole. J. Cutan. Pathol. 2016, 43, 383–387. [Google Scholar] [CrossRef]
- Ritchie, E.B.; Taylor, T.E. A Study of Tinea Nigra Palmaris: Report of a Case and Inoculation Experiments. Arch. Dermatol. 1964, 89, 601–603. [Google Scholar] [CrossRef]
- Rout, N.; Nanda, B.; Gangopadhyaya, S. Experimental pheohyphomycosis and mycotoxicosis by Curvularia lunata in albino rats. Indian J. Pathol. Microbiol. 1989, 32, 1–6. [Google Scholar]
- Ismail, Y.; Johnson, R.H.; Wells, M.V.; Pusavat, J.; Douglas, K.; Arsura, E.L. Invasive sinusitis with intracranial extension caused by Curvularia lunata. Arch. Intern. Med. 1993, 153, 1604–1606. [Google Scholar] [CrossRef]
- Cavanna, C.; Seminari, E.; Pusateri, A.; Mangione, F.; Lallitto, F.; Esposto, M.C.; Pagella, F. Allergic fungal rhinosinusitis due to Curvularia lunata. New Microbiol. 2014, 37, 241–245. [Google Scholar]
- Nasu, S.; Satoh, S.; Shimizu, K.; Matsuno, O.; Morishita, H.; Yaguchi, T.; Kawahara, K.; Matsuoka, H. Spontaneous Regression of Allergic Bronchopulmonary Mycosis Due to Curvularia lunata. Intern. Med. 2018, 57, 243–246. [Google Scholar] [CrossRef]
- Alex, D.; Li, D.; Calderone, R.; Peters, S.M. Identification of Curvularia lunata by polymerase chain reaction in a case of fungal endophthalmitis. Med. Mycol. Case Rep. 2013, 2, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Akiti, T.; Horta, R.A.; Morizot Leite-Filho, L.A.; Gené, J.; Ferreira-Gomes, S.; Aguilar, C.; Ortoneda, M. Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J. Clin. Microbiol. 1999, 37, 4170–4173. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gao, Y.; Li, S.; Wang, M.; Wang, T.; Gao, H. Clinical and Etiological Studies of Five Cases of Curvularia lunata Keratitis. Chin. J. Lab. Med. 2012, 5, 469–471. [Google Scholar]
- da Cunha, K.C.; Sutton, D.; Fothergill, A.W.; Gené, J.; Cano, J.; Madrid, H.; Hoog Sd Crous, P.W.; Guarro, J. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagn. Microbiol. Infect. Dis. 2013, 76, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Nizam, T.M.; Binting, R.; Saari, S.M.; Kumar, T.V.; Muhammad, M.; Satim, H.; Yusoff, H.; Santhanam, J. In vitro antifungal activities against molds isolated from dermatological specimens. Malays J. Med. Sci. 2016, 23, 32–39. [Google Scholar]
- Krizsán, K.; Tóth, E.; Nagy, L.G.; Galgóczy, L.; Manikandan, P.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C.; Papp, T. Molecular identification and antifungal susceptibility of Curvularia australiensis, C. hawaiiensis and C. spicifera isolated from human eye infections. Mycoses 2015, 58, 603–609. [Google Scholar] [CrossRef]




| Isolates and Drugs | MIC Range | GM |
|---|---|---|
| (μg/mL) | (μg/mL) | |
| C. lunata (n = 2) | ||
| FLC | 8–16 | 11.3 |
| AMB | 2 | 2 |
| ITC | 2–4 | 2.8 |
| VRC | 0.25–0.5 | 0.35 |
| TRB | 1 | 1 |
| KCZ | 1–2 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Odaini, N.; Pan, K.-s.; Liao, L.-w.; Mo, N.-f.; Jiang, Z.-w.; Li, T.-t.; Li, X.-y.; He, X.-j.; Zheng, D.-y.; Cao, C.-w. Experimental Phaeohyphomycosis of Curvularia lunata. J. Clin. Med. 2022, 11, 5393. https://doi.org/10.3390/jcm11185393
Al-Odaini N, Pan K-s, Liao L-w, Mo N-f, Jiang Z-w, Li T-t, Li X-y, He X-j, Zheng D-y, Cao C-w. Experimental Phaeohyphomycosis of Curvularia lunata. Journal of Clinical Medicine. 2022; 11(18):5393. https://doi.org/10.3390/jcm11185393
Chicago/Turabian StyleAl-Odaini, Najwa, Kai-su Pan, Liu-wei Liao, Nan-fang Mo, Zhi-wen Jiang, Tian-tian Li, Xiu-ying Li, Xiao-juan He, Dong-yan Zheng, and Cun-wei Cao. 2022. "Experimental Phaeohyphomycosis of Curvularia lunata" Journal of Clinical Medicine 11, no. 18: 5393. https://doi.org/10.3390/jcm11185393
APA StyleAl-Odaini, N., Pan, K.-s., Liao, L.-w., Mo, N.-f., Jiang, Z.-w., Li, T.-t., Li, X.-y., He, X.-j., Zheng, D.-y., & Cao, C.-w. (2022). Experimental Phaeohyphomycosis of Curvularia lunata. Journal of Clinical Medicine, 11(18), 5393. https://doi.org/10.3390/jcm11185393





