The Effect of COVID-19 on the Perioperative Course of Acute Coronary Syndrome in Poland: The Estimation of Perioperative Prognosis and Neural Network Analysis in 243,515 Cases from 2020 to 2021
Abstract
:1. Introduction
2. Material and Methods
3. Statistical Methods
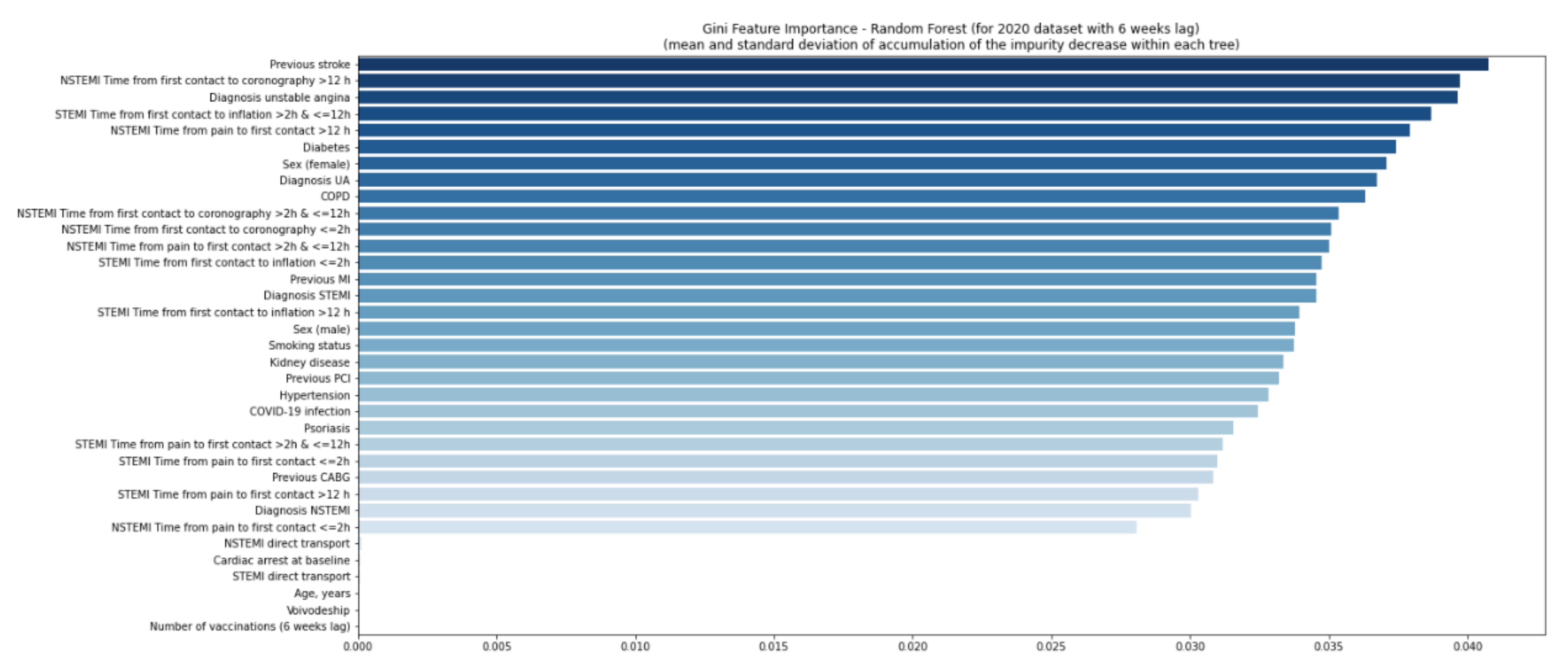
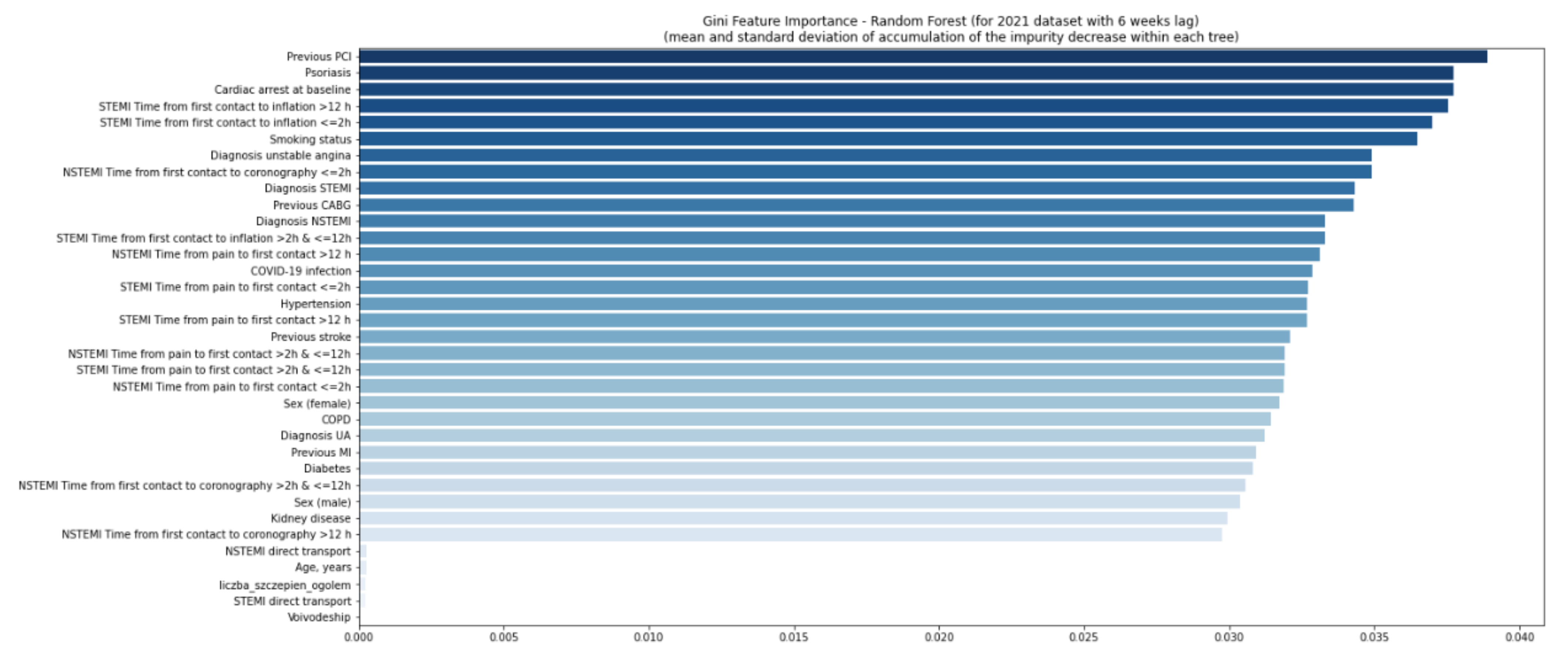
4. Results
5. Discussion
6. Conclusions
7. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Lindmark, K.; Connolly, A.-M.F. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study . Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef]
- Lee, C.C.E.; Ali, K.; Connell, D.; Mordi, I.R.; George, J.; Lang, E.M.; Lang, C.C. COVID-19-Associated Cardiovascular Complications. Diseases 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Conforti, G.; Iovine, M.; Carbone, A.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients. Cancers 2020, 12, 3316. [Google Scholar] [CrossRef]
- Quagliariello, V.; Paccone, A.; Iovine, M.; Cavalcanti, E.; Berretta, M.; Maurea, C.; Canale, M.L.; Maurea, N. Interleukin-1 blocking agents as promising strategy for prevention of anticancer drug-induced cardiotoxicities: Possible implications in cancer patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6797–6812. [Google Scholar]
- Yin, J.; Wang, S.; Liu, Y.; Chen, J.; Li, D.; Xu, T. Coronary microvascular dysfunction pathophysiology in COVID-19. Microcirculation 2021, 28, e12718. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti-Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Nuzzi, V.; Castrichini, M.; Collini, V.; Roman-Pognuz, E.; Di Bella, S.; Luzzati, R.; Berlot, G.; Confalonieri, M.; Merlo, M.; Stolfo, D.; et al. Impaired Right Ventricular Longitudinal Strain Without Pulmonary Hypertension in Patients Who Have Recovered From COVID-19. Circ. Cardiovasc. Imaging 2021, 14, e012166. [Google Scholar] [CrossRef]
- Kaziród-Wolski, K.; Sielski, J.; Sidło, J.; Januszek, R.; Siudak, Z. The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry. Biomedicines 2021, 9, 1813. [Google Scholar] [CrossRef]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19) Pandemic. [Cited 2022 Feb 5]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 12 May 2022).
- Available online: https://www.gov.pl/web/szczepimysie/raport-szczepien-przeciwko-covid-19 (accessed on 4 June 2022).
- Crisp, K.M.; Sutter, E.N.; Westerberg, J.A. Pencil-and-Paper Neural Networks: An Undergraduate Laboratory Exercise in Computational Neuroscience. J. Undergrad. Neurosci. Educ. 2015, 14, A13–A22. [Google Scholar] [PubMed]
- Kriegeskorte, N.; Golan, T. Neural network models and deep learning. Curr. Biol. 2019, 29, R231–R236. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, J.T.; Cieśla, D.; Wojakowski, W.; Gierlotka, M.; Dudek, D.; Witkowski, A.; Zdrojewski, T.; Lesiak, M.; Buszman, P.; Gąsior, M. Is neural network better than logistic regression in death prediction in patients after ST-segment elevation myocardial infarction? Kardiol. Pol. 2021, 79, 1353–1361. [Google Scholar] [CrossRef]
- Arslan, F.; Damman, P.; Zwart, B.; Appelman, Y.; Voskuil, M.; de Vos, A.; van Royen, N.; Jukema, J.W.; Waalewijn, R.; Hermanides, R.S.; et al. 2020 ESC Guidelines on acute coronary syndrome without ST-segment elevation: Recommendations and critical appraisal from the Dutch ACS and Interventional Cardiology working groups. Neth. Heart J. 2021, 29, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Mallela, A.; Neumann, J.; Miller, E.F. Bayesian Inference of State-Level COVID-19 Basic Reproduction Numbers across the United States. Viruses 2022, 14, 157. [Google Scholar] [CrossRef]
- Matsushita, K.; Hess, S.; Marchandot, B.; Sato, C.; Truong, D.P.; Kim, N.T.; Weiss, A.; Jesel, L.; Ohlmann, P.; Morel, O. Clinical features of patients with acute coronary syndrome during the COVID-19 pandemic. J. Thromb. Thrombolysis 2021, 52, 95–104. [Google Scholar] [CrossRef]
- Showkathali, R.; Yalamanchi, R.; Narra, L.; Vinayagamoorthy, L.N.N.; Gunasekaran, S.; Nayak, R.; Reddy, Y.V.; Mahilmaran, A.; Srinivasan, K.N.; Oomman, A.; et al. Coronary thrombo-embolic events after Covid-19 vaccination- a single centre study. Indian Heart J. 2022, 74, 131–134. [Google Scholar] [CrossRef]
- Tajstra, M.; Jaroszewicz, J.; Gąsior, M. Acute Coronary Tree Thrombosis After Vaccination for COVID-19. JACC Cardiovasc. Interv. 2021, 14, e103–e104. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e00341-21. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- Wang, M.; Wen, W.; Zhou, M.; Wang, C.; Feng, Z.H. Meta-Analysis of Risk of Myocarditis After Messenger RNA COVID-19 Vaccine. Am. J. Cardiol. 2022, 167, 155–157. [Google Scholar]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome . Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sielski, J.; Kaziród-Wolski, K.; Siudak, Z. Risk of perioperative death and sudden cardiac arrest: A study of 113,456 cases from the National Registry of Invasive Cardiology Procedures (ORPKI) for estimation of the perioperative prognosis. Kardiol. Pol. 2021, 79, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Sielski, J.; Kaziród-Wolski, K.; Siudak, Z. Out-of-hospital cardiac arrest: Data from the National Registry of Invasive Cardiology Procedures (ORPKI) in a long-term survival analysis of patients with acute coronary syndromes in a Polish region. Kardiol. Pol. 2020, 78, 412–419. [Google Scholar] [CrossRef]
- Siudak, Z.; Dudek, D.; Grygier, M.; Araszkiewicz, A.; Dąbrowski, M.; Kusa, J.; Hawranek, M.; Huczek, Z.; Kralisz, P.; Roleder, T.; et al. Interventional cardiology in Poland in 2020—Impact of the COVID-19 pandemic. Annual summary report of the Association of Cardiovascular Interventions of the Polish Cardiac Society and Jagiellonian University Medical College. Adv. Interv. Cardiol. 2021, 17, 131–134. [Google Scholar] [CrossRef]
- Weber, B.; Merola, J.F.; Husni, M.E.; Di Carli, M.; Berger, J.S.; Garshick, M.S. Psoriasis and Cardiovascular Disease: Novel Mechanisms and Evolving Therapeutics. Curr. Atheroscler. Rep. 2021, 23, 67. [Google Scholar] [CrossRef]
- Mehta, N.N.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the General Practice Research Database. Eur. Heart J. 2010, 31, 1000–1006. [Google Scholar] [CrossRef]
- Egeberg, A.; Thyssen, J.P.; Jensen, P.; Gislason, G.H.; Skov, L. Risk of Myocardial Infarction in Patients with Psoriasis and Psoriatic Arthritis: A Nationwide Cohort Study. Acta Derm. Venereol. 2017, 97, 819–824. [Google Scholar] [CrossRef]
- Mahil, S.K.; Yates, M.; Yiu, Z.Z.N.; Langan, S.M.; Tsakok, T.; Dand, N.; Mason, K.J.; McAteer, H.; Meynell, F.; Coker, B.; et al. Describing the burden of the COVID-19 pandemic in people with psoriasis: Findings from a global cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e636–e640. [Google Scholar] [CrossRef] [PubMed]
- Von Stebut, E.; Reich, K.; Thaçi, D.; Koenig, W.; Pinter, A.; Körber, A.; Rassaf, T.; Waisman, A.; Mani, V.; Yates, D.; et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J. Investig. Dermatol. 2019, 139, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.; Motreff, P.; Rangé, G. COVID-19 et SCA ST [COVID-19 and STEMI]. Ann. Cardiol. Angeiol. 2020, 69, 355–359. [Google Scholar] [CrossRef]
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: The pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853. [Google Scholar] [CrossRef]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabro, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leor, O.; Cid-Álvarez, B.; Ojeda, S.; Martín-Moreiras, J.; Rumoroso, J.R.; López-Palop, R.; Serrador, A.; Cequier, A.; Romaguera, R.; Cruz, I. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv. Cardiol. 2020, 2, 82–89. [Google Scholar] [CrossRef]
- Solomon, M.D.; McNulty, E.J.; Rana, J.S.; Leong, T.K.; Lee, C.; Sung, S.H.; Ambrosy, A.P.; Sidney, S.; Go, A.S. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N. Engl. J. Med. 2020, 383, 691–693. [Google Scholar] [CrossRef]
- Xiang, D.; Xiang, X.; Zhang, W.; Yi, S.; Zhang, J.; Gu, X.; Xu, Y.; Huang, K.; Su, X.; Yu, B.; et al. Management and Outcomes of Patients With STEMI During the COVID-19 Pandemic in China. J. Am. Coll. Cardiol. 2020, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.S.; Fanous, E.J.; Tan, W.; Lee, J.; Trinh, T.; Rafique, A.M.; Parikh, R.V.; Press, M.C. Trajectory of Cardiac Catheterization for Acute Coronary Syndrome and Out-of-Hospital Cardiac Arrest During the COVID-19 Pandemic. Cardiol. Res. 2021, 12, 47–50. [Google Scholar] [CrossRef]
- Bellis, A.; Mauro, C.; Barbato, E.; Ceriello, A.; Cittadini, A.; Morisco, C. Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 775. [Google Scholar] [CrossRef]
- Alahmari, S.S.; Goldgof, D.B.; Mouton, P.R.; Hall, L.O. Challenges for the Repeatability of Deep Learning Models. IEEE Access 2020, 8, 211860–211868. [Google Scholar] [CrossRef]
- Fort, S.; Hu, H.; Lakshminarayanan, B. Deep Ensembles: A Loss Landscape Perspective. arXiv 2019, arXiv:1912.02757. [Google Scholar]
- D’Amour, A.; Heller, K.; Moldovan, D.; Adlam, B.; Alipanahi, B.; Beutel, A.; Chen, C.; Deaton, J.; Eisenstein, J.; Hoffman, M.D.; et al. Underspecification Presents Challenges for Credibility in Modern Machine Learning. arXiv 2020, arXiv:2011.03395. [Google Scholar]
- Shamir, G.I.; Lin, D. Real World Large Scale Recommendation Systems Reproducibility and Smooth Activations. arXiv 2022, arXiv:2202.06499v1. [Google Scholar]
- Hartley, M.; Olsson, T.S. dtoolAI: Reproducibility for Deep Learning. Patterns 2020, 1, 100073. [Google Scholar] [CrossRef]


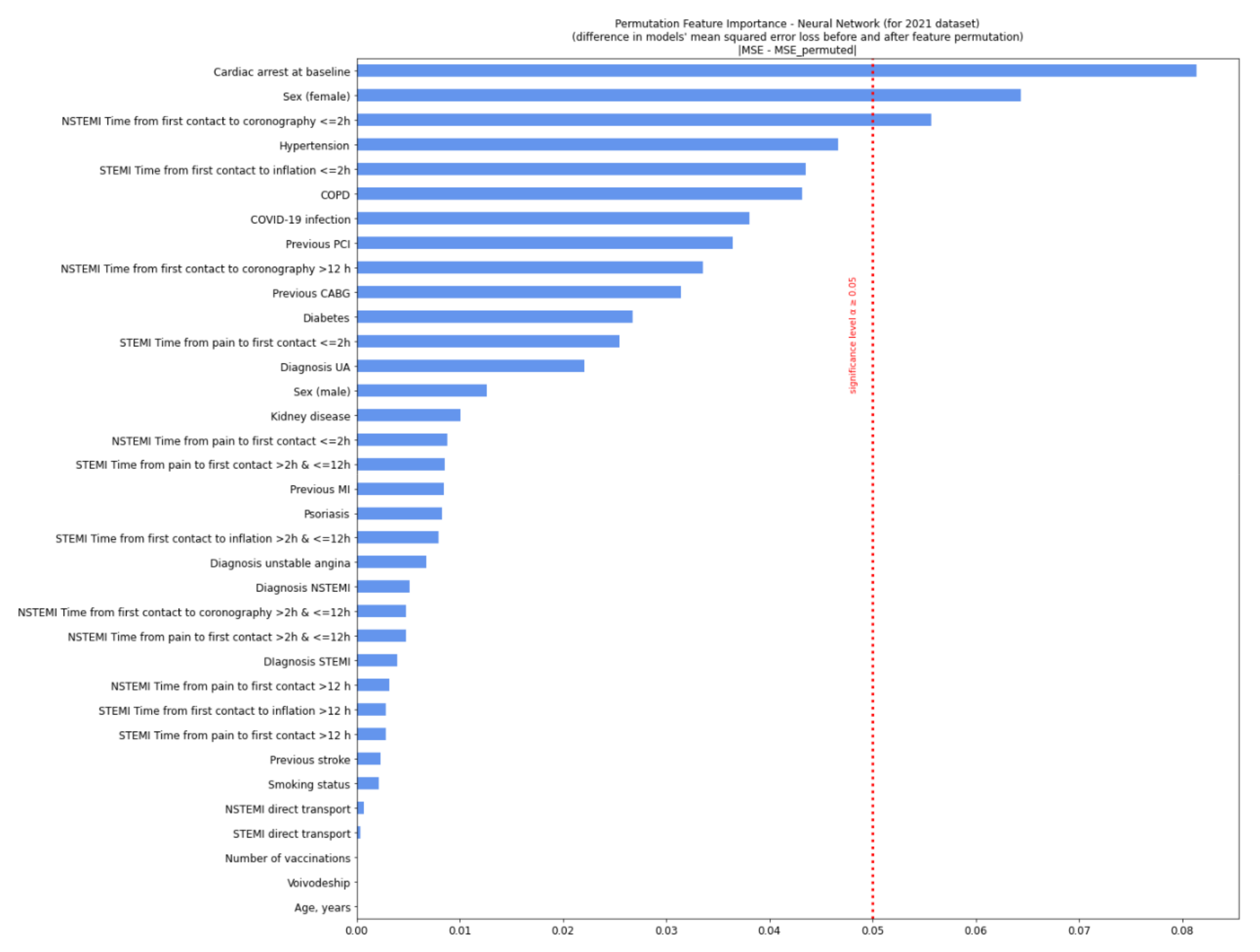

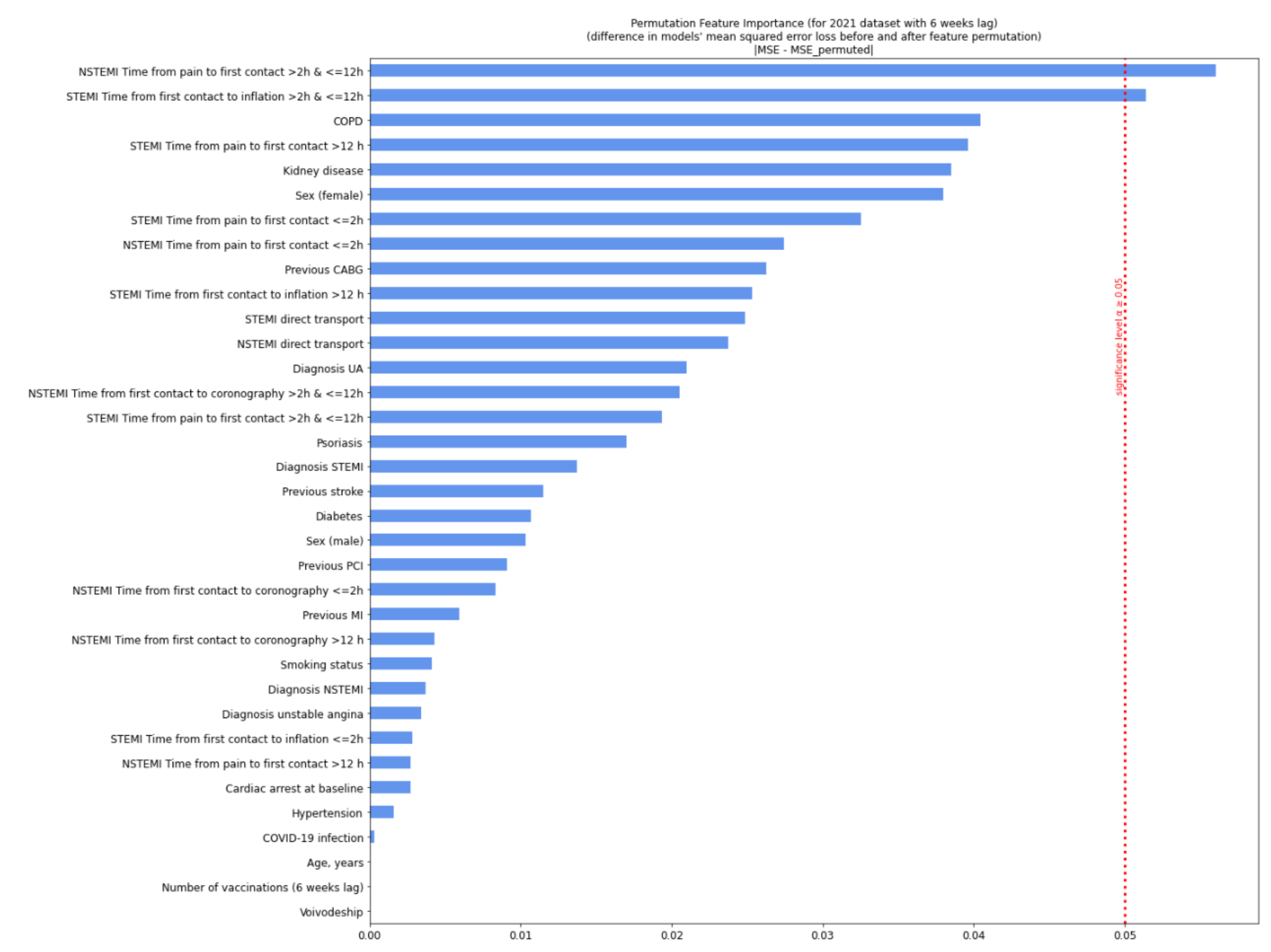
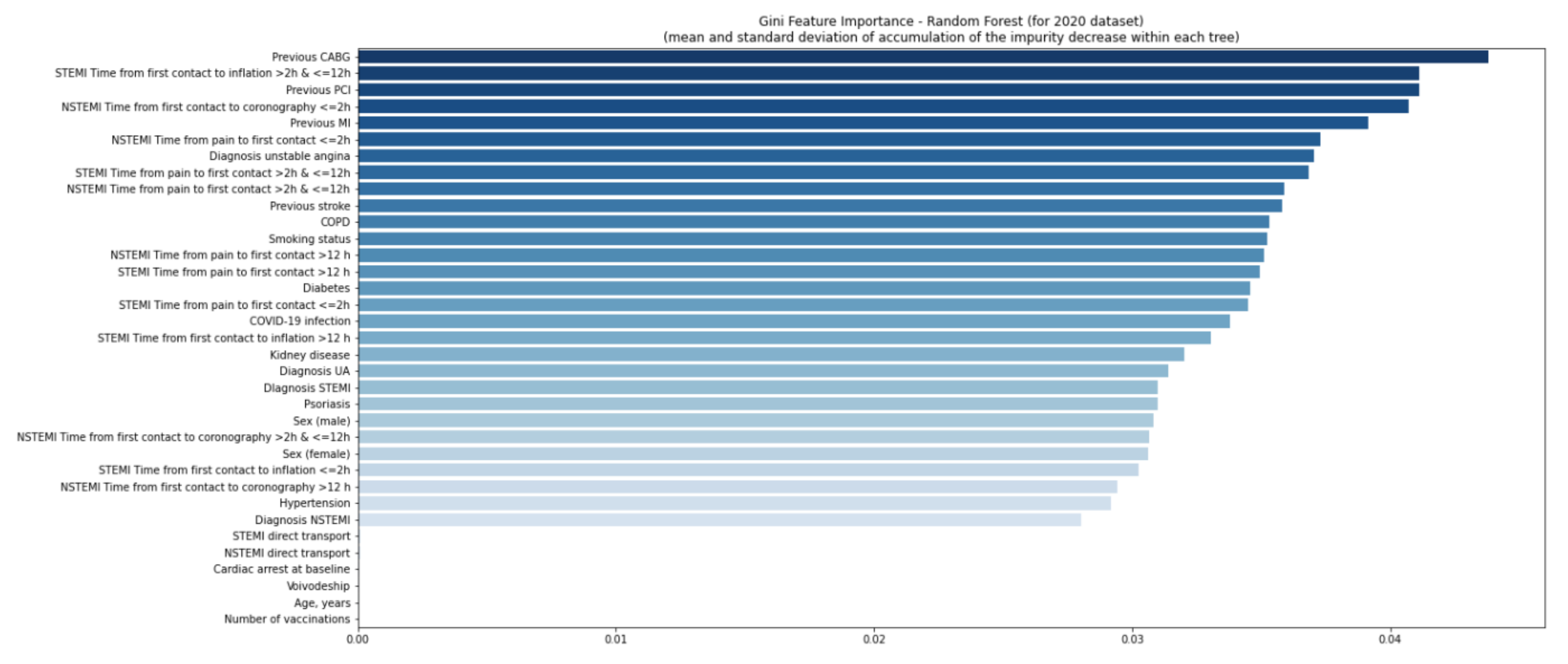
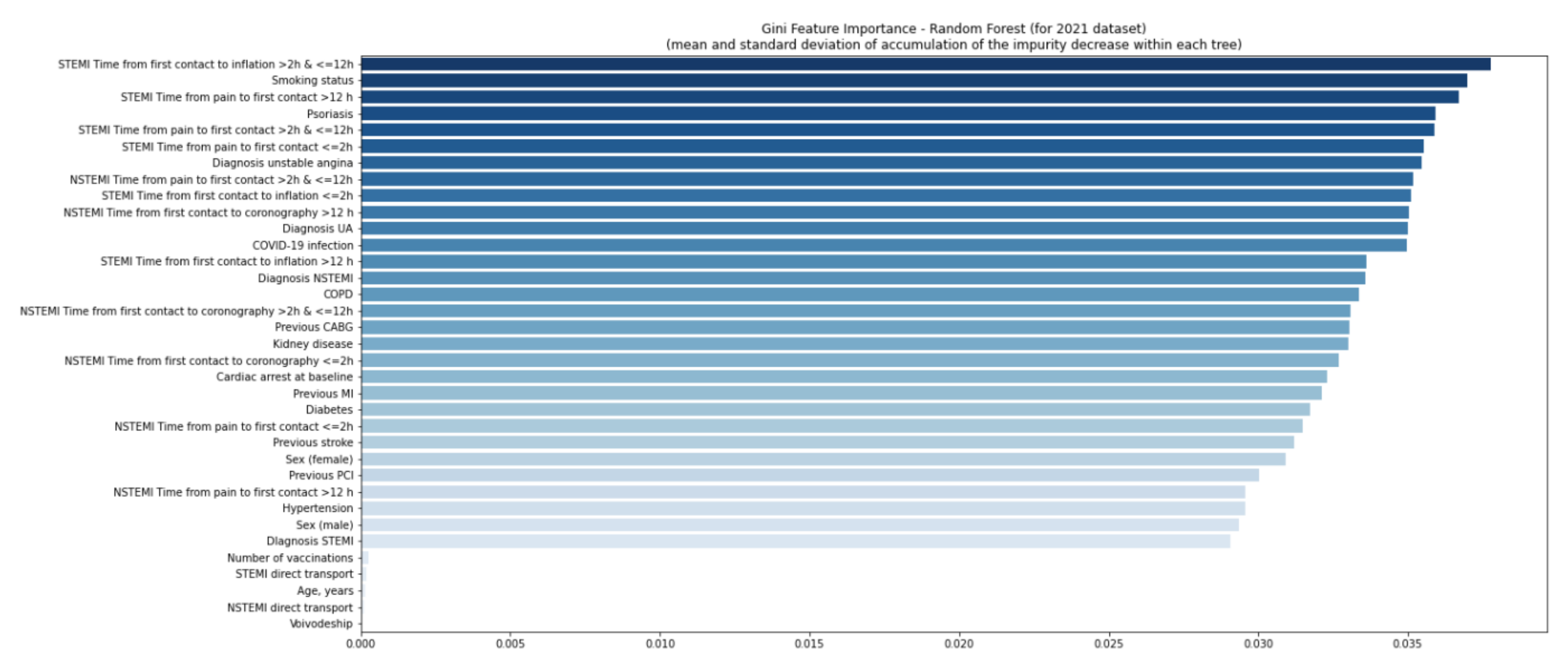
| Missing | Overall | COVID-19 (−) | COVID-19 (+) | p-Value | |
|---|---|---|---|---|---|
| n | 78,689 | 50,710 | 3104 | ||
| Age, years, median [Q1, Q3] | 52 | 67.0 [60.0, 74.0] | 67.0 [60.0, 74.0] | 66.0 [60.0, 74.0] | 0.001 |
| Sex (male), n (%) | 454 | 50,704 (64.8) | 32,850 (64.9) | 2150 (69.7) | <0.001 |
| Diabetes, n (%) | 0 | 17,204 (21.9) | 11,356 (22.4) | 662 (21.3) | 0.173 |
| Previous stroke, n (%) | 0 | 2400 (3.0) | 1516 (3.0) | 110 (3.5) | 0.090 |
| Previous MI, n (%) | 0 | 19,918 (25.3) | 13,410 (26.4) | 518 (16.7) | <0.001 |
| Previous PCI, n (%) | 0 | 23,332 (29.7) | 15,657 (30.9) | 507 (16.3) | <0.001 |
| Previous CABG, n (%) | 0 | 3972 (5.0) | 2537 (5.0) | 107 (3.4) | <0.001 |
| Smoking status, n (%) | 0 | 16,880 (21.5) | 11,018 (21.7) | 762 (24.5) | <0.001 |
| Hypertension, n (%) | 0 | 52,579 (66.8) | 34,288 (67.6) | 1847 (59.5) | <0.001 |
| Kidney disease, n (%) | 0 | 4301 (5.5) | 2914 (5.7) | 182 (5.9) | 0.817 |
| COPD, n (%) | 0 | 2614 (3.3) | 1729 (3.4) | 125 (4.0) | 0.075 |
| Diagnosis STEMI, n (%) | 0 | 17,989 (22.9) | 11,468 (22.6) | 1469 (47.3) | <0.001 |
| Diagnosis NSTEMI, n (%) | 0 | 22,372 (28.4) | 14,505 (28.6) | 966 (31.1) | 0.003 |
| Diagnosis UA, n (%) * | 0 | 34,195 (43.5) | 21,958 (43.3) | 636 (20.5) | <0.001 |
| Diagnosis unstable angina, n (%) * | 0 | 4133 (5.3) | 2779 (5.5) | 33 (1.1) | <0.001 |
| STEMI direct transport, n (%) | 8457 | 4609 (6.6) | 2895 (6.4) | 548 (18.3) | <0.001 |
| NSTEMI direct transport, n (%) | 8457 | 1190 (1.7) | 727 (1.6) | 89 (3.0) | <0.001 |
| Cardiac arrest at baseline, n (%) | 8457 | 945 (1.3) | 489 (1.1) | 237 (7.9) | <0.001 |
| Death during the procedure, n (%) | 0 | 276 (0.4) | 178 (0.4) | 27 (0.9) | <0.001 |
| STEMI time from pain to first contact ≤ 2 h, n (%) | 0 | 7114 (9.0) | 4491 (8.9) | 706 (22.7) | <0.001 |
| STEMI time from pain to first contact > 2 h and ≤12 h, n (%) | 0 | 13,844 (17.6) | 8828 (17.4) | 1263 (40.7) | <0.001 |
| STEMI time from pain to first contact > 12 h, n (%) | 0 | 1958 (2.5) | 1240 (2.4) | 177 (5.7) | <0.001 |
| STEMI time from first contact to inflation ≤ 2 h, n (%) | 0 | 10,047 (12.8) | 6476 (12.8) | 909 (29.3) | <0.001 |
| STEMI time from first contact to inflation > 2 h and ≤12 h, n (%) | 0 | 13,583 (17.3) | 8674 (17.1) | 1269 (40.9) | <0.001 |
| STEMI time from first contact to inflation > 12 h, n (%) | 0 | 418 (0.5) | 269 (0.5) | 34 (1.1) | <0.001 |
| NSTEMI time from pain to first contact ≤ 2 h, n (%) | 0 | 4824 (6.1) | 3130 (6.2) | 224 (7.2) | 0.022 |
| NSTEMI time from pain to first contact > 2 h and ≤12 h, n (%) | 0 | 15,448 (19.6) | 10,029 (19.8) | 702 (22.6) | <0.001 |
| NSTEMI time from pain to first contact > 12 h, n (%) | 0 | 15,108 (19.2) | 9826 (19.4) | 676 (21.8) | 0.001 |
| NSTEMI time from first contact to coronarography ≤ 2 h, n (%) | 0 | 3214 (4.1) | 2007 (4.0) | 224 (7.2) | <0.001 |
| NSTEMI time from first contact to coronarography > 2 h and ≤12 h, n (%) | 0 | 15,562 (19.8) | 10,062 (19.8) | 780 (25.1) | <0.001 |
| NSTEMI time from first contact to coronarography > 12 h, n (%) | 0 | 5460 (6.9) | 3677 (7.3) | 199 (6.4) | 0.085 |
| Number of vaccinations, mean (SD) | 0 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0 |
| Missing | Overall | COVID-19 (−) | COVID-19 (+) | p-Value | |
|---|---|---|---|---|---|
| n | 164,826 | 139,885 | 4303 | ||
| Age, years, median [Q1,Q3] | 90 | 68.0 [61.0, 74.0] | 68.0 [61.0, 74.0] | 67.0 [60.0, 74.5] | 0.001 |
| Sex (male), n (%) | 587 | 106,320 (64.7) | 90,455 (64.7) | 2855 (66.5) | 0.019 |
| Diabetes, n (%) | 0 | 35,001 (21.2) | 30,169 (21.6) | 1002 (23.3) | 0.007 |
| Previous stroke, n (%) | 0 | 4260 (2.6) | 3595 (2.6) | 135 (3.1) | 0.024 |
| Previous MI, n (%) | 0 | 42,593 (25.8) | 37,119 (26.5) | 811 (18.8) | <0.001 |
| Previous PCI, n (%) | 0 | 52,549 (31.9) | 45,883 (32.8) | 820 (19.1) | <0.001 |
| Previous CABG, n (%) | 0 | 8465 (5.1) | 7301 (5.2) | 191 (4.4) | 0.025 |
| Smoking status, n (%) | 0 | 28,729 (17.4) | 24,819 (17.7) | 933 (21.7) | <0.001 |
| Hypertension, n (%) | 0 | 113,095 (68.6) | 97,198 (69.5) | 2707 (62.9) | <0.001 |
| Kidney disease, n (%) | 0 | 8570 (5.2) | 7425 (5.3) | 227 (5.3) | 0.953 |
| COPD, n (%) | 0 | 4660 (2.8) | 4064 (2.9) | 132 (3.1) | 0.563 |
| Diagnosis STEMI, n (%) | 0 | 18,011 (10.9) | 14,330 (10.2) | 1538 (35.7) | <0.001 |
| Diagnosis NSTEMI, n (%) | 0 | 21,886 (13.3) | 18,106 (12.9) | 943 (21.9) | <0.001 |
| Diagnosis UA, n (%) * | 0 | 37,721 (22.9) | 31,186 (22.3) | 979 (22.8) | 0.489 |
| Diagnosis unstable angina, n (%) * | 0 | 3864 (2.3) | 3279 (2.3) | 73 (1.7) | 0.006 |
| STEMI direct transport, n (%) | 21,596 | 4556 (3.2) | 3594 (3.0) | 392 (9.8) | <0.001 |
| NSTEMI direct transport, n (%) | 21,596 | 1259 (0.9) | 1005 (0.8) | 93 (2.3) | <0.001 |
| Cardiac arrest at baseline, n (%) | 0 | 253 (0.2) | 190 (0.1) | 21 (0.5) | <0.001 |
| Death during the procedure, n (%) | 0 | 324 (0.2) | 250 (0.2) | 37 (0.9) | <0.001 |
| STEMI time from pain to first contact ≤ 2 h, n (%) | 0 | 6919 (4.2) | 5529 (4.0) | 650 (15.1) | <0.001 |
| STEMI time from pain to first contact > 2 h and ≤12 h, n (%) | 0 | 13,505 (8.2) | 10,815 (7.7) | 1243 (28.9) | <0.001 |
| STEMI time from pain to first contact > 12 h, n (%) | 0 | 2087 (1.3) | 1627 (1.2) | 212 (4.9) | <0.001 |
| STEMI time from first contact to inflation ≤ 2 h, n (%) | 0 | 9746 (5.9) | 7861 (5.6) | 843 (19.6) | <0.001 |
| STEMI time from first contact to inflation > 2 h and ≤12 h, n (%) | 0 | 13,241 (8.0) | 10,627 (7.6) | 1204 (28.0) | <0.001 |
| STEMI Time from first contact to inflation > 12 h, n (%) | 0 | 388 (0.2) | 304 (0.2) | 48 (1.1) | <0.001 |
| NSTEMI time from pain to first contact ≤ 2 h, n (%) | 0 | 4175 (2.5) | 3441 (2.5) | 243 (5.6) | <0.001 |
| NSTEMI time from pain to first contact > 2 h and ≤12 h, n (%) | 0 | 14,514 (8.8) | 12,097 (8.6) | 749 (17.4) | <0.001 |
| NSTEMI time from pain to first contact > 12 h, n (%) | 0 | 14,196 (8.6) | 11,836 (8.5) | 720 (16.7) | <0.001 |
| NSTEMI time from first contact to coronarography ≤ 2 h, n (%) | 0 | 2767 (1.7) | 2237 (1.6) | 201 (4.7) | <0.001 |
| NSTEMI time from first contact to coronarography > 2 h and ≤12 h, n (%) | 0 | 14,561 (8.8) | 12,147 (8.7) | 753 (17.5) | <0.001 |
| NSTEMI time from first contact to coronarography > 12 h, n (%) | 0 | 5204 (3.2) | 4434 (3.2) | 191 (4.4) | <0.001 |
| Number of vaccinations, mean (SD) | 0 | 9529.3 (11,504.0) | 8901.8 (10,584.9) | 9402.6 (10,470.8) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaziród-Wolski, K.; Zając, P.; Zabojszcz, M.; Kołodziej, A.; Sielski, J.; Siudak, Z. The Effect of COVID-19 on the Perioperative Course of Acute Coronary Syndrome in Poland: The Estimation of Perioperative Prognosis and Neural Network Analysis in 243,515 Cases from 2020 to 2021. J. Clin. Med. 2022, 11, 5394. https://doi.org/10.3390/jcm11185394
Kaziród-Wolski K, Zając P, Zabojszcz M, Kołodziej A, Sielski J, Siudak Z. The Effect of COVID-19 on the Perioperative Course of Acute Coronary Syndrome in Poland: The Estimation of Perioperative Prognosis and Neural Network Analysis in 243,515 Cases from 2020 to 2021. Journal of Clinical Medicine. 2022; 11(18):5394. https://doi.org/10.3390/jcm11185394
Chicago/Turabian StyleKaziród-Wolski, Karol, Patrycja Zając, Michał Zabojszcz, Agnieszka Kołodziej, Janusz Sielski, and Zbigniew Siudak. 2022. "The Effect of COVID-19 on the Perioperative Course of Acute Coronary Syndrome in Poland: The Estimation of Perioperative Prognosis and Neural Network Analysis in 243,515 Cases from 2020 to 2021" Journal of Clinical Medicine 11, no. 18: 5394. https://doi.org/10.3390/jcm11185394
APA StyleKaziród-Wolski, K., Zając, P., Zabojszcz, M., Kołodziej, A., Sielski, J., & Siudak, Z. (2022). The Effect of COVID-19 on the Perioperative Course of Acute Coronary Syndrome in Poland: The Estimation of Perioperative Prognosis and Neural Network Analysis in 243,515 Cases from 2020 to 2021. Journal of Clinical Medicine, 11(18), 5394. https://doi.org/10.3390/jcm11185394






