Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ethics Approval
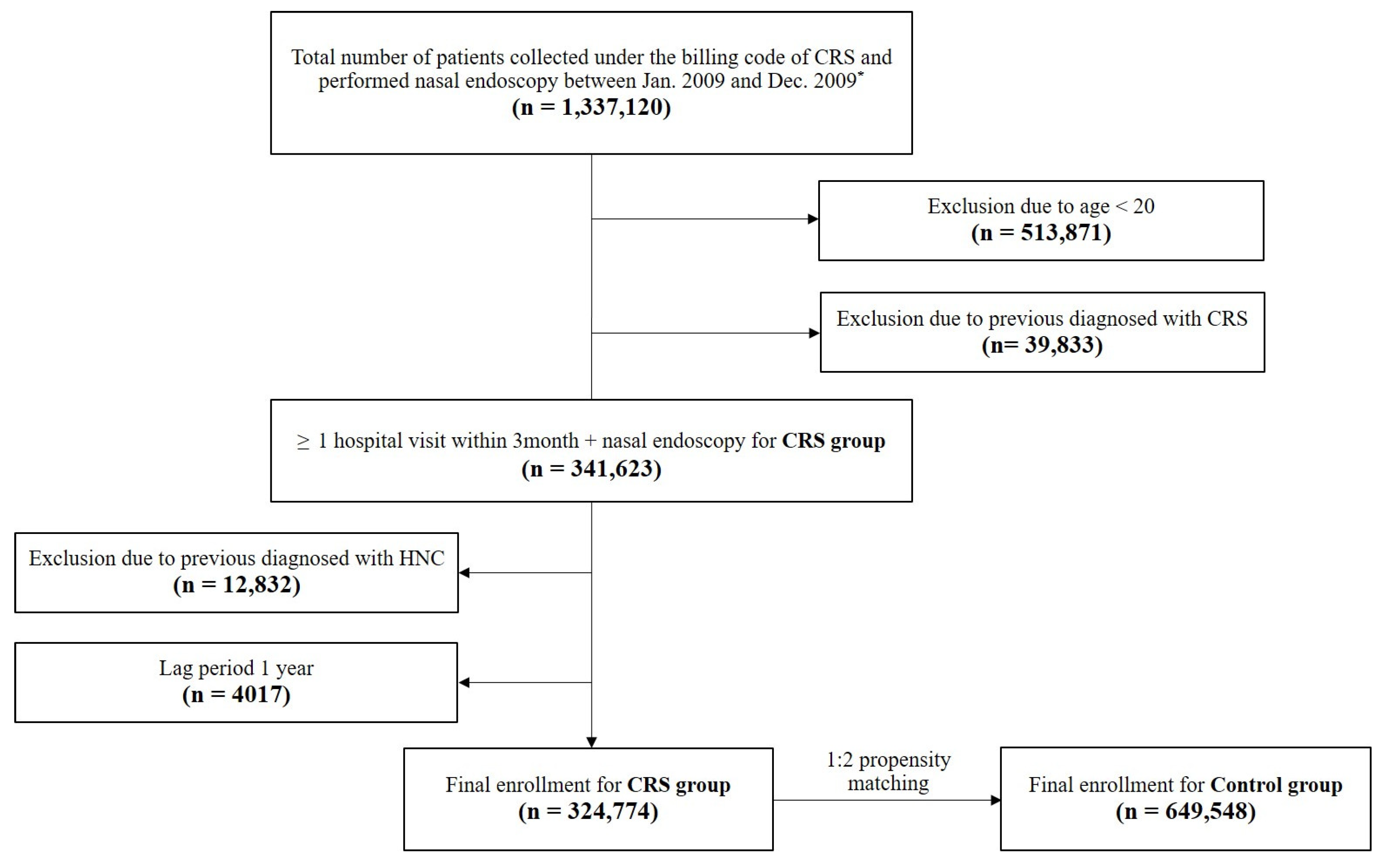
2.3. Study Population and Setting
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Subjects
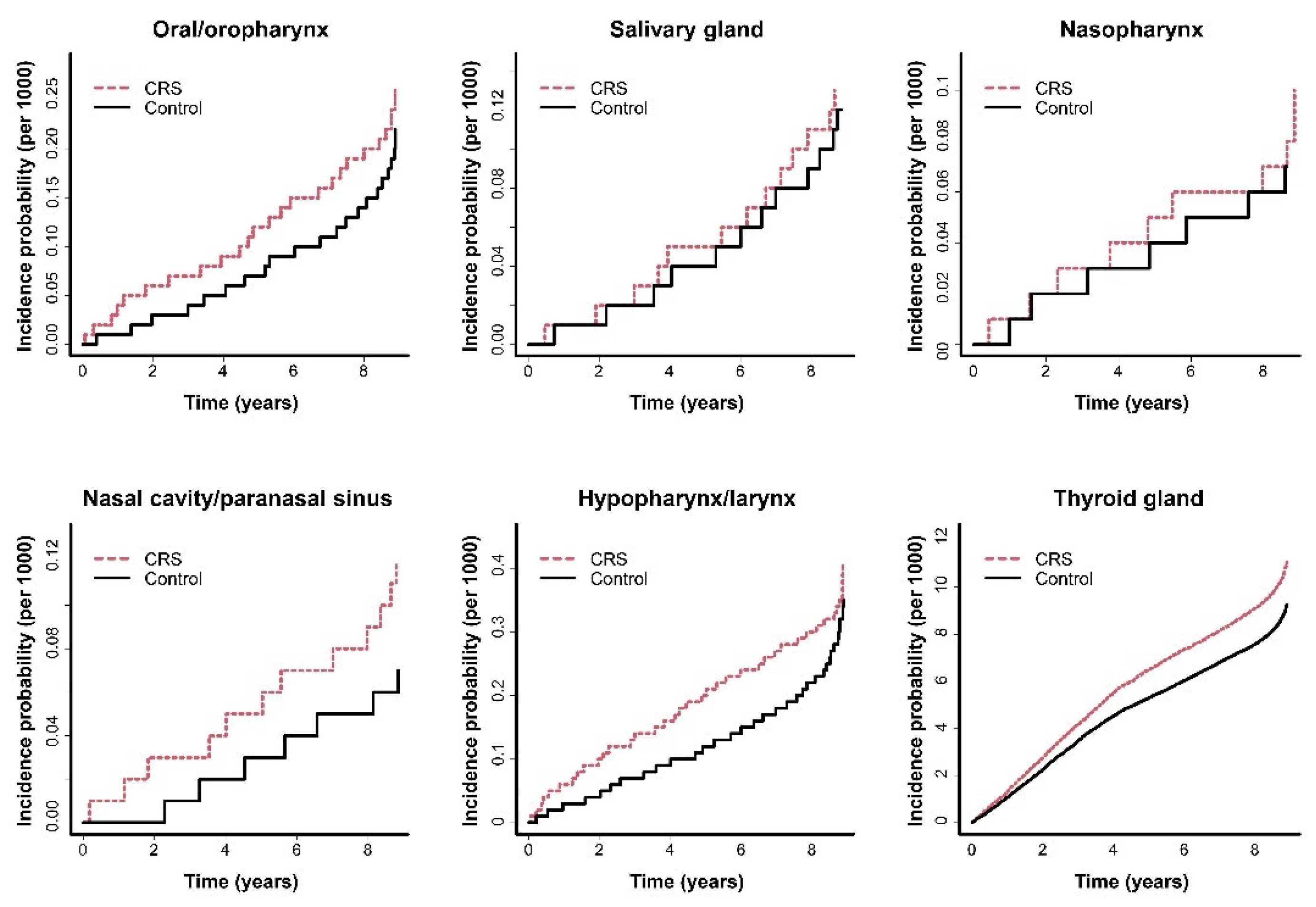
3.2. Comparison of the Incidence of HNCs between the CRS and Control Groups
3.3. Incidence of HNCs among Subjects with CRS According to Several Covariates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Cauwenberge, P.; Watelet, J.B. Epidemiology of chronic rhinosinusitis. Thorax 2000, 55 (Suppl. S2), S20–S21. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kim, J.W.; Lim, J.S.; Kong, I.G.; Choi, H.G. Chronic rhinosinusitis increases the risk of hemorrhagic and ischemic stroke: A longitudinal follow-up study using a national sample cohort. PLoS ONE 2018, 13, e0193886. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.F.; McMains, K.C.; Orlandi, R.R. The association between allergy and chronic rhinosinusitis with and without nasal polyps: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2014, 4, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chien, C.Y.; Tai, S.Y.; Huang, C.M.; Lee, C.T. Asthma associated with chronic rhinosinusitis: A population-based study. Int. Forum Allergy Rhinol. 2016, 6, 1284–1293. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.; Lim, H.; Kim, M.; Kong, I.G.; Choi, H.G. Gastroesophageal reflux disease increases the risk of chronic rhinosinusitis: A nested case-control study using a national sample cohort. Int. Forum Allergy Rhinol. 2019, 9, 357–362. [Google Scholar] [CrossRef]
- Wee, J.H.; Yoo, D.M.; Byun, S.H.; Hong, S.J.; Park, M.W.; Choi, H.G. Association between neurodegenerative dementia and chronic rhinosinusitis: A nested case-control study using a national health screening cohort. Medicine 2020, 99, e22141. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, Y.D.; Park, C.S.; Han, K.D.; Joo, Y.H. Hypertension is associated with oral, laryngeal, and esophageal cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 10291. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Han, K.D.; Joo, Y.H. Metabolic Syndrome and Incidence of Laryngeal Cancer: A Nationwide Cohort Study. Sci. Rep. 2019, 9, 667. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, L.; He, Q.; Jiang, K.; Yuan, J.; Huang, X. The effect of metabolic syndrome on head and neck cancer incidence risk: A population-based prospective cohort study. Cancer Metab. 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; Engels, E.A. Chronic Sinusitis and Risk of Head and Neck Cancer in the US Elderly Population. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 25–31. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, H.S.; Kang, T.; Bachert, C.; Song, W.J. Nasal polyps and future risk of head and neck cancer: A nationwide population-based cohort study. J. Allergy Clin. Immunol. 2019, 144, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, H.Y.; Suh, J.D.; Han, K.D.; Kim, J.K.; Lim, Y.C.; Hong, S.C.; Cho, J.H. Uvulopalatopharyngoplasty reduces the incidence of cardiovascular complications caused by obstructive sleep apnea: Results from the national insurance service survey 2007–2014. Sleep Med. 2018, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Han, K.; Choi, K.S.; Ko, S.H.; Rhee, E.J.; Park, C.Y.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Task Force Team for Diabetes Fact Sheet of the Korean Diabetes, A. Trends in diabetic retinopathy and related medical practices among type 2 diabetes patients: Results from the National Insurance Service Survey 2006–2013. J. Diabetes Investig. 2018, 9, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Suh, J.D.; Han, K.D.; Lee, H.M. Uvulopalatopharyngoplasty reduces the incidence of depression caused by obstructive sleep apnea. Laryngoscope 2019, 129, 1005–1009. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, I.; Kim, M.S.; Yu, M.S.; Cho, B.J.; Kim, D.K. Association of Chronic Rhinosinusitis With Depression and Anxiety in a Nationwide Insurance Population. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 313–319. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, I.; Kim, M.S.; Kim, D.W.; Cho, B.J.; Kim, D.K. Relationship of Chronic Rhinosinusitis with Asthma, Myocardial Infarction, Stroke, Anxiety, and Depression. J. Allergy Clin. Immunol. Pract. 2020, 8, 721–727. [Google Scholar] [CrossRef]
- Rhee, T.M.; Choi, E.K.; Han, K.D.; Lee, S.R.; Oh, S. Impact of the Combinations of Allergic Diseases on Myocardial Infarction and Mortality. J. Allergy Clin. Immunol. Pract. 2021, 9, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; In, S.M.; Kim, J.Y.; Hong, J.Y.; Han, K.D.; Kim, J.S.; Jung, Y.G. Association of nasal septal deviation with the incidence of anxiety, depression, and migraine: A national population-based study. PLoS ONE 2021, 16, e0259468. [Google Scholar] [CrossRef]
- Wise, S.K.; Wise, J.C.; DelGaudio, J.M. Association of nasopharyngeal and laryngopharyngeal reflux with postnasal drip symptomatology in patients with and without rhinosinusitis. Am. J. Rhinol. 2006, 20, 283–289. [Google Scholar] [CrossRef]
- Brown, H.J.; Kuhar, H.N.; Plitt, M.A.; Husain, I.; Batra, P.S.; Tajudeen, B.A. The Impact of Laryngopharyngeal Reflux on Patient-reported Measures of Chronic Rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2020, 129, 886–893. [Google Scholar] [CrossRef]
- Takabayashi, T.; Schleimer, R.P. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J. Allergy Clin. Immunol. 2020, 145, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Forster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziaber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e1444. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.; Patsias, A.; Posner, M.; Sikora, A. The role of inflammation in head and neck cancer. Adv. Exp. Med. Biol. 2014, 816, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.J.; Liu, Y.C.; Zhou, M.L.; Zhou, S.H. The role of innate lymphoid cells in nasal inflammation and cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10588–10599. [Google Scholar] [CrossRef]
- Wang, P.C.; Lin, H.C.; Kang, J.H. Chronic rhinosinusitis confers an increased risk of acute myocardial infarction. Am. J. Rhinol. Allergy 2013, 27, e178–e182. [Google Scholar] [CrossRef]
- Hao, W.R.; Lin, H.W.; Chao, P.Z.; Wu, C.W.; Yen, T.H.; Liu, J.C.; Liou, T.H. Risk of myocardial infarction in patients with rhinosinusitis. Atherosclerosis 2013, 226, 263–268. [Google Scholar] [CrossRef]
- Xia, C.X.; Kao, Y.W.; Qin, L.; Chen, M.C.; Shia, B.C.; Wu, S.Y. Cancer risk in chronic rhinosinusitis: A propensity score matched case-control cohort study. Am. J. Transl. Res. 2019, 11, 7146–7156. [Google Scholar]
- Huang, P.W.; Chiou, Y.R.; Wu, S.L.; Liu, J.C.; Chiou, K.R. Risk of nasopharyngeal carcinoma in patients with chronic rhinosinusitis: A nationwide propensity score matched study in Taiwan. Asia Pac. J. Clin. Oncol. 2021, 17, 442–447. [Google Scholar] [CrossRef]
- Resta, O.; Carpanano, G.E.; Lacedonia, D.; Di Gioia, G.; Giliberti, T.; Stefano, A.; Bonfitto, P. Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA). Respir. Med. 2005, 99, 91–96. [Google Scholar] [CrossRef]
- Murphy, B.A.; Wulff-Burchfield, E.; Ghiam, M.; Bond, S.M.; Deng, J. Chronic Systemic Symptoms in Head and Neck Cancer Patients. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz004. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Su, K.; Fu, Z.; Wang, P.; Shi, H. Anxiety and depression in patients with nasal septal deviation. Am. J. Otolaryngol. 2020, 41, 102450. [Google Scholar] [CrossRef] [PubMed]
- Valsamidis, K.; Titelis, K.; Karkos, P.; Markou, K.; Constantinidis, J.; Triaridis, S. Predictive factors of patients’ general quality of life after nasal septoplasty. Eur. Arch. Otorhinolaryngol. 2019, 276, 429–438. [Google Scholar] [CrossRef] [PubMed]
| Disease | Working Definition |
|---|---|
| Chronic rhinusinitis | J32 + ≥1 out-patient visit within 3 months + nasal endoscopy (E7530, E7540, E7550 or E7560) |
| Diabetes | At least one claim under ICD-10 E11-14 + ≥2 out-patient visits or ≥1 admission within 1 year |
| Hypertension | At least one claim under ICD-10 I10-13 or I15 + ≥2 out-patient visits or ≥1 admission within 1 year |
| Dyslipidemia | E78 + ≥2 out-patient visits or ≥1 admission within 1 year |
| Rhinitis | J30 + ≥3 out-patient visits within 1 year |
| Variable | Control (n = 649,548) | CRS (n = 324,774) | p Value |
|---|---|---|---|
| Male | 267,046 (41.11%) | 133,523 (41.11%) | 1.00 |
| Mean age (year) | 45.31 ± 15.35 | 45.31 ± 15.35 | 1.00 |
| Income lowest quintile | 138,945 (21.39%) | 69,684 (21.46%) | 0.46 |
| Urban residency | 306,502 (41.19%) | 157,269 (48.42%) | <0.001 |
| Diabetes | 34,010 (5.24%) | 19,565 (6.02%) | <0.001 |
| Hypertension | 107,391 (16.53%) | 61,466 (18.93%) | <0.001 |
| Dyslipidemia | 50,800 (7.82%) | 32,155 (9.9%) | <0.001 |
| Rhinitis | 83,857 (12.91%) | 189,440 (58.33%) | <0.001 |
| HNC | Groups | N | Events | Duration | Rate | Model 1 | Model 2 | Model 3 | Mode 4 |
|---|---|---|---|---|---|---|---|---|---|
| Oral/oropharynx | Control | 649,548 | 124 | 5,416,889.91 | 0.0229 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 71 | 2,650,612.95 | 0.0268 | 1.175 (0.878–1.574) | 1.230 (0.919–1.648) | 1.212 (0.904–1.624) | 1.250 (0.8968–1.745) | |
| Salivary gland | Control | 649,548 | 73 | 5,416,889.91 | 0.0135 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 38 | 2,650,612.95 | 0.0143 | 1.07 (0.723–1.584) | 1.103 (0.745–1.633) | 1.076 (0.726–1.594) | 1.155 (0.739–1.804) | |
| NPC | Control | 649,548 | 44 | 5,416,889.91 | 0.0081 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 29 | 2,650,612.95 | 0.0109 | 1.348 (0.843–2.154) | 1.384 (0.866–2.213) | 1.392 (0.870–2.226) | 1.165 (0.672–2.019) | |
| NCPSC | Control | 649,548 | 41 | 5,416,889.91 | 0.0076 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 38 | 2,650,612.95 | 0.0143 | 1.900 (1.222–2.954) | 2.031 (1.305–3.160) | 2.041 (1.311–3.179) | 1.809 (1.085–3.016) | |
| HPLC | Control | 649,548 | 197 | 5,416,889.91 | 0.0364 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 109 | 2,650,612.95 | 0.0411 | 1.135 (0.898–1.434) | 1.259 (0.996–1.592) | 1.247 (0.985–1.577) | 1.343 (1.031–1.748) | |
| Thyroid | Control | 649,548 | 5651 | 5,416,889.91 | 1.0432 | 1 | 1 | 1 | 1 |
| CRS | 324,774 | 3305 | 2,650,612.95 | 1.2469 | 1.195 (1.145–1.247) | 1.186 (1.136–1.238) | 1.186 (1.136–1.238) | 1.116 (1.063–1.173) |
| HNC | Groups | N | Events | Duration | Rate | Model 1 | Model 2 | Model 3 | Mode 4 |
|---|---|---|---|---|---|---|---|---|---|
| Oral/oropharynx | CRSsNP | 305,907 | 66 | 2,496,904.8 | 0.0264 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 5 | 153,708.15 | 0.03225 | 1.231 (0.496–3.056) | 0.941 (0.378–2.344) | 0.952 (0.382–2.371) | 0.961 (0.379–2.441) | |
| Salivary gland | CRSsNP | 305,907 | 34 | 2,496,904.8 | 0.0136 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 4 | 153,708.15 | 0.026 | 1.908 (0.677–5.376) | 1.597 (0.562–4.539) | 1.595 (0.561–4.535) | 1.608 (0.549–4.710) | |
| NPC | CRSsNP | 305,907 | 28 | 2,496,904.8 | 0.0112 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 1 | 153,708.15 | 0.0065 | 0.569 (0.077–4.185) | 0.385 (0.052–2.836) | 0.379 (0.051–2.795) | 0.336 (0.045–2.526) | |
| NCPSC | CRSsNP | 305,907 | 30 | 2,496,904.8 | 0.012 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 8 | 153,708.15 | 0.052 | 4.340 (1.989–9.468) | 3.321 (1.510–7.306) | 3.332 (1.513–7.336) | 3.140 (1.383–7.128) | |
| HPLC | CRSsNP | 305,907 | 103 | 2,496,904.8 | 0.0413 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 6 | 153,708.15 | 0.039 | 0.946 (0.416–2.154) | 0.654 (0.2887–1.491) | 0.655 (0.287–1.495) | 0.750 (0.327–1.721) | |
| Thyroid | CRSsNP | 305,907 | 3140 | 2,496,904.8 | 1.2576 | 1 | 1 | 1 | 1 |
| CRSwNP | 18,867 | 165 | 153,708.15 | 1.0735 | 0.848 (0.725–0.992) | 1.143 (0.976–1.338) | 1.144 (0.977–1.339) | 1.112 (0.947–1.305) |
| Variable | Oral/Oropharynx | Salivary Gland | NPC | NCPSC | HPLC | Thyroid | |
|---|---|---|---|---|---|---|---|
| Sex | Male | 1.224 (0.824–1.817) | 1.515 (0.830–2.768) | 1.540 (0.827–2.2866) | 1.830 (1.014–3.302) | 1.329 (1.011–1.747) | 1.169 (1.045–1.307) |
| Female | 1.304 (0.768–2.215) | 0.905 (0.499–1.642) | 0.554 (0.197–1.561) | 1.761 (0.753–4.123) | 1.500 (0.668–3.366) | 1.107 (1.051–1.167) | |
| Age | <50 | 0.784 (0.429–1.435) | 1.252 (0.658–2.383) | 1.005 (0.445–2.273) | 1.547 (0.571–4.194) | 0.827 (0.344–1.988) | 1.095 (1.033–1.161) |
| ≥50 | 1.475 (1.013–2.148) | 1.095 (0.628–1.908) | 1.267 (0.655–2.449) | 1.858 (1.064–3.243) | 1.371 (1.045–1.800) | 1.161 (1.074–1.254) | |
| Residency | Rural | 1.035 (0.665–1.609) | 1.217 (0.680–2.177) | 1.505 (0.757–2.990) | 1.117 (0.542–2.301) | 1.473 (1.058–2.051) | 1.158 (1.085–1.236) |
| Urban | 1.542 (0.983–2.418) | 1.090 (0.594–2.001) | 0.844 (0.387–1.839) | 2.712 (1.392–5.285) | 1.190 (0.814–1.742) | 1.074 (1.006–1.147) | |
| Diabetes | No | 1.243 (0.876–1.762) | 1.260 (0.795–1.998) | 1.026 (0.578–1.820) | 1.813 (1.070–3.071) | 1.177 (0.882–1.572) | 1.111 (1.056–1.168) |
| Yes | 1.314 (0.531–3.253) | 0.462 (0.099–2.165) | 6.620 (0.729–60.102) | 1.769 (0.351–8.916) | 2.517 (1.410–4.493) | 1.243 (1.012–1.526) | |
| Hypertension | No | 1.098 (0.745–1.618) | 1.006 (0.580–1.743) | 1.186 (0.633–2.222) | 1.303 (0.704–2.409) | 1.064 (0.754–1.502) | 1.102 (1.045–1.162) |
| Yes | 1.715 (0.975–3.015) | 1.446 (0.743–2.817) | 1.114 (0.440–2.2822) | 3.446 (1.486–7.992) | 1.799 (1.242–2.606) | 1.188 (1.066–1.324) | |
| Dyslipidemia | No | 1.268 (0.877–1.833) | 1.217 (0.749–1.978) | 1.217 (0.689–2.148) | 1.685 (0.977–2.907) | 1.275 (0.957–1.698) | 1.109 (1.054–1.167) |
| Yes | 1.190 (0.622–2.277) | 0.935 (0.370–2.363) | 0.728 (0.131–4.044) | 2.776 (0.796–9.677) | 1.713 (0.966–3.038) | 1.192 (1.027–1.384) | |
| Rhinitis | No | 1.543 (1.056–2.254) | 1.211 (0.722–2.034) | 1.139 (0.563–2.302) | 1.567 (0.827–2.969) | 1.190 (0.857–1.654) | 1.202 (1.131–1.279) |
| Yes | 0.793 (0.457–1.375) | 1.027 (0.460–2.292) | 1.207 (0.501–2.908) | 2.476 (0.939–6.527) | 1.757 (1.070–2.885) | 0.992 (0.918–1.071) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.-D.; Park, S.-H.; Son, S.; Kim, S.-H.; Kim, I.; Kim, J.-Y.; In, S.-M.; Kim, Y.-S.; Lee, K.-I. Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study. J. Clin. Med. 2022, 11, 5316. https://doi.org/10.3390/jcm11185316
Han K-D, Park S-H, Son S, Kim S-H, Kim I, Kim J-Y, In S-M, Kim Y-S, Lee K-I. Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study. Journal of Clinical Medicine. 2022; 11(18):5316. https://doi.org/10.3390/jcm11185316
Chicago/Turabian StyleHan, Kyung-Do, Sang-Hyun Park, Sumin Son, Seung-Ho Kim, Ikhee Kim, Jong-Yeup Kim, Seung-Min In, Yeon-Soo Kim, and Ki-Il Lee. 2022. "Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study" Journal of Clinical Medicine 11, no. 18: 5316. https://doi.org/10.3390/jcm11185316
APA StyleHan, K.-D., Park, S.-H., Son, S., Kim, S.-H., Kim, I., Kim, J.-Y., In, S.-M., Kim, Y.-S., & Lee, K.-I. (2022). Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study. Journal of Clinical Medicine, 11(18), 5316. https://doi.org/10.3390/jcm11185316








