Diagnostic Value of Middle Meatal Cultures versus Maxillary Sinus Culture in Acute and Chronic Sinusitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration and Search Strategy
2.2. Selection Criteria
2.3. Analysis Methods and Bias Assessment
2.4. Statistical Analysis and Outcomes
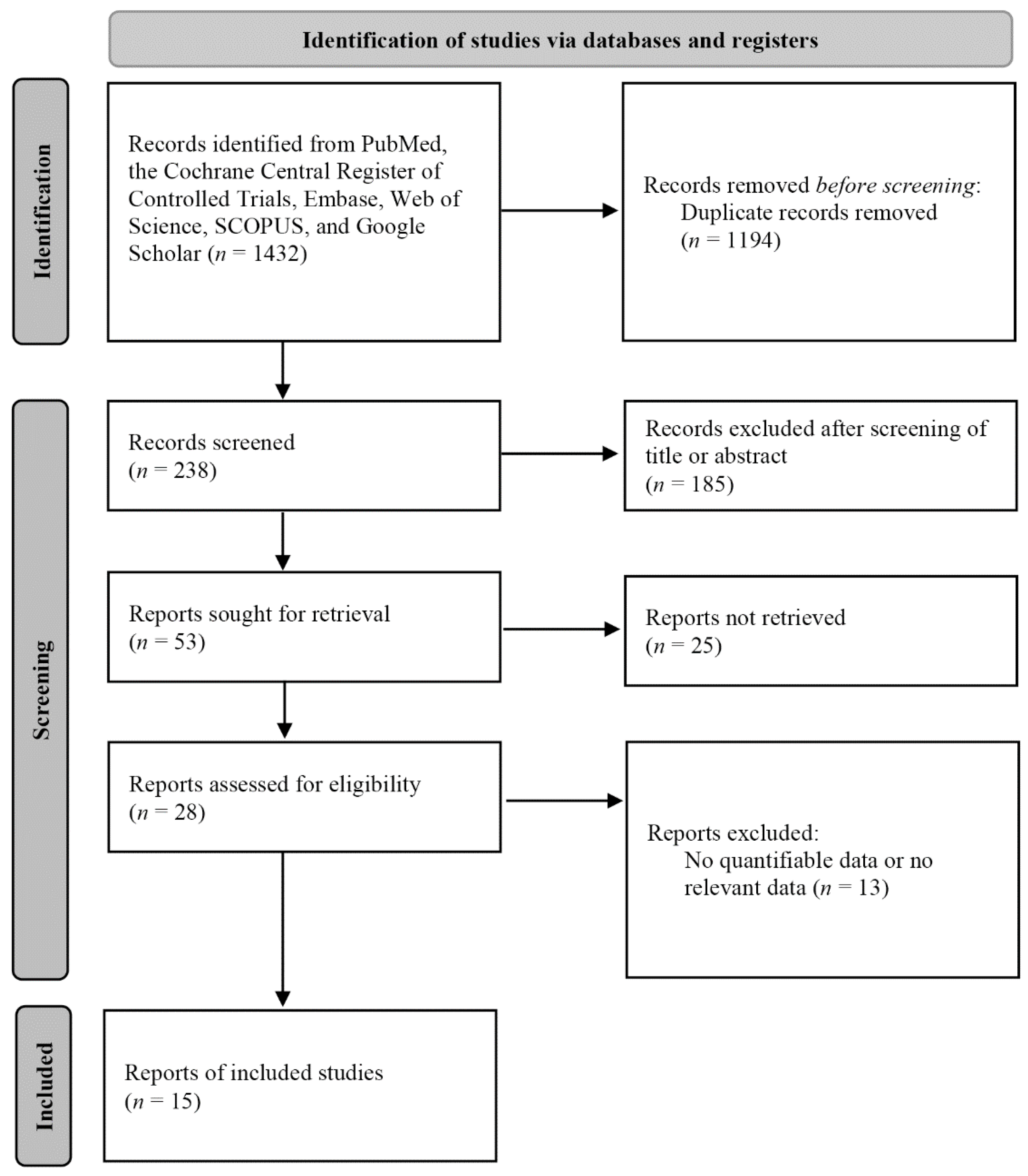
3. Results
3.1. Correlations between Middle Meatus and Maxillary Sinus Cultures
3.2. Diagnostic Accuracy of Middle Meatus and Maxillary Sinus Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirtsreesakul, V.; Chatwiwat, Y.; Laohaprertthisan, V. A comparison between endoscopically middle meatal aspiration culture using modified aspiration instrument and direct maxillary antral tap culture in chronic rhinosinusitis. J. Med. Assoc. Thai. 2005, 88, 1591–1597. [Google Scholar] [PubMed]
- Dubin, M.G.; Ebert, C.S.; Coffey, C.S.; Melroy, C.T.; Sonnenburg, R.E.; Senior, B.A. Concordance of middle meatal swab and maxillary sinus aspirate in acute and chronic sinusitis: A meta-analysis. Am. J. Rhinol. 2005, 19, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Tami, T.A. Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope 1997, 107, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Szaleniec, J.; Gibała, A.; Hartwich, P.; Hydzik-Sobocińska, K.; Konior, M.; Gosiewski, T.; Szaleniec, M. Challenging the gold standard: Methods of sampling for microbial culture in patients with chronic rhinosinusitis. Eur. Arch. Otorhinolaryngol. 2021, 278, 4795–4803. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Vogan, J.C.; Bolger, W.E.; Keyes, A.S. Endoscopically guided sinonasal cultures: A direct comparison with maxillary sinus aspirate cultures. Otolaryngol. Head Neck Surg. 2000, 122, 370–373. [Google Scholar] [CrossRef]
- Klossek, J.M.; Dubreuil, L.; Richet, H.; Richet, B.; Beutter, P. Bacteriology of chronic purulent secretions in chronic rhinosinusitis. J. Laryngol. Otol. 1998, 112, 1162–1166. [Google Scholar] [CrossRef]
- Talbot, G.H.; Kennedy, D.W.; Scheld, W.M.; Granito, K. Rigid nasal endoscopy versus sinus puncture and aspiration for microbiologic documentation of acute bacterial maxillary sinusitis. Clin. Infect. Dis. 2001, 33, 1668–1675. [Google Scholar] [CrossRef]
- Benninger, M.S.; Payne, S.C.; Ferguson, B.J.; Hadley, J.A.; Ahmad, N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: A meta-analysis. Otolaryngol. Head Neck Surg. 2006, 134, 3–9. [Google Scholar] [CrossRef]
- Araujo, E.; Dall, C.; Cantarelli, V.; Pereira, A.; Mariante, A.R. Microbiology of middle meatus in chronic rhinosinusitis. Braz. J. Otorhinolaryngol. 2007, 73, 549–555. [Google Scholar] [CrossRef]
- Hsin, C.H.; Chen, T.H.; Su, M.C.; Jiang, R.S.; Liu, C.M. Aspiration technique improves reliability of endoscopically directed middle meatal cultures in pediatric rhinosinusitis. Am. J. Rhinol. Allergy 2010, 24, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, U.; Engström, K.; Olaison, S.; Hugosson, S. Anterior rhinoscopy and middle meatal culture in acute rhinosinusitis. J. Laryngol. Otol. 2013, 127, 1088–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsin, C.H.; Tsao, C.H.; Su, M.C.; Chou, M.C.; Liu, C.M. Comparison of maxillary sinus puncture with endoscopic middle meatal culture in pediatric rhinosinusitis. Am. J. Rhinol. 2008, 22, 280–284. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Stybayeva, G.; Lim, S.Y.; Hwang, S.H. Predictive Value of Olfactory and Taste Symptoms in the Diagnosis of COVID-19: A Systematic Review and Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2021, 14, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.; Kim, S.W.; Hwang, S.H. The Efficacy of Hypotensive Agents on Intraoperative Bleeding and Recovery Following General Anesthesia for Nasal Surgery: A Network Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2021, 14, 200–209. [Google Scholar] [CrossRef]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- Casiano, R.R.; Cohn, S.; Villasuso, E., 3rd; Brown, M.; Memari, F.; Barquist, E.; Namias, N. Comparison of antral tap with endoscopically directed nasal culture. Laryngoscope 2001, 111, 1333–1337. [Google Scholar] [CrossRef]
- Kountakis, S.E.; Skoulas, I.G. Middle meatal vs antral lavage cultures in intensive care unit patients. Otolaryngol. Head Neck Surg. 2002, 126, 377–381. [Google Scholar] [CrossRef]
- Joniau, S.; Vlaminck, S.; Van Landuyt, H.; Kuhweide, R.; Dick, C. Microbiology of sinus puncture versus middle meatal aspiration in acute bacterial maxillary sinusitis. Am J Rhinol 2005, 19, 135–140. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Straka, M.B. Endoscopically directed middle meatal aspirate compared to antral tap in the diagnosis of acute and chronic bacterial rhinosinusitis. In Proceedings of the Annual Meeting of the American Rhinologic Society, New Orleans, LA, USA, 26 September 1999. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, S.W.; Song, E.A.; Lee, J.; Kim, D.H. Methylene Blue as a Diagnosis and Screening Tool for Oral Cancer and Precancer. Otolaryngol. Head Neck Surg. 2021, 164, 271–276. [Google Scholar] [CrossRef] [PubMed]
- DeMuri, G.P.; Wald, E.R. Microbiology of Acute, Subacute, and Chronic Rhinosinusitis in Children. In Diseases of the Sinuses; Chang, C., Incaudo, G., Gershwin, M., Eds.; Springer: New York, NY, USA, 2013; pp. 89–97. [Google Scholar]
- Payne, S.C.; Benninger, M.S. Staphylococcus aureus is a major pathogen in acute bacterial rhinosinusitis: A meta-analysis. Clin. Infect. Dis. 2007, 45, e121–e127. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Pignataro, L.; Torretta, S. Microbiological Aspects of Acute and Chronic Pediatric Rhinosinusitis. J. Clin. Med. 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Hopp, R.J.; Allison, J.; Brooks, D. Fifty Years of Chronic Rhinosinusitis in Children: The Accepted, the Unknown, and Thoughts for the Future. Pediatr. Allergy Immunol. Pulmonol. 2016, 29, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, E.; Cheng, J. A Pilot Investigation of the Pediatric Nasal Microbiome. Otolaryngol. Head Neck Surg. 2021, 165, 895–898. [Google Scholar] [CrossRef]
- Stapleton, A.L.; Shaffer, A.D.; Morris, A.; Li, K.; Fitch, A.; Methé, B.A. The microbiome of pediatric patients with chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2021, 11, 31–39. [Google Scholar] [CrossRef]
- Javer, A.R.; Genoway, K.; Tsaparas, Y. Comparison of swabs versus suction traps for endoscopically guided sinus cultures. J. Otolaryngol. Head Neck Surg. 2008, 37, 185–191. [Google Scholar]
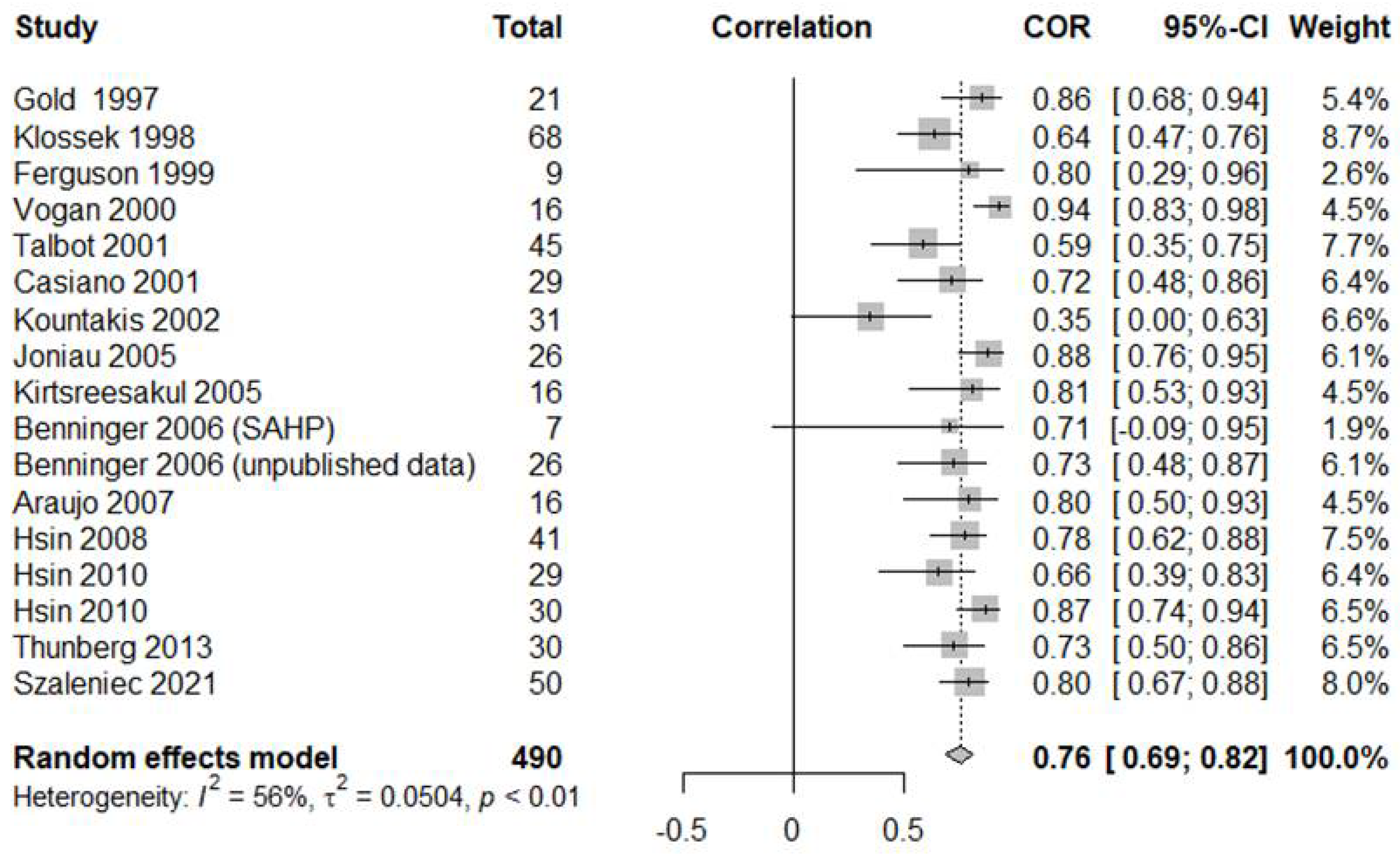

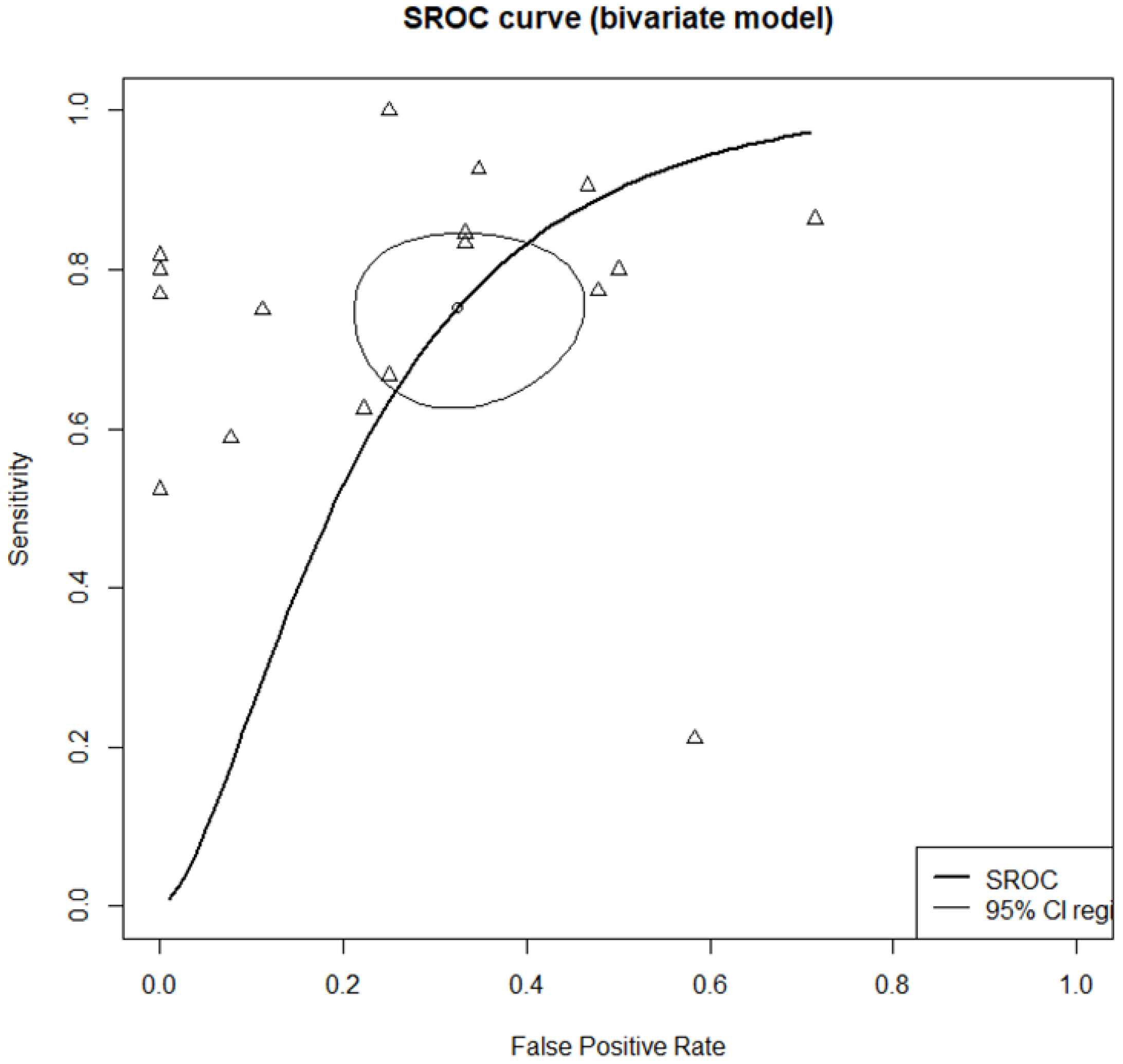
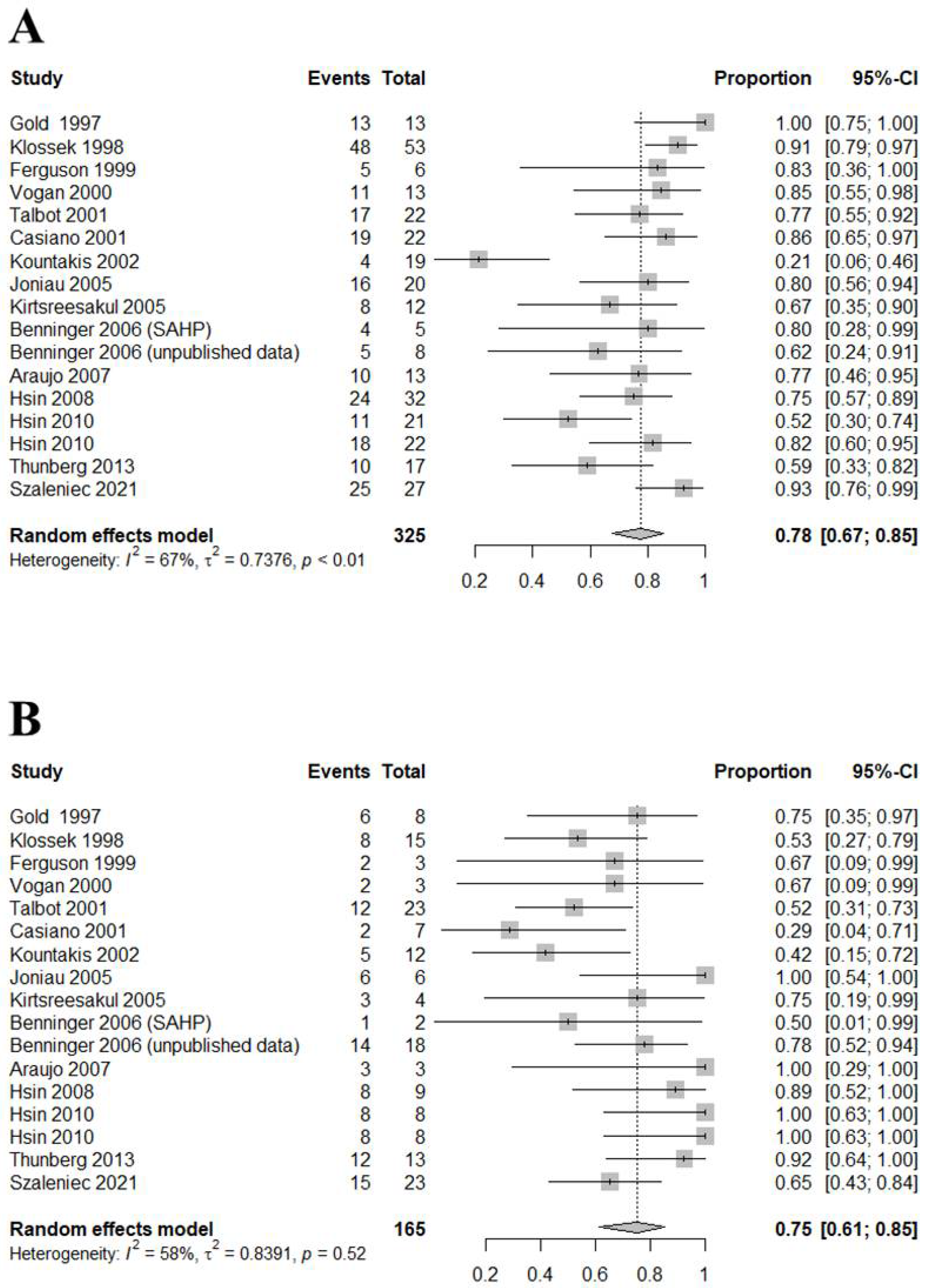
| Sensitivity | Specificity | DOR | Correlation | |
|---|---|---|---|---|
| Total (n = 17) | 0.7759 [0.6744; 0.8526]; I2 = 67.4% | 0.7514 [0.6110; 0.8534]: I2 = 58.2% | 8.5475 [3.9238; 18.6199]; I2 = 51.0% | 0.7613 [0.6917; 0.8169]; I2 = 56.0% |
| Adult (n = 14) | 0.7931 [0.6734; 0.8769]; I2 = 68.0% | 0.6704 [0.5461; 0.7747]; I2 = 38.5% | 6.8726 [2.9181; 16.1863]; I2 = 54.1% | 0.7563 [0.6716; 0.8215]; I2 = 58.7% |
| Children (n = 3) | 0.7079 [0.5600; 0.8219]; I2 = 36.5% | 0.9600 [0.7645; 0.9944]; I2 = 0.0% | 29.5081 [6.3469; 137.1903]; I2 = 0.0% | 0.7849 [0.6468; 0.8732]; I2 = 48.4% |
| p = 0.3152 | p = 0.0193 | p = 0.1045 | p = 0.6809 | |
| Acute sinusitis (n = 9) | 0.7184 [0.5553; 0.8391]; I2 = 63.4% | 0.6694 [0.4740; 0.8197]; I2 = 55.1% | 4.2660 [1.3833; 13.1559]; I2 = 57.8% | 0.7458 [0.6040; 0.8418]; I2 = 67.9% |
| Chronic sinusitis (n = 8) | 0.8219 [0.7039; 0.8995]; I2 = 63.0% | 0.8226 [0.6144; 0.9310]; I2 = 50.8% | 18.2397 [8.3954; 39.6272]; I2 = 0.0% | 0.7745 [0.7038; 0.8300]; I2 = 31.5% |
| p = 0.2338 | p = 0.2256 | p = 0.0373 | p = 0.6637 | |
| Biopsy (n = 1) | 0.8636 [0.6521; 0.9554]; I2 = NA | 0.2857 [0.0720; 0.6734]; I2 = NA | 2.5333 [0.3286; 19.5311]; I2 = NA | 0.7200 [0.5416; 0.8984]; I2 = NA |
| Suction (n = 7) | 0.7979 [0.7047; 0.8672]; I2 = 0.0% | 0.8400 [0.7114; 0.9179]: I2 = 0.0% | 15.1597 [5.4793; 41.9424]; I2 = 0.0% | 0.8353 [0.7734; 0.8814]; I2 = 0.0% |
| Swab (n = 9) | 0.7386 [0.5637; 0.8607]; I2 = 78.5% | 0.7000 [0.5256; 0.8308]: I2 = 56.2% | 6.7136 [2.0918; 21.5475]; I2 = 67.9% | 0.7115 [0.5944; 0.7990]; I2 = 65.0% |
| p = 0.5355 | p = 0.0158 | p = 0.2553 | p = 0.0547 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Kim, S.W.; Basurrah, M.A.; Hwang, S.H. Diagnostic Value of Middle Meatal Cultures versus Maxillary Sinus Culture in Acute and Chronic Sinusitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6069. https://doi.org/10.3390/jcm11206069
Kim DH, Kim SW, Basurrah MA, Hwang SH. Diagnostic Value of Middle Meatal Cultures versus Maxillary Sinus Culture in Acute and Chronic Sinusitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(20):6069. https://doi.org/10.3390/jcm11206069
Chicago/Turabian StyleKim, Do Hyun, Sung Won Kim, Mohammed Abdullah Basurrah, and Se Hwan Hwang. 2022. "Diagnostic Value of Middle Meatal Cultures versus Maxillary Sinus Culture in Acute and Chronic Sinusitis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 20: 6069. https://doi.org/10.3390/jcm11206069
APA StyleKim, D. H., Kim, S. W., Basurrah, M. A., & Hwang, S. H. (2022). Diagnostic Value of Middle Meatal Cultures versus Maxillary Sinus Culture in Acute and Chronic Sinusitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(20), 6069. https://doi.org/10.3390/jcm11206069








