Leisure-Time Physical Activity Has a More Favourable Impact on Carotid Artery Stiffness Than Vigorous Physical Activity in Hypertensive Human Beings
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Procedures
2.3. Statistics
3. Results
3.1. Carotid Stiffness Parameters
3.2. Multiple Regression Analysis
4. Discussion
Role of Heart Rate
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2016. Diabetes Care 2016, 39 (Suppl. S1), S1–S111. [Google Scholar]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Graniero, G.; Mormino, P.; Nicolosi, L.; Mos, L.; Visentin, P.; Pessina, A.C. Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST trial. Circulation 1994, 90, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Jurik, R.; Stastny, P.J. Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review. Clin. Med. 2019, 8, 1393. [Google Scholar] [CrossRef]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef]
- Palatini, P.; Puato, M.; Rattazzi, M.; Pauletto, P. Effect of regular physical activity on Carotid Intima-Media Thickness. Results from a 6-year Prospective Study in the early stage of hypertension. Blood Press 2011, 20, 37–44. [Google Scholar] [CrossRef]
- Saladini, F.; Benetti, E.; Mos, L.; Mazzer, A.; Casiglia, E.; Palatini, P. Regular physical activity is associated with improved small artery distensibility in young to middle-age stage 1 hypertensives. Vasc. Med. 2014, 19, 458–464. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar]
- Lee, D.H.; Rezende, L.F.M.; Joh, H.; Keum, N.; Ferrari, G.; Rey-Lopez, J.P.; Rimm, E.B.; Tabung, F.K.; Giovannucci, E.L. Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. Circulation 2022, 146, 523–534. [Google Scholar] [CrossRef]
- Vriz, O.; Magne, J.; Jarosh, J.; Bossone, E.; Aboyans, V.; Palatini, P. Local carotid arterial stiffness is an independent determinant of left ventricular remodeling in never-treated hypertensive patients. Blood Press 2019, 28, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Favretto, S.; Jaroch, J.; Wojciech, R.; Bossone, E.; Driussi, C.; Antonini-Canterin, F.; Palatini, P.; Loboz-Grudzien, K. Left Ventricular Function Assessed by One-Point Carotid Wave Intensity in Newly Diagnosed Untreated Hypertensive Patients. J. Ultrasound Med. 2017, 36, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [PubMed]
- Tanaka, M.; Sugawara, M.; Ogasawara, Y.; Suminoe, I.; Izumi, T.; Niki, K.; Kajiya, F. Noninvasive evaluation of left ventricular force-frequency relationships by measuring carotid arterial wave intensity during exercise stress. J. Med. Ultrason. 2015, 42, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Zito, C.; di Bello, V.A.; La Carrubba, S.; Driussi, S.; Carerj, S.; Bossone, E.; Antonini-Canterin, F. Non-invasive one-point carotid wave intensity in a large group of healthy subjects: A ventricular-arterial coupling parameter. Heart Vessel. 2016, 31, 360–369. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Tomoto, T.; Liu, J.; Tseng, B.Y.; Pasha, E.P.; Cardim, D.; Tarumi, T.; Hynan, L.S.; Munro Cullum, C.; Zhang, R. One-Year Aerobic Exercise Reduced Carotid Arterial Stiffness and Increased Cerebral Blood Flow in Amnestic Mild Cognitive Impairment. J. Alzheimers. Dis. 2021, 80, 841–853. [Google Scholar] [CrossRef]
- Saz-Lara, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Reina-Gutiérrez, S.; Sequí-Domínguez, I.; Ruiz, J.R.; Martínez-Vizcaíno, V. What type of physical exercise should be recommended for improving arterial stiffness on adult population? A network meta-analysis. Eur. J. Cardiovasc. Nurs. 2021, 20, 696–716. [Google Scholar] [CrossRef]
- Sugawara, J.; Otsuki, T.; Tanabe, T.; Hayashi, K.; Maeda, S.; Matsuda, M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am. J. Hypertens 2006, 19, 1032–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, L.; Xu, L.; Sun, X.; Liu, W.; Zhou, S.; van de Vosse, F.; Greenwald, S.E. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0200829. [Google Scholar] [CrossRef] [PubMed]
- Kollet, D.P.; Marenco, A.B.; Bellé, N.L.; Barbosa, E.; Boll, L.; Eibel, B.; Waclawovsky, G.; Lehnen, A.M. Aerobic exercise, but not isometric handgrip exercise, improves endothelial function and arterial stiffness in patients with myocardial infarction undergoing coronary intervention: A randomized pilot study. BMC Cardiovasc. Disord. 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Shoemaker, J.K.; Overend, T.J.; Petrella, R.J. Effects of lifestyle modification on central artery stiffness in metabolic syndrome subjects with pre-hypertension and/or pre-diabetes. Diabetes Res. Clin. Pract. 2009, 83, 249–256. [Google Scholar] [CrossRef]
- Palatini, P.; Visentin, P.; Dorigatti, F.; Guarnieri, C.; Santonastaso, M.; Cozzio, S.; Pegoraro, F.; Bortolazzi, A.; Vriz, O.; Mos, L.; et al. Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur. Heart J. 2009, 30, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Li, M.Y.; Li, K.H.; Yang, X.; Yang, Y.Y.; Zhao, X.X.; Yan, T.; Li, M.M.; Luo, S.Q.; Zhang, M.L.; et al. Effect of Exercise Prescription Implementation Rate on Cardiovascular Events. Front. Cardiovasc. Med. 2022, 8, 753672. [Google Scholar] [CrossRef]
- Rossi, A.; Dikareva, A.; Bacon, S.L.; Daskalopoulou, S.S. The impact of physical activity on mortality in patients with high blood pressure: A systematic review. J. Hypertens 2012, 30, 1277–1288. [Google Scholar] [CrossRef]
- Joseph, G.; Marott, J.L.; Torp-Pedersen, C.; Biering-Sørensen, T.; Nielsen, G.; Christensen, A.E.; Johansen, M.B.; Schnohr, P.; Sogaard, P.; Mogelvang, R. Dose-Response Association between Level of Physical Activity and Mortality in Normal, Elevated, and High Blood Pressure. Hypertension 2019, 74, 1307–1315. [Google Scholar] [CrossRef]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; De Gonzalez, A.B.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Schnohr, P.; O’Keefe, J.H.; Lavie, C.J.; Holtermann, A.; Lange, P.; Jensen, G.B.; Marott, J.L. U-Shaped Association between Duration of Sports Activities and Mortality: Copenhagen City Heart Study. Mayo Clin. Proc. 2021, 96, 3012–3020. [Google Scholar] [CrossRef]
- Lee, D.C.; Sui, X.; Artero, E.G.; Lee, I.M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W., III; Blair, S.N. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: The Aerobics Center Longitudinal Study. Circulation 2011, 124, 2483–2490. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Gao, F. Prospective Advances in Beneficial Effects of Exercise on Human Health. Adv. Exp. Med. Biol. 2020, 1228, 455–459. [Google Scholar]
- Lee, D.H.; Rezende, L.F.; Ferrari, G.; Aune, D.; Keum, N.; Tabung, F.K.; Giovannucci, E.L. Physical activity and all-cause and cause-specific mortality: Assessing the impact of reverse causation and measurement error in two large prospective cohorts. Eur. J. Epidemiol. 2021, 36, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Saladini, F.; Palatini, P. Arterial Distensibility, Physical Activity, and the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 39–46. [Google Scholar] [CrossRef]
- Völz, S.; Svedlund, S.; Andersson, B.; Li-Ming, G.; Rundqvist, B. Coronary flow reserve in patients with resistant hypertension. Clin. Res. Cardiol. 2017, 106, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; De Ciuceis, C.; Porteri, E.; Paiardi, S.; Boari, G.E.; Mortini, P.; Cornali, C.; Cenzato, M.; Rodella, L.F.; Borsani, E.; et al. Altered structure of small cerebral arteries in patients with essential hypertension. J. Hypertens 2009, 27, 838–845. [Google Scholar] [CrossRef]
- Paiardi, S.; Rodella, L.F.; De Ciuceis, C.; Porteri, E.; Boari, G.E.; Rezzani, R.; Rizzardi, N.; Platto, C.; Tiberio, G.A.; Giulini, S.M.; et al. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin. Hemorheol. Microcirc. 2009, 42, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Buess, R.; Nyberg, M.; Hoppeler, H.; Odriozola, A.; Thaning, P.; Hellsten, Y.; Baum, O.; Mortensen, S.P. Capillary growth, ultrastructure remodelling and exercise training in skeletal muscle of essential hypertensive patients. Acta Physiol. 2015, 214, 210–220. [Google Scholar] [CrossRef]
- Dekleva, M.; Lazic, J.; Arandjelovic, A.; Mazic, S. Beneficial and harmful effects of exercise in hypertensive patients: The role of oxidative stress. Hypertens Res. 2017, 40, 15–20. [Google Scholar] [CrossRef]
- Pierce, G.L. Aortic Stiffness in Aging and Hypertension: Prevention and Treatment with Habitual Aerobic Exercise. Curr. Hypertens Rep. 2017, 19, 90–99. [Google Scholar] [CrossRef]
- Kraft, K.A.; Arena, R.; Arrowood, J.A.; Fei, D.Y. High aerobic capacity does not attenuate aortic stiffness in hypertensive subjects. Am. Heart J. 2007, 154, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Lantelme, P.; Mestre, C.; Lievre, M.; Gressard, A.; Milon, H. Heart rate: An important confounder of pulse wave velocity assessment. Hypertension 2002, 39, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.; Spronck, B.; Kiat, H.; Barin, E.; Reesink, K.D.; Delhaas, T.; Avolio, A.P.; Butlin, M. Heart rate dependency of large artery stiffness. Hypertension 2016, 68, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, H.; Hashimoto, H.; Tanaka, H.; Matsumoto, C.; Odaira, M.; Yamada, J.; Yoshida, M.; Shiina, K.; Nagata, M.; Yamashina, A. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: A prospective study. J. Hypertens 2010, 28, 687–694. [Google Scholar] [CrossRef]
- Benetos, A.; Adamopoulos, C.; Bureau, J.M.; Temmar, M.; Labat, C.; Bean, K.; Thomas, F.; Pannier, B.; Asmar, R.; Zureik, M.; et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002, 105, 1202–1207. [Google Scholar] [CrossRef]
- Tanaka, H. Antiaging effects of aerobic exercise on systemic arteries. Hypertension 2019, 74, 237–243. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Tan, X.; Hu, M.; Shen, B. Exercise restores impaired endothelium-derived hyperpolarizing factor-mediated vasodilation in aged rat aortic arteries via the TRPV4-KCa2.3 signaling complex. Clin. Interv. Aging 2019, 14, 1579–1587. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, C.; Xu, G.; Tang, Z.; Zhang, Z.; Liu, Y.; Wang, Z. Effects of aerobic exercise on the expressions and activities of nitric oxide synthases in the blood vessel endothelium in prediabetes mellitus. Exp. Ther. Med. 2019, 17, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.P.; Nascimento, A.R.; Lopes, G.O.; Medeiros-Lima, D.J.M.; Coelho, M.P.; Nascimento, P.M.C.; Kopiler, D.A.; Matsuura, C.; Mediano, M.F.F.; Tibirica, E. The impact of exercise frequency upon microvascular endothelium function and oxidative stress among patients with coronary artery disease. Clin. Physiol. Funct. Imaging 2018, 38, 840–846. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012, 87, 587–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
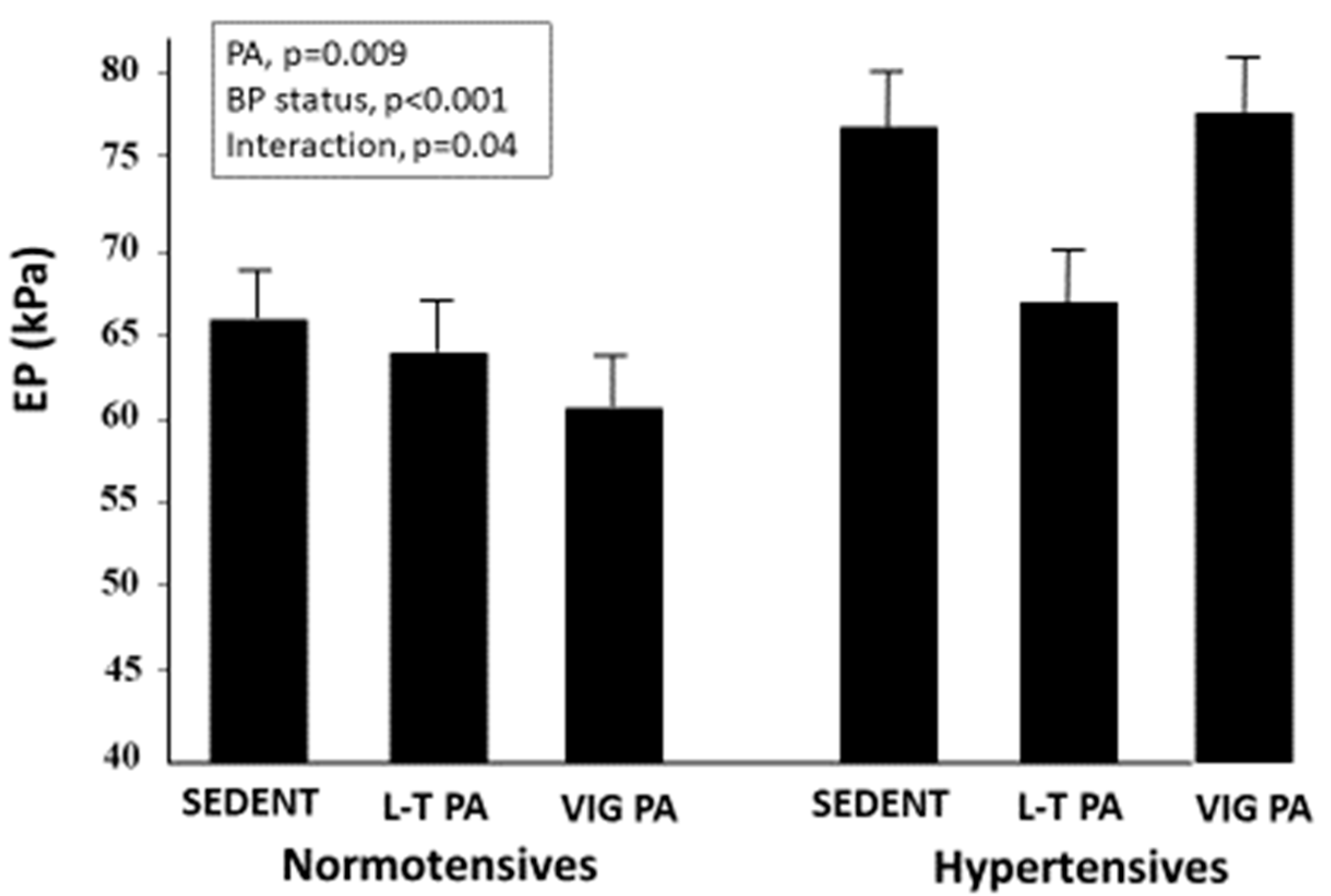
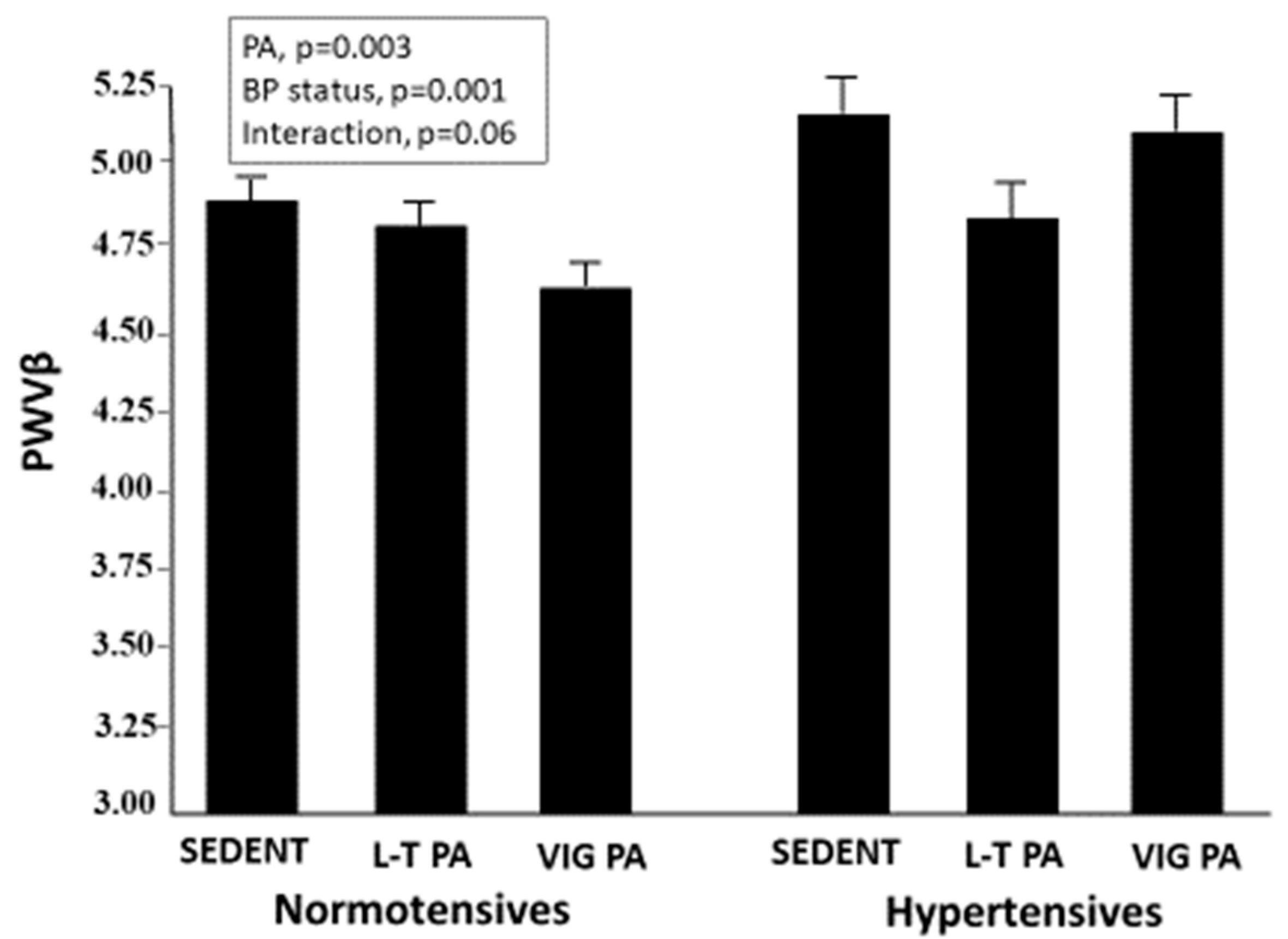
| Sedentary | Leisure-Time PA | Competitive Athletes | p-Value | |
|---|---|---|---|---|
| N | 120 | 120 | 120 | |
| Age, years | 33.9 (13.1) | 31.8 (12.8) | 29.6 (14.1) | 0.042 |
| Sex, men | 77.5% | 77.5% | 83.3% | 0.435 |
| BMI, kg/m2 | 24.4 (3.7) | 23.7 (3.7) | 22.8 (2.8) | 0.002 |
| Office Systolic BP, mmHg | 123.8 (12.5) | 123.9 (12.8) | 122.2 (12.4) | 0.57 |
| Office Diastolic BP, mmHg | 75.5 (10.2) | 73.8 (10.3) | 72.2 (9.8) | 0.19 |
| Heart rate, bpm | 70.6 (11.1) | 66.1 (9.3) | 59.5 (12.5) | <0.001 |
| Smoking, yes | 10.0% | 17.5% | 7.5% | 0.043 |
| Predictors | Coefficient | Standard Error | T | p-Value | R Partial |
|---|---|---|---|---|---|
| Age, years | 1.1225 | 0.0886 | 12.664 | <0.0001 | 0.5595 |
| Sex | 3.6221 | 2.7703 | 1.307 | 0.19 | 0.0695 |
| Body mass index, Kg/m2 | 0.5522 | 0.3482 | 1.586 | 0.11 | 0.0842 |
| Smoking, yes/no | 3.4167 | 3.1723 | 1.077 | 0.28 | 0.0573 |
| Systolic blood pressure, mmHg | 0.5364 | 0.0896 | 5.986 | <0.0001 | 0.3040 |
| Physical activity, no/yes | −4.7026 | 2.3142 | −2.032 | 0.042 | −0.1077 |
| Heart rate, bpm | 0.3189 | 0.0947 | 3.367 | 0.0008 | 0.1766 |
| Predictors | Coefficient | Standard Error | T | p-Value | R Partial |
|---|---|---|---|---|---|
| Age, years | 0.0417 | 0.0029 | 14.337 | <0.0001 | 0.6072 |
| Sex | 0.1700 | 0.0909 | 1.870 | 0.062 | 0.0992 |
| Body mass index, Kg/m2 | 0.0260 | 0.0114 | 2.273 | 0.024 | 0.1203 |
| Smoking, yes/no | 0.1050 | 0.1041 | 1.008 | 0.31 | 0.0537 |
| Systolic blood pressure, mmHg | 0.0184 | 0.0029 | 6.251 | <0.0001 | 0.3161 |
| Physical activity, no/yes | −0.2054 | 0.0760 | −2.703 | 0.007 | −0.1426 |
| Heart rate, bpm | 0.0104 | 0.0031 | 3.338 | 0.0009 | 0.1752 |
| Models | AIC | Delta AIC | BIC |
|---|---|---|---|
| EP | |||
| Model including PA | 3386.7 | 3398.4 | |
| Base Model * (M0) | 3163.9 | 222.8 | 3195.0 |
| Model 1 (M0 + Heart rate) | 3154.5 | 9.4 | 3189.5 |
| PWVβ | |||
| Model including PA | 968.3 | 978.0 | |
| Base Model * (M0) | 703.9 | 264.4 | 735.0 |
| Model 1 (M0 + Heart rate) | 694.7 | 9.2 | 729.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vriz, O.; Mos, L.; Palatini, P. Leisure-Time Physical Activity Has a More Favourable Impact on Carotid Artery Stiffness Than Vigorous Physical Activity in Hypertensive Human Beings. J. Clin. Med. 2022, 11, 5303. https://doi.org/10.3390/jcm11185303
Vriz O, Mos L, Palatini P. Leisure-Time Physical Activity Has a More Favourable Impact on Carotid Artery Stiffness Than Vigorous Physical Activity in Hypertensive Human Beings. Journal of Clinical Medicine. 2022; 11(18):5303. https://doi.org/10.3390/jcm11185303
Chicago/Turabian StyleVriz, Olga, Lucio Mos, and Paolo Palatini. 2022. "Leisure-Time Physical Activity Has a More Favourable Impact on Carotid Artery Stiffness Than Vigorous Physical Activity in Hypertensive Human Beings" Journal of Clinical Medicine 11, no. 18: 5303. https://doi.org/10.3390/jcm11185303
APA StyleVriz, O., Mos, L., & Palatini, P. (2022). Leisure-Time Physical Activity Has a More Favourable Impact on Carotid Artery Stiffness Than Vigorous Physical Activity in Hypertensive Human Beings. Journal of Clinical Medicine, 11(18), 5303. https://doi.org/10.3390/jcm11185303





