Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Variable Measures
2.3. Imaging Data Acquisition and Processing
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
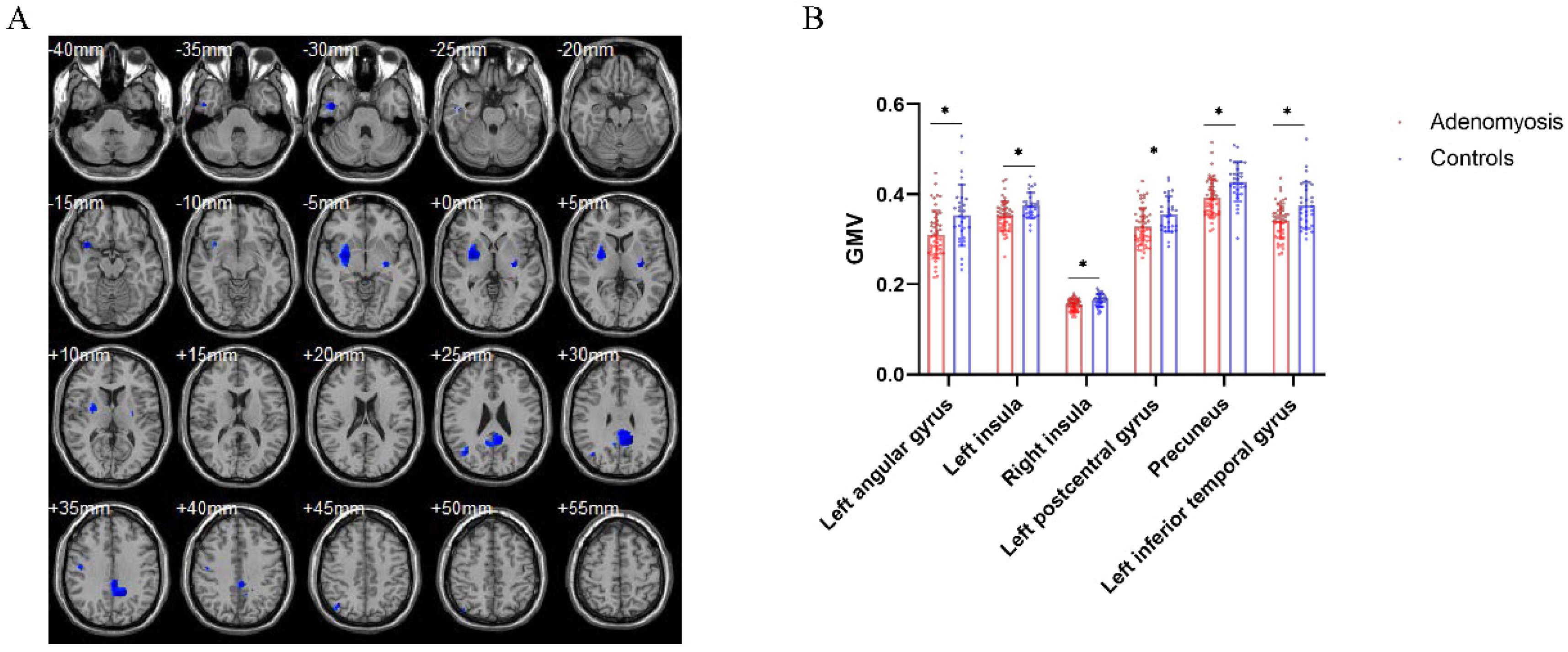
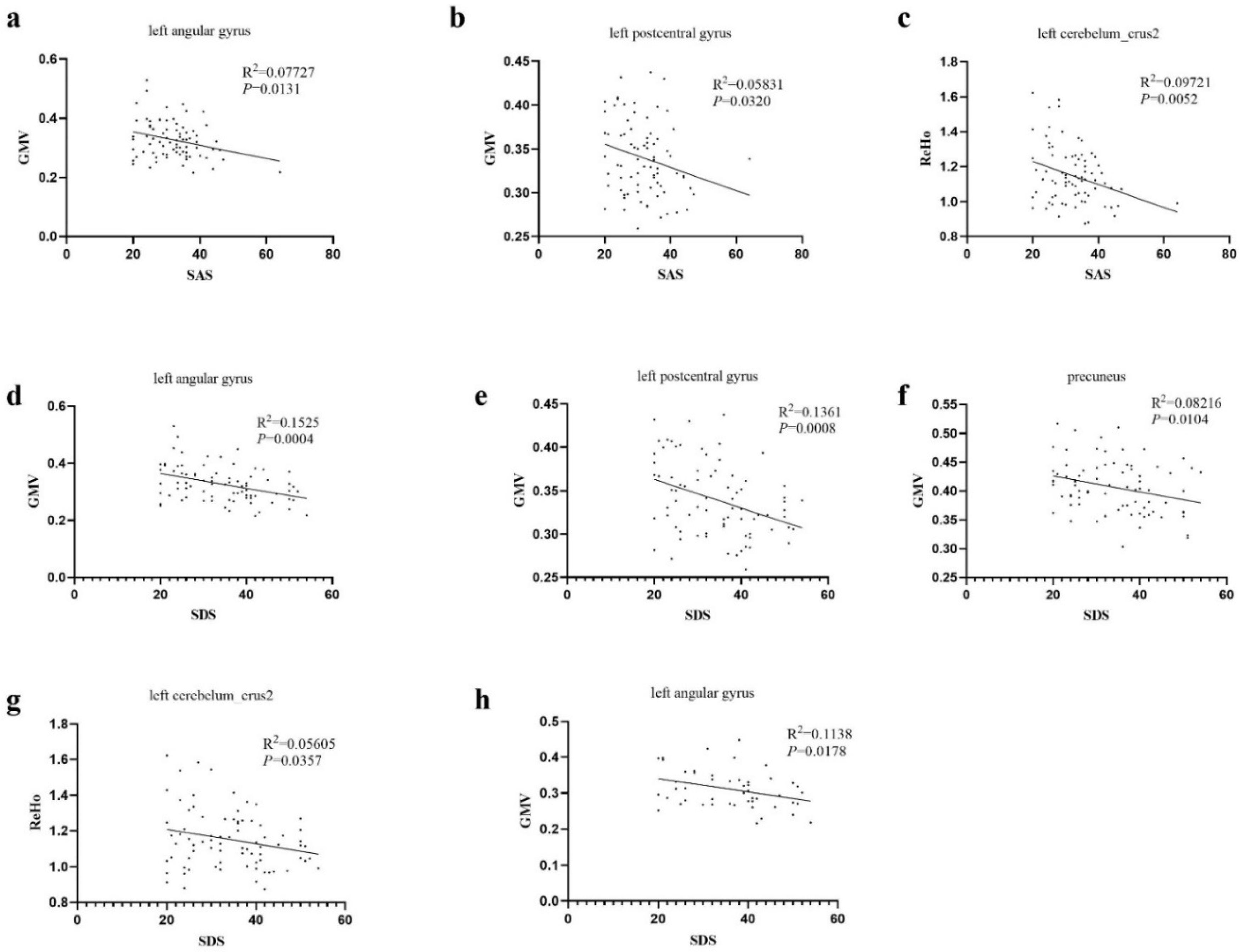
3.2. Brain Structural Differences between Patients with AM and Pain-Free Controls
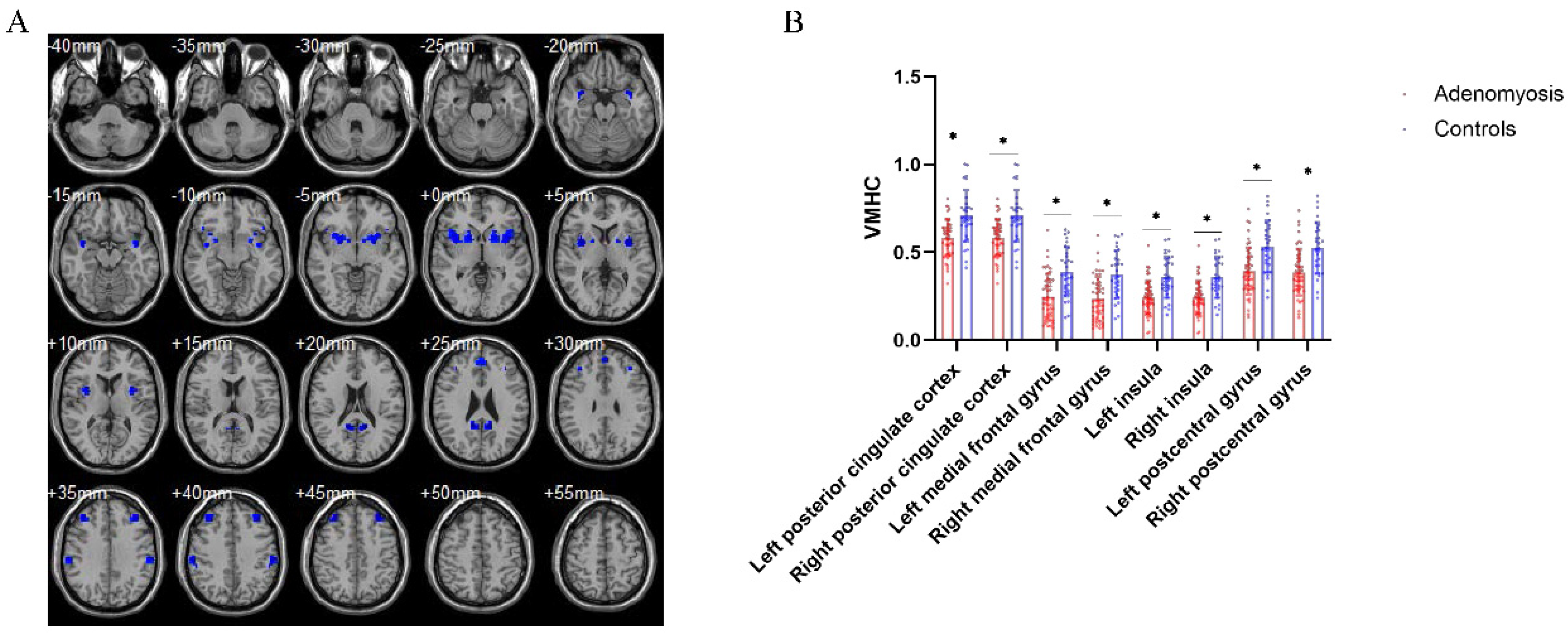
3.3. Aberrant Interhemispheric Functional Connectivity between AM Patients and Pain-Free Controls
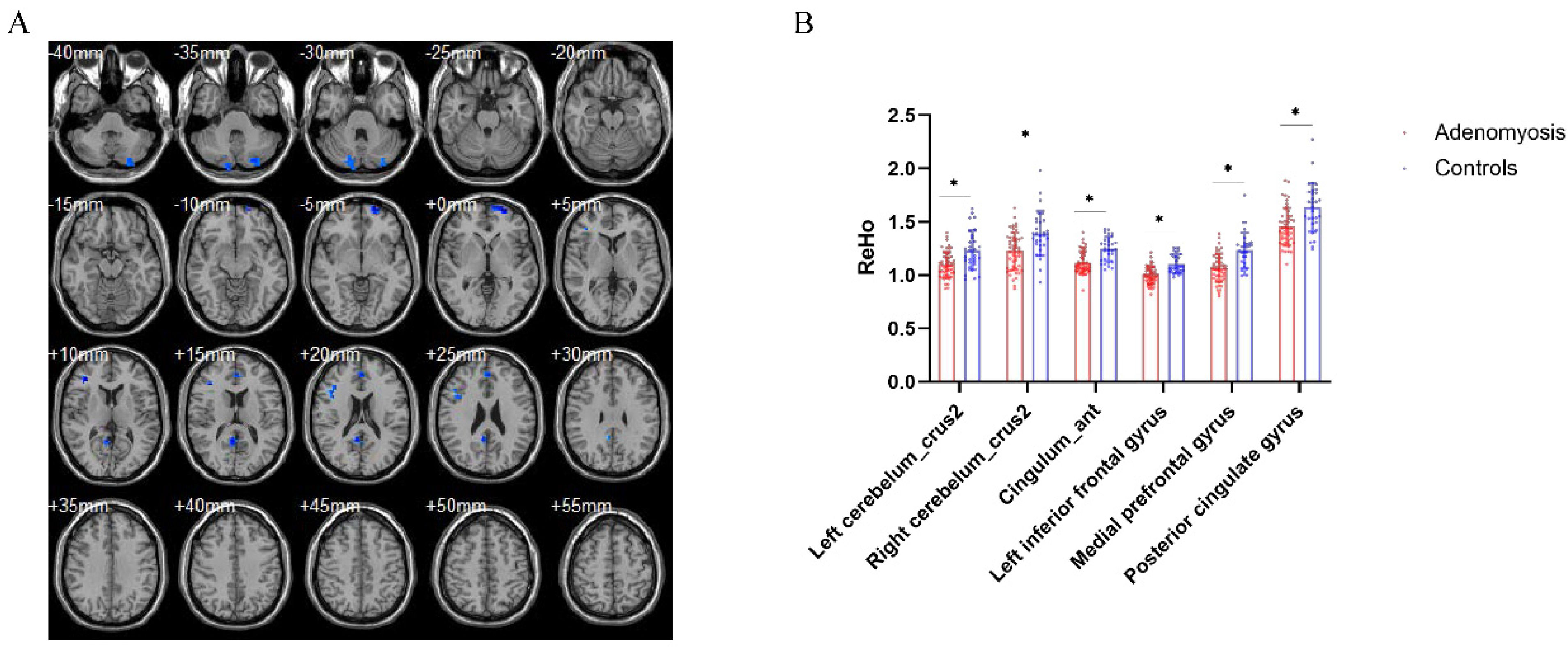
3.4. Brain Regional Homogeneity Differences between AM Patients and Pain-Free Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.W.; Just, P.A.; Noël, J.C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.M.; Mechsner, S. Pathogenesis of endometriosis: The origin of pain and subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Marcellin, L.; Maignien, C.; Maitrot-Mantelet, L.; Bordonne, C.; Plu Bureau, G.; Chapron, C. Adenomyosis: An update regarding its diagnosis and clinical features. J. Gynecol. Obstet. Hum. Reprod 2021, 50, 102228. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.S.; Staud, R.; Price, D.D. Individual differences in pain sensitivity: Measurement, causation, and consequences. J. Pain 2009, 10, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, N.; Montanari, G.; Benfenati, A.; Leonardi, D.; Bertoldo, V.; Monti, G.; Raimondo, D.; Seracchioli, R. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod Biol. 2014, 181, 289–293. [Google Scholar] [CrossRef]
- Barcena de Arellano, M.L.; Oldeweme, J.; Arnold, J.; Schneider, A.; Mechsner, S. Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis. Fertil. Steril. 2013, 100, 801–809. [Google Scholar] [CrossRef]
- Xu, X.; Cai, X.; Liu, X.; Guo, S.W. Possible involvement of neuropeptide and neurotransmitter receptors in adenomyosis. Reprod Biol. Endocrinol. 2021, 19, 25. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Morioka, S.; Niiro, E.; Shigemitsu, A.; Ito, F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch. Gynecol. Obstet. 2014, 289, 13–21. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef]
- Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, A.M.; Martínez-Zamora, M.Á.; Gracia, M.; Ros, C.; Rius, M.; Nicolás, I.; Carmona, F. Impact of adenomyosis on women’s psychological health and work productivity: A comparative cross-sectional study. J. Womens Health 2021, 30, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yuan, M.; Li, Q.; Ji, M.; Jiao, X.; Wang, G. Higher Risk of Anxiety and Depression in Women with Adenomyosis as Compared with Those with Uterine Leiomyoma. J. Clin. Med. 2022, 7, 2638. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.E.; Glover, G.H. Functional magnetic resonance imaging methods. Neuropsychol. Rev. 2015, 25, 289–313. [Google Scholar] [CrossRef]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional homogeneity approach to fMRI data analysis. NeuroImage 2004, 22, 394–400. [Google Scholar] [CrossRef]
- Li, J.L.; Yan, C.Q.; Wang, X.; Zhang, S.; Zhang, N.; Hu, S.Q.; Wang, L.Q.; Liu, C.Z. Brain functional alternations of the pain-related emotional and cognitive regions in patients with chronic shoulder pain. J. Pain Res. 2020, 13, 575–583. [Google Scholar] [CrossRef]
- Huang, S.; Wakaizumi, K.; Wu, B.; Shen, B.; Wu, B.; Fan, L.; Baliki, M.N.; Zhan, G.; Apkarian, A.V.; Huang, L. Whole-brain functional network disruption in chronic pain with disk herniation. Pain 2019, 160, 2829–2840. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Chen, N.; Zhao, Z.; Guo, M.; Liu, H.; Feng, J.; Zhang, D.; Yao, Z.; Hu, B. Inter-hemispheric functional connections are more vulnerable to attack than structural connection in patients with irritable bowel syndrome. J. Neurogastroenterol. Motil. 2021, 27, 426–435. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Xue, C.; Zhang, S.; Huang, Q.; Liu, W. A Radiomics approach to predicting Parkinson’s disease by incorporating whole-brain functional activity and gray matter structure. Front Neurosci. 2020, 14, 751. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.; Yang, Z.; Huang, H.; Zheng, R.; Wang, W.; Wei, Y.; Cheng, J.; Han, S.; Zhang, Y. Shared gray matter alterations in subtypes of addiction: A voxel-wise meta-analysis. Psychopharmacology 2021, 238, 2365–2379. [Google Scholar] [CrossRef]
- Barroso, J.; Vigotsky, A.D.; Branco, P.; Reis, A.M.; Schnitzer, T.J.; Galhardo, V.; Apkarian, A.V. Brain gray matter abnormalities in osteoarthritis pain: A cross-sectional evaluation. Pain 2020, 161, 2167–2178. [Google Scholar] [CrossRef]
- Chronic Pelvic Pain: ACOG Practice Bulletin, Number 218. Obstet. Gynecol. 2020, 135, e98–e109. [CrossRef] [PubMed]
- Price, D.D.; Bush, F.M.; Long, S.; Harkins, S.W. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994, 56, 217–226. [Google Scholar] [CrossRef]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of endometriosis on quality of life and mental health: Pelvic pain makes the difference. J. Psychosom. Obstet. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef]
- Dunstan, D.A.; Scott, N. Norms for Zung’s self-rating anxiety scale. BMC Psychiatry 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Thurber, S.; Snow, M.; Honts, C.R. The Zung Self-Rating Depression Scale: Convergent validity and diagnostic discrimination. Assessment 2002, 9, 401–405. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data processing & analysis for (Resting-State) brain imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.N.; Kelly, C.; Di Martino, A.; Mennes, M.; Margulies, D.S.; Bangaru, S.; Grzadzinski, R.; Evans, A.C.; Zang, Y.F.; Castellanos, F.X.; et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010, 30, 15034–15043. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.A.; Baranowski, A.P.; Berghmans, B.; Borovicka, J.; Cottrell, A.M.; Dinis-Oliveira, P.; Elneil, S.; Hughes, J.; Messelink, B.; de Williams, A.C.; et al. Management of chronic primary pelvic pain syndromes. BJU Int. 2022, 129, 572–581. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [Green Version]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Ashar, Y.K.; Gordon, A.; Schubiner, H.; Uipi, C.; Knight, K.; Anderson, Z.; Carlisle, J.; Polisky, L.; Geuter, S.; Flood, T.F.; et al. Effect of pain reprocessing therapy vs placebo and usual care for patients with chronic back pain: A randomized clinical trial. JAMA Psychiatry 2021, 29, e212669. [Google Scholar] [CrossRef] [PubMed]
- Geuter, S.; Boll, S.; Eippert, F.; Büchel, C. Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. eLife 2017, 6, e24770. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Grabner, R.H.; Ansari, D.; Koschutnig, K.; Reishofer, G.; Ebner, F. The function of the left angular gyrus in mental arithmetic: Evidence from the associative confusion effect. Hum. Brain Mapp. 2013, 34, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Sosa, Y.; Krauss, B.R.; Thomas, P.S.; Fredrickson, B.E.; Levy, R.E.; Harden, R.N.; Chialvo, D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004, 108, 129–136. [Google Scholar] [CrossRef]
- Naylor, B.; Hesam-Shariati, N.; McAuley, J.H.; Boag, S.; Newton-John, T.; Rae, C.D.; Gustin, S.M. Reduced glutamate in the medial prefrontal cortex is associated with emotional and cognitive dysregulation in people with chronic pain. Front Neurol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Kang, D.; Hesam-Shariati, N.; McAuley, J.H.; Alam, M.; Trost, Z.; Rae, C.D.; Gustin, S.M. Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in people with chronic pain. Eur. J. Pain 2021, 25, 2242–2256. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Chen, Y.; Liang, Z.; Jiang, J.; Misrani, A.; Su, Y.; Peng, Y.; Chen, J.; Tang, B.; et al. Pelvic pain alters functional connectivity between anterior cingulate cortex and hippocampus in both humans and a rat model. Front Syst. Neurosci. 2021, 15, 642349. [Google Scholar] [CrossRef]
- Xiao, X.; Ding, M.; Zhang, Y.Q. Role of the anterior cingulate cortex in translational pain research. Neurosci. Bull 2021, 37, 405–422. [Google Scholar] [CrossRef]
- Naegel, S.; Holle, D.; Desmarattes, N.; Theysohn, N.; Diener, H.C.; Katsarava, Z.; Obermann, M. Cortical plasticity in episodic and chronic cluster headache. NeuroImage Clin. 2014, 6, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | Adenomyosis (n = 49) | Controls (n = 30) | p-Value |
|---|---|---|---|
| Age (y) | 40.52 ± 6.28 | 39.27 ± 7.33 | 0.420 |
| BMI (kg/m2) | 24.67 ± 3.69 | 24.77 ± 3.43 | 0.906 |
| Age of menarche (y) | 13.40 ± 1.43 | 13.27 ± 1.20 | 0.914 |
| Menstrual cycle (d) | 29.88 ± 8.85 | 28.80 ± 4.06 | 0.534 |
| Menstruation (d) | 6.35 ± 2.33 | 5.87 ± 1.40 | 0.508 |
| Menorrhagia n (%) | 26 (52.00%) | 4 (13.33%) | 0.001 * |
| Gravidity | 2.98 ± 1.70 | 3.27 ± 1.72 | 0.437 |
| Uterine volume (cm3) | 327.19 ± 147.05 | 98.59 ± 45.32 | <0.001 * |
| HGB (g/L) | 104.53 ± 20.07 | 122.27 ± 16.59 | <0.001 * |
| NRS | 8.40 ± 1.78 | 0.43 ± 0.82 | <0.001 * |
| SAS | 33.38 ± 8.17 | 29.16 ± 6.29 | 0.018 * |
| SDS | 35.96 ± 9.80 | 31.13 ± 8.32 | 0.027 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Yuan, M.; Li, Q.; Huang, Y.; Ji, M.; Li, J.; Yan, S.; Sun, H.; Wang, X.; Pan, Z.; et al. Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study. J. Clin. Med. 2022, 11, 5286. https://doi.org/10.3390/jcm11185286
Jiao X, Yuan M, Li Q, Huang Y, Ji M, Li J, Yan S, Sun H, Wang X, Pan Z, et al. Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study. Journal of Clinical Medicine. 2022; 11(18):5286. https://doi.org/10.3390/jcm11185286
Chicago/Turabian StyleJiao, Xue, Ming Yuan, Qiuju Li, Yufei Huang, Miaomiao Ji, Jing Li, Shumin Yan, Hao Sun, Xinyu Wang, Zangyu Pan, and et al. 2022. "Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study" Journal of Clinical Medicine 11, no. 18: 5286. https://doi.org/10.3390/jcm11185286
APA StyleJiao, X., Yuan, M., Li, Q., Huang, Y., Ji, M., Li, J., Yan, S., Sun, H., Wang, X., Pan, Z., Ren, Q., Wang, D., & Wang, G. (2022). Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study. Journal of Clinical Medicine, 11(18), 5286. https://doi.org/10.3390/jcm11185286











