The Prevalence and Clinical Impact of Adenomyosis in Pregnancy-Related Hysterectomy
Abstract
1. Introduction
2. Materials and Methods
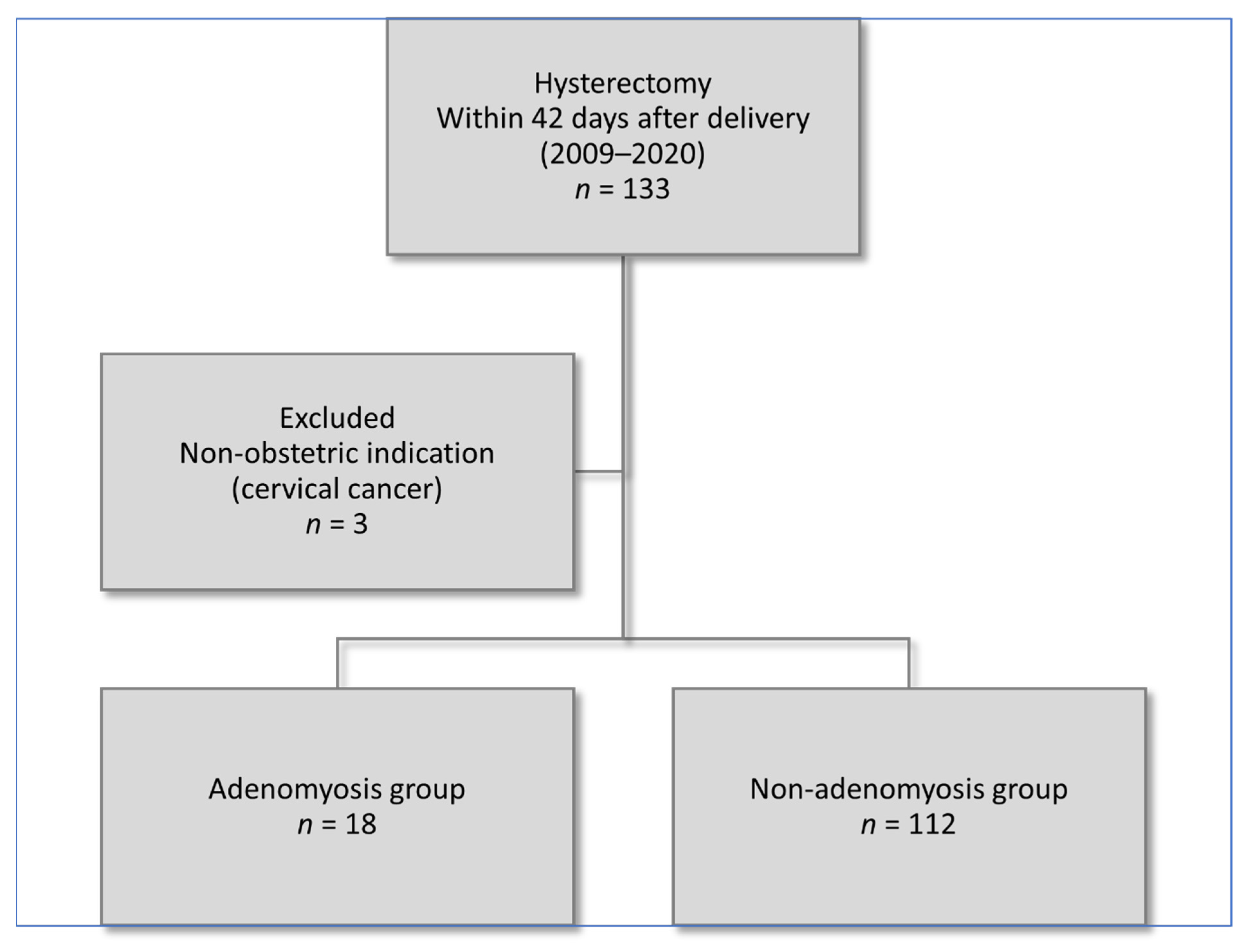
2.1. Study Population
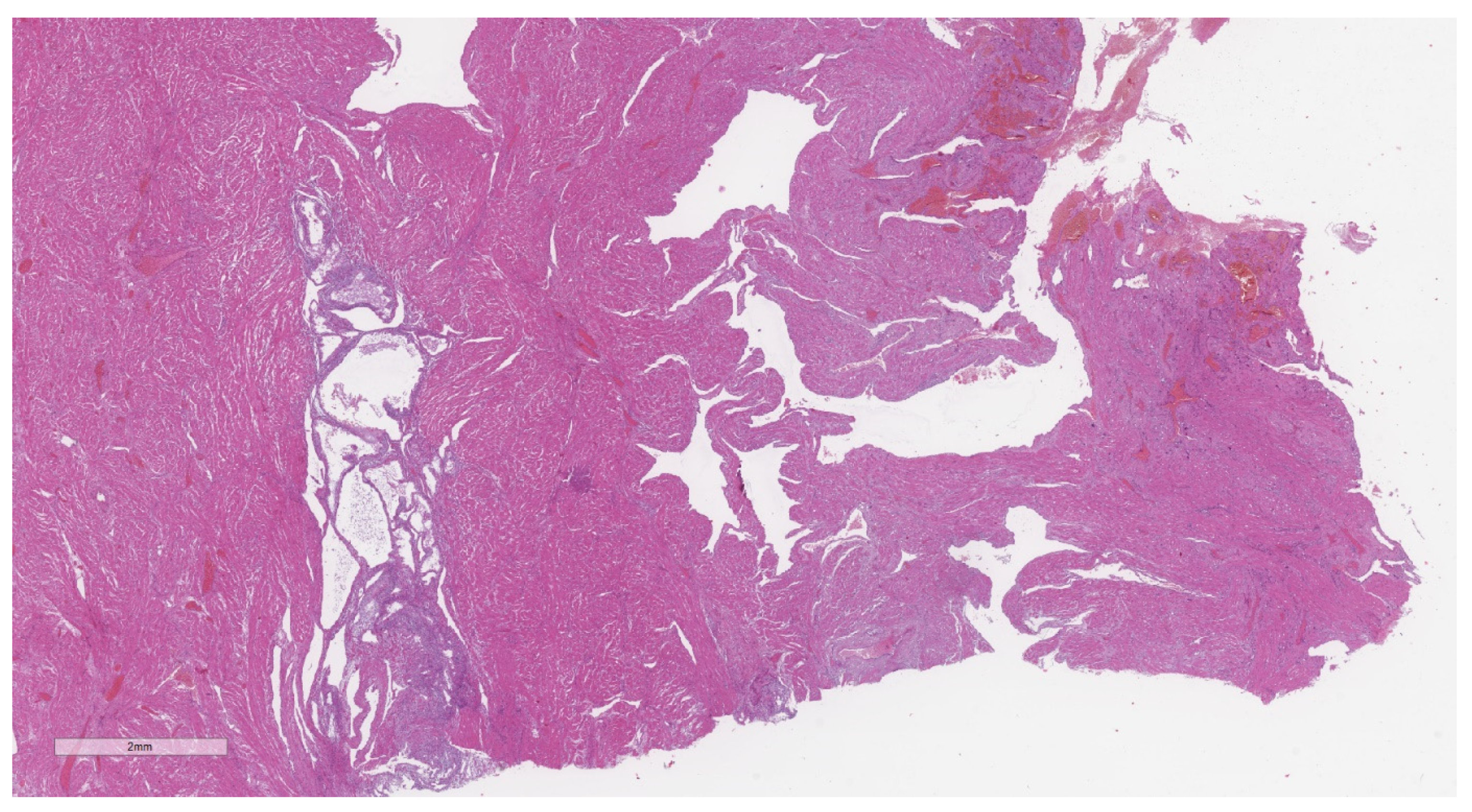
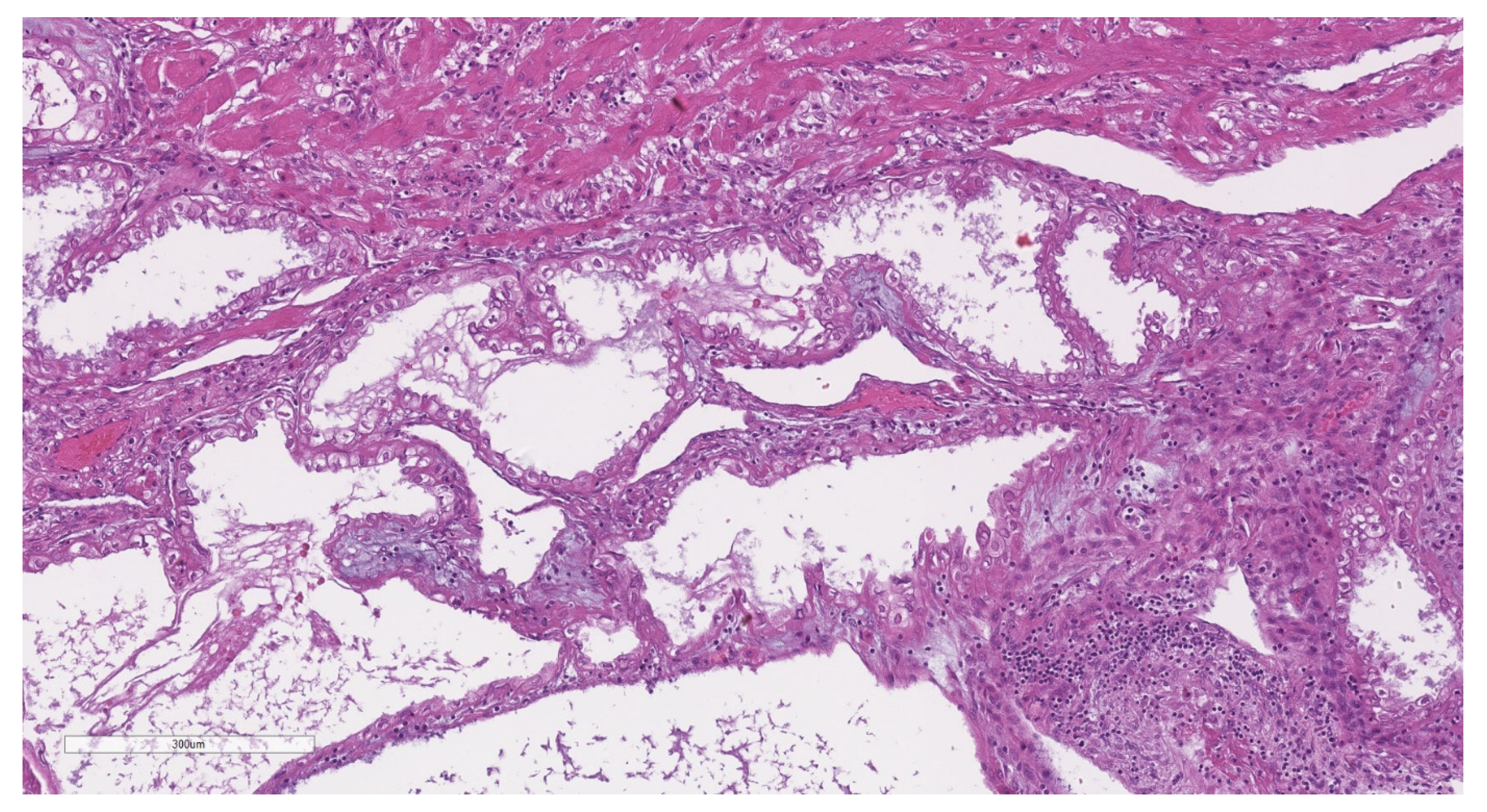
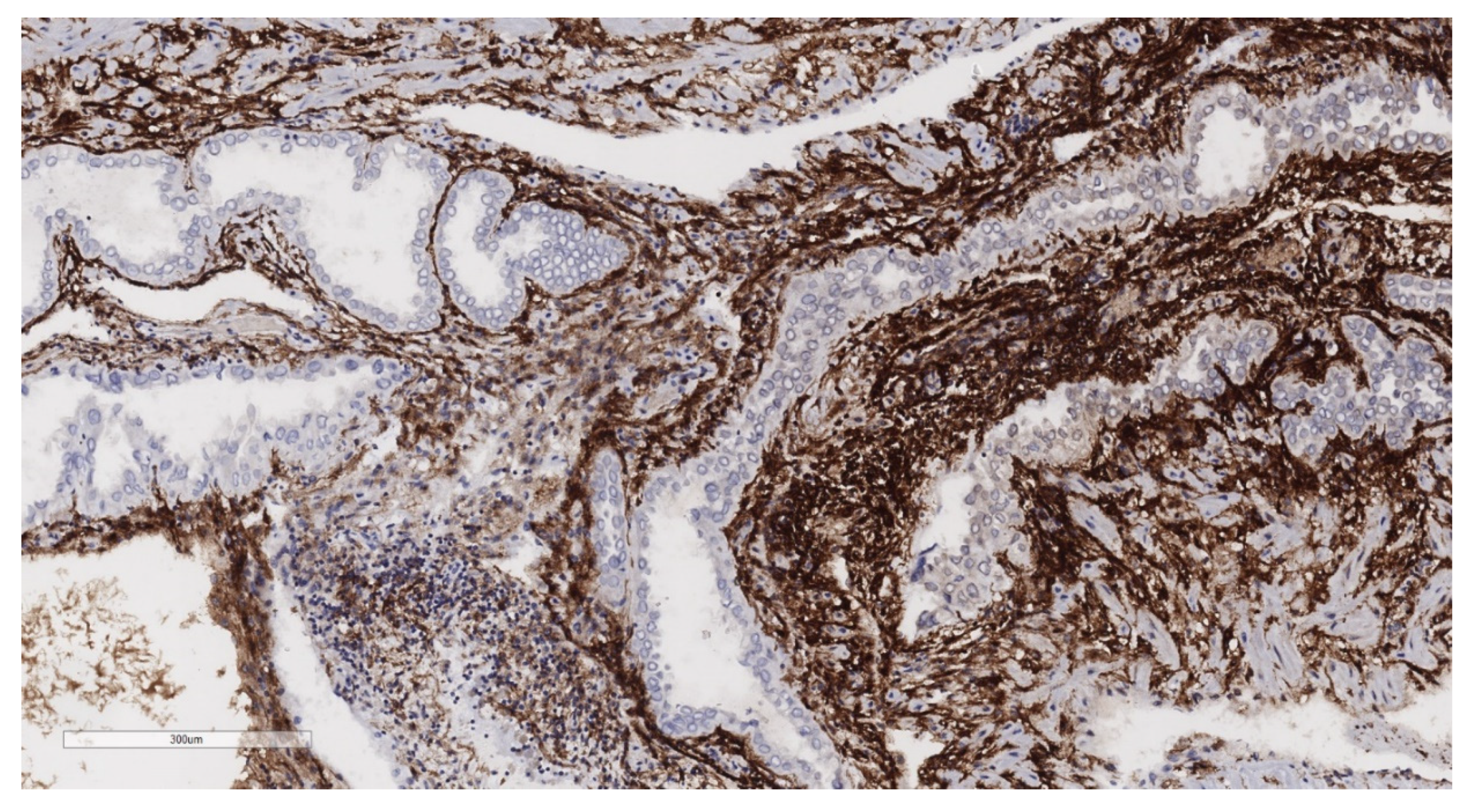
2.2. Histopathological Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I. History of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Camerini, G.; Menada, M.V.; Biscaldi, E.; Ragni, N.; Remorgida, V. Uterine Ade-nomyosis in Persistence of Dysmenorrhea after Surgical Excision of Pelvic Endometriosis and Colorectal Resection. J. Reprod. Med. 2009, 54, 366–372. [Google Scholar] [PubMed]
- Naftalin, J.; Hoo, W.; Pateman, K.; Mavrelos, D.; Foo, X.; Jurkovic, D. Is adenomyosis associated with menorrhagia? Hum. Reprod. 2014, 29, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 593–633. [Google Scholar] [CrossRef]
- Buggio, L.; Dridi, D.; Barbara, G. Adenomyosis: Impact on Fertility and Obstetric Outcomes. Reprod. Sci. 2021, 28, 3081–3084. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, S.; Okuda, Y.; Hirata, S.; Suzuki, K. Adenomyosis as a Potential Risk Factor for Adverse Pregnancy Outcomes: A Multicenter Case-Control Study. Tohoku J. Exp. Med. 2020, 251, 231–239. [Google Scholar] [CrossRef]
- Cozzolino, M.; Basile, F.; Pontrelli, G. Effects of adenomyosis on obstetric outcomes. Minerva Ginecol. 2019, 71, 146–154. [Google Scholar] [CrossRef]
- Martone, S.; Centini, G.; Exacoustos, C.; Zupi, E.; Afors, K.; Zullo, F.; Maneschi, F.; Habib, N.; Lazzeri, L. Pathophysiologic mechanisms by which adenomyosis predisposes to postpartum haemorrhage and other obstetric complications. Med. Hypotheses 2020, 143, 109833. [Google Scholar] [CrossRef]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological changes associated with adenomyosis: A systematic review. Hum. Reprod. Updat. 2021, 27, 108–129. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.V.D.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Tellum, T.; Nygaard, S.; Lieng, M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta-analysis of Diagnostic Accuracy in Imaging. J. Minim. Invasive Gynecol. 2020, 27, 408–418.e3. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477. [Google Scholar] [CrossRef]
- Upson, K.; Missmer, S.A. Epidemiology of Adenomyosis. Semin. Reprod. Med. 2020, 38, 89–107. [Google Scholar] [CrossRef]
- Movilla, P.; Morris, S.; Isaacson, K. A Systematic Review of Tissue Sampling Techniques for the Diagnosis of Adenomyosis. J. Minim. Invasive Gynecol. 2020, 27, 344–351. [Google Scholar] [CrossRef]
- Luciano, D.E.; Exacoustos, C.; Albrecht, L.; LaMonica, R.; Proffer, A.; Zupi, E.; Luciano, A.A. Three-Dimensional Ultrasound in Diagnosis of Adenomyosis: Histologic Correlation with Ultrasound Targeted Biopsies of the Uterus. J. Minim. Invasive Gynecol. 2013, 20, 803–810. [Google Scholar] [CrossRef]
- Nam, J.-H.; Lyu, G.-S. Abdominal Ultrasound-Guided Transvaginal Myometrial Core Needle Biopsy for the Definitive Diagnosis of Suspected Adenomyosis in 1032 Patients: A Retrospective Study. J. Minim. Invasive Gynecol. 2015, 22, 395–402. [Google Scholar] [CrossRef]
- Tellum, T.; Qvigstad, E.; Skovholt, E.K.; Lieng, M. In Vivo Adenomyosis Tissue Sampling Using a Transvaginal Ultrasound–guided Core Biopsy Technique for Research Purposes: Safety, Feasibility, and Effectiveness. J. Minim. Invasive Gynecol. 2019, 26, 1357–1362. [Google Scholar] [CrossRef]
- Loughlin, A.M.; Chiuve, S.E.; Reznor, G.; Doherty, M.; Missmer, S.A.; Chomistek, A.K.; Enger, C. Method used to identify adenomyosis and potentially undiagnosed adenomyosis in a large, U.S. electronic health record database. Pharmacoepidemiol. Drug Saf. 2021, 30, 1675–1686. [Google Scholar] [CrossRef]
- Schaap, T.; Bloemenkamp, K.; Deneux-Tharaux, C.; Knight, M.; Langhoff-Roos, J.; Sullivan, E.; Akker, T.V.D. Inoss Defining definitions: A Delphi study to develop a core outcome set for conditions of severe maternal morbidity. BJOG: Int. J. Obstet. Gynaecol. 2019, 126, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Souza, J.P.; Pattinson, R.C.; WHO Working Group on Maternal Mortality and Mor-Bidity Classifications. Maternal near miss—Towards a standard tool for monitoring quality of maternal health care. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 287–296. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluating the Quality of Care for Severe Pregnancy Complications: The WHO Near-Miss Approach for Maternal Health; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-150222-1. [Google Scholar]
- Orsi, M.; Ossola, M.W.; Iurlaro, E.; Perugino, G.; Somigliana, E.; Ferrazzi, E. The impact of a multilevel approach to reduce emergency hysterectomy for postpartum haemorrhage: Insights from a tertiary referral centre in Northern Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 152–157. [Google Scholar] [CrossRef]
- Zaloudek, C.J.; Hendrickson, M.R.; Soslow, R.A. Mesenchymal Tumors of the Uterus. In Blaustein’s Pathology of the Female Genital Tract; Kurman, R.J., Ellenson, L.H., Ronnett, B.M., Eds.; Springer US: Boston, MA, USA, 2011; pp. 453–527. ISBN 978-1-4419-0489-8. [Google Scholar]
- Aas-Eng, M.K.; Montanari, E.; Lieng, M.; Keckstein, J.; Hudelist, G. Transvaginal Sonographic Imaging and Associated Techniques for Diagnosis of Ovarian, Deep Endometriosis, and Adenomyosis: A Comprehensive Review. Semin. Reprod. Med. 2020, 38, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Nirgianakis, K.; Kalaitzopoulos, D.R.; Schwartz, A.S.K.; Spaanderman, M.; Kramer, B.W.; Mueller, M.D.; Mueller, M. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 185–206. [Google Scholar] [CrossRef]
- Frincu, F.; Carp-Veliscu, A.; Petca, A.; Badiu, D.-C.; Bratila, E.; Cirstoiu, M.; Mehedintu, C. Maternal–Fetal Outcomes in Women with Endometriosis and Shared Pathogenic Mechanisms. Medicina 2021, 57, 1258. [Google Scholar] [CrossRef]
- Vercellini, P.; Parazzini, F.; Pietropaolo, G.; Cipriani, S.; Frattaruolo, M.P.; Fedele, L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: A retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1538–1543. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Ueda, Y.; Lee, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Endo, M.; et al. The association of endometriosis with placenta previa and postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100417. [Google Scholar] [CrossRef]
- Lalani, S.; Choudhry, A.J.; Firth, B.; Bacal, V.; Walker, M.; Wen, S.W.; Singh, S.; Amath, A.; Hodge, M.; Chen, I. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum. Reprod. 2018, 33, 1854–1865. [Google Scholar] [CrossRef]
- Benaglia, L.; Candotti, G.; Papaleo, E.; Pagliardini, L.; Leonardi, M.; Reschini, M.; Quaranta, L.; Munaretto, M.; Vigano, P.; Candiani, M.; et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum. Reprod. 2016, 31, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Berlanda, N.; Alio, W.; Angioni, S.; Bergamini, V.; Bonin, C.; Boracchi, P.; Candiani, M.; Centini, G.; D’alterio, M.N.; Del Forno, S.; et al. Impact of endometriosis on obstetric outcome after natural conception: A multicenter Italian study. Arch. Gynecol. Obstet. 2022, 305, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: Results from a prospective multicentric study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.C.; Lim, E.K.; Liang, D.; Bullwinkel, R. Cesarean Section as a Risk Factor for the Development of Adenomyosis Uteri. J. Reprod. Med. 2014, 59, 20–24. [Google Scholar] [PubMed]
- Davis, A.A. A womb like a broken heart. BMJ Case Rep. 2018, 2018, bcr2017222075. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Sergent, F.; Roman, H.; Verspyck, E.; Marpeau, L. Late complications of operative hysteroscopy: Predicting patients at risk of uterine rupture during subsequent pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; De Guibert, F.; Salari, K.; Crochet, P.; Bretelle, F.; Gamerre, M. Adverse Obstetric Outcomes at Term after Hysteroscopic Metroplasty. J. Minim. Invasive Gynecol. 2009, 16, 454–457. [Google Scholar] [CrossRef]
- Ono, S.; Kuwabara, Y.; Matsuda, S.; Yonezawa, M.; Watanabe, K.; Akira, S.; Takeshita, T. Is hysteroscopic metroplasty using the incision method for septate uterus a risk factor for adverse obstetric outcomes? J. Obstet. Gynaecol. Res. 2019, 45, 634–639. [Google Scholar] [CrossRef]
- Šuster, N.K.; Gergolet, M. Does hysteroscopic metroplasty for septate uterus represent a risk factor for adverse outcome during pregnancy and labor? Gynecol. Surg. 2016, 13, 37–41. [Google Scholar] [CrossRef][Green Version]
- Kim, M.S.; Jang, J.H.; Park, S.; Ahn, E.H.; Jung, S.H. Effect of adenomyosis on adverse obstetrical outcomes in twin pregnancies achieved with assisted reproductive technology. J. Obstet. Gynaecol. 2021, 41, 1225–1229. [Google Scholar] [CrossRef]


| Characteristics | Adenomyosis | Non-A. | p-Value | ||
|---|---|---|---|---|---|
| n = 18 | % or SD | n = 112 | % or SD | ||
| Maternal age (years) | 39.7 | 5.2 | 37.6 | 4.5 | 0.08 |
| Country of origin | |||||
| Italy | 13 | 72.2 | 71 | 63.4 | 0.47 |
| Others | 5 | 27.8 | 41 | 36.6 | |
| Level of education | |||||
| Lower level | 5 | 27.8 | 12 | 10.7 | 0.1 |
| High school | 4 | 22.2 | 25 | 22.3 | 0.76 |
| University education | 6 | 33.3 | 45 | 40.2 | 0.58 |
| Missed | 3 | 16.7 | 30 | 26.8 | |
| Occupational status | |||||
| Working | 13 | 72.2 | 78 | 69.6 | 0.78 |
| No-working | 3 | 16.7 | 15 | 13.4 | |
| Missed | 2 | 11.1 | 19 | 17.0 | |
| Marital status | |||||
| Married | 12 | 66.7 | 78 | 69.6 | 0.8 |
| Unmarried | 6 | 33.3 | 34 | 30.4 | |
| Obstetric history | |||||
| Nulliparous | 15 | 83.3 | 84 | 75.0 | 0.44 |
| Previous vaginal delivery | 3 | 16.7 | 28 | 25.0 | 0.45 |
| One previous caesarean | 6 | 33.3 | 25 | 22.3 | 0.31 |
| Two or more previous caesarean | 4 | 22.2 | 27 | 24.1 | 0.86 |
| Previous curettage for abortion | 8 | 44.4 | 32 | 28.6 | 0.17 |
| Gynecological disease | |||||
| Previous surgery for uterine fibroid * | 2 | 11.1 | 6 | 5.4 | 0.35 |
| Uterine fibroid ** | 4 | 22.2 | 7 | 6.3 | 0.07 |
| Previous surgery for endometriosis *** | 3 | 16.7 | 3 | 2.7 | 0.004 |
| Previous adenomyomectomy **** | 1 | 5.6 | 0 | 0.0 | 0.13 |
| Endometriosis ** | 5 | 27.8 | 9 | 8.0 | 0.035 |
| Previous hysteroscopic metroplasty for uterine septum ***** | 3 | 16.7 | 2 | 1.8 | 0.017 |
| Characteristics | Adenomyosis | Non-A. | p-Value | ||
|---|---|---|---|---|---|
| n = 18 | % or SD | n = 112 | % or SD | ||
| Pregnancy complications | |||||
| Twin gestation | 4 | 22.2 | 23 | 20.5 | 0.87 |
| Assisted reproductive technology | 9 | 50.0 | 22 | 19.6 | 0.01 |
| Hypertensive disorder of pregnancy | 1 | 5.6 | 10 | 8.9 | 0.71 |
| Placenta previa | 14 | 77.8 | 51 | 45.5 | 0.01 |
| Placenta accreta spectrum | 12 | 66.7 | 60 | 53.6 | 0.3 |
| Preterm premature rupture of membrane | 2 | 11.1 | 6 | 5.4 | 0.34 |
| Antepartum bleeding | 3 | 16.7 | 10 | 8.9 | 0.31 |
| Mode of delivery | |||||
| Vaginal delivery | 0 | 0.0 | 15 | 13.4 | 0.13 |
| Elective caesarean | 12 | 66.7 | 63 | 56.3 | 0.41 |
| Emergency caesarean | 6 | 33.3 | 34 | 30.4 | 0.8 |
| Clinical indication for hysterectomy | |||||
| Haemorrhage: placenta previa or accreta spectrum | 11 | 61.1 | 57 | 50.9 | 0.3 |
| Haemorrhage: uterine atony | 4 | 22.2 | 49 | 43.8 | 0.14 |
| Uterine rupture | 2 | 11.1 | 3 | 2.7 | 0.28 |
| Sepsis | 1 | 5.6 | 3 | 2.7 | 0.51 |
| Maternal and fetal outcomes | |||||
| Estimated blood loss (ml, SD) | 4072 | 2055.0 | 4726 | 3195.0 | 0.41 |
| Packed red cells units transfused | 7.3 | 5.6 | 9.5 | 6.4 | 0.17 |
| Major surgical complications * | 5 | 27.8 | 20 | 17.9 | 0.34 |
| Medical complications ** | 0 | 0.0 | 8 | 7.1 | 0.59 |
| Gestational age at delivery (weeks, SD) | 32 | 4.6 | 35.5 | 3.6 | 0.0004 |
| Small for gestational age at birth | 2 | 11.1 | 7 | 6.3 | 0.6 |
| Chorionamnionitis | 5 | 27.8 | 6 | 5.4 | 0.008 |
| Stillbirth | 2 | 11.1 | 1 | 0.9 | 0.07 |
| Intrauterine fetal demise in twin pregnancy | 2 | 50.0 | 1 | 4.5 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsi, M.; Somigliana, E.; Cribiù, F.M.; Lopez, G.; Buggio, L.; Ossola, M.W.; Ferrazzi, E. The Prevalence and Clinical Impact of Adenomyosis in Pregnancy-Related Hysterectomy. J. Clin. Med. 2022, 11, 4814. https://doi.org/10.3390/jcm11164814
Orsi M, Somigliana E, Cribiù FM, Lopez G, Buggio L, Ossola MW, Ferrazzi E. The Prevalence and Clinical Impact of Adenomyosis in Pregnancy-Related Hysterectomy. Journal of Clinical Medicine. 2022; 11(16):4814. https://doi.org/10.3390/jcm11164814
Chicago/Turabian StyleOrsi, Michele, Edgardo Somigliana, Fulvia Milena Cribiù, Gianluca Lopez, Laura Buggio, Manuela Wally Ossola, and Enrico Ferrazzi. 2022. "The Prevalence and Clinical Impact of Adenomyosis in Pregnancy-Related Hysterectomy" Journal of Clinical Medicine 11, no. 16: 4814. https://doi.org/10.3390/jcm11164814
APA StyleOrsi, M., Somigliana, E., Cribiù, F. M., Lopez, G., Buggio, L., Ossola, M. W., & Ferrazzi, E. (2022). The Prevalence and Clinical Impact of Adenomyosis in Pregnancy-Related Hysterectomy. Journal of Clinical Medicine, 11(16), 4814. https://doi.org/10.3390/jcm11164814







