Association between High Normal TSH Levels and Obesity in Women with Anti-Thyroid Autoantibodies (ATAs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sample Size
- (a)
- Overt or subclinical hyper/hypothyroidism and/or current or previous specific treatment for thyroid diseases (medical, surgical, radiometabolic) (236 patients);
- (b)
- History of intake of medications potentially affecting TSH levels (32 patients);
- (c)
- Diabetes mellitus (223 patients).
2.2. Anthropometric Data
- (a)
- Height (expressed in cm), measured without shoes, with the participant’s head being in the “Frankfort plane”, by means of a stadiometer (SECA);
- (b)
- Body weight (expressed in kg), measured by wearing light clothes, by means of a mechanical scale (SECA 700);
- (c)
- BMI (Body Mass Index, expressed in kg/m2), calculated by the ratio of weight in kilograms and height in meters squared;
- (d)
- Waist circumference (WC) (expressed in cm), measured using recommendations of the Airlie conference (according to WHO criteria), considering as cut-off the International Diabetes Federation (IDF) values (94 cm for men and 80 cm for women) [78].
2.3. Blood Sampling and Analytes Measurements
- (a)
- TSH was analyzed via an ultra-sensitive automatic chemiluminescence method (Ortho-Clinical Diagnostic SpA, Milan, Italy). The sensitivity was 0.004 µUI/L, and normal values were 0.4–4.1 µUI/L.
- (b)
- ATAs (thyroid peroxidase autoantibodies, TPOAb) were measured with a chemiluminescent method (Immulite 2000’s; Diagnostic Products Corporation, Los Angeles, CA, USA; Distributor Medical Systems Corporation, Genoa, Italy), and normal range values were 10–35 IU/mL.
2.4. Ethical Committee
2.5. Statistical Analysis
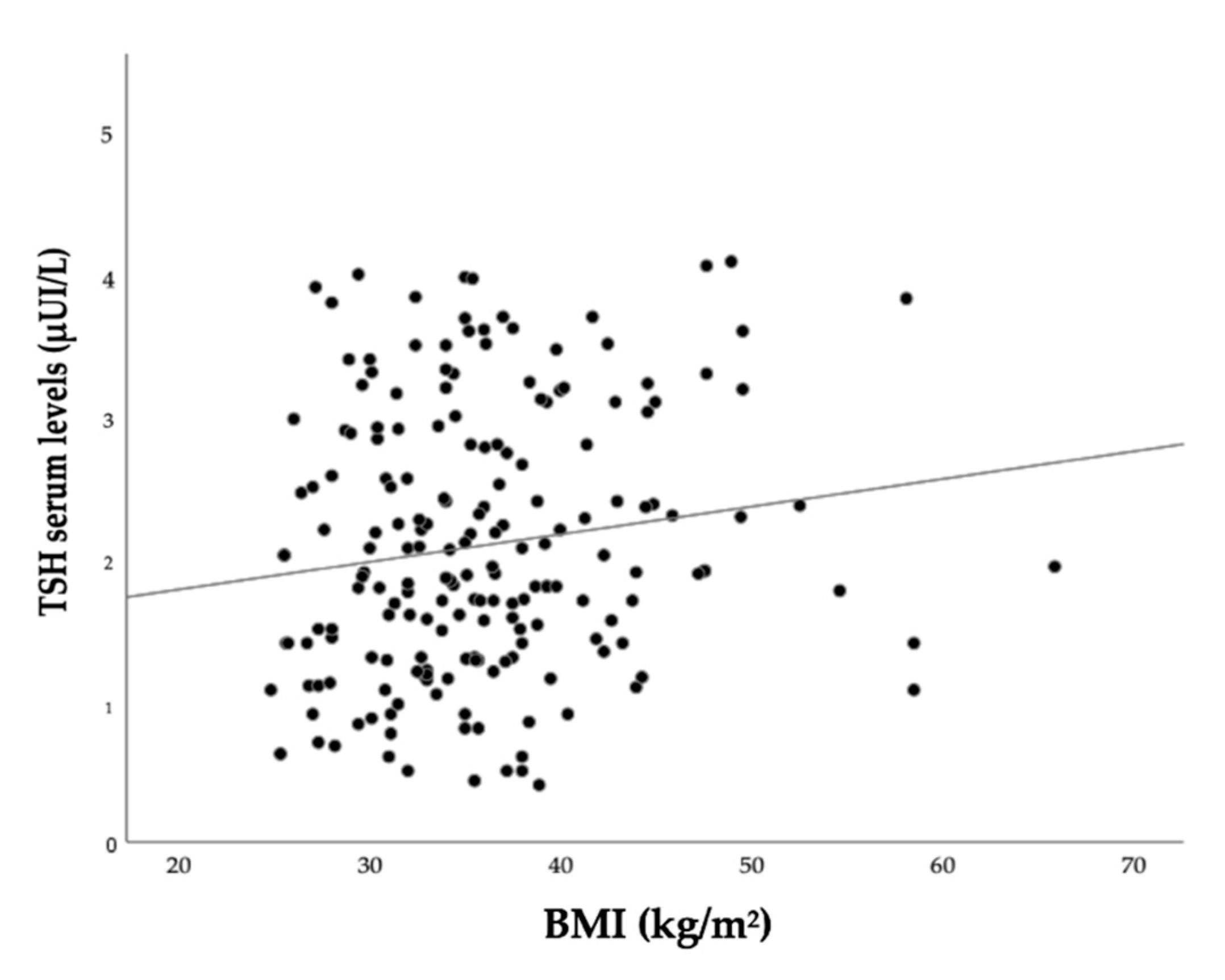
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, Z.J.; Willett, W.C.; Hu, F.B.; Pacheco, L.S.; Long, M.W.; Gortmaker, S.L. Excess mortality associated with elevated body weight in the USA by state and demographic subgroup: A modelling study. EClinicalMedicine 2022, 48, 101429. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Qin, B.; Poti, J.; Sokol, R.; Gordon-Larsen, P. Epidemiology of Obesity in Adults: Latest Trends. Curr. Obes. Rep. 2018, 7, 276–288. [Google Scholar] [CrossRef]
- World Health Organisation. Obesity and Overweight—Key Facts. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 November 2018).
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Canaris, G.J.; Manowitz, N.R.; Mayor, G.; Ridgway, E.C. The Colorado Thyroid Disease Prevalence Study. Arch. Intern. Med. 2000, 160, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.; Yuen, A.D.; Du, L.; Ro, C.C.; Seger, C.; Yeh, M.W.; Leung, A.M.; Rhee, C.M. Impact of Hypothyroidism and Heart Failure on Hospitalization Risk. Thyroid 2018, 28, 1094–1100. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Mattace-Raso, F.U.S.; Terzikhan, N.; Kavousi, M.; Ikram, M.A.; Peeters, R.P.; Franco, O.H. Thyroid function and life expectancy with and without noncommunicable diseases: A population-based study. PLoS Med. 2019, 16, e1002957. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Biondi, B. Thyroid and Obesity: An Intriguing Relationship. J. Clin. Endocrinol. Metab. 2010, 95, 3614–3617. [Google Scholar] [CrossRef]
- Pasquali, R.; Casanueva, F.; Haluzik, M.; Van Hulsteijn, L.; LeDoux, S.; Monteiro, M.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. Eur. J. Endocrinol. 2020, 182, G1–G32. [Google Scholar] [CrossRef] [PubMed]
- Valdés, S.; Maldonado-Araque, C.; Lago-Sampedro, A.M.; Lillo-Muñoz, J.A.; Garcia-Fuentes, E.; Perez-Valero, V.; Gutiérrez-Repiso, C.; Garcia-Escobar, E.; Goday, A.; Urrutia, I.; et al. Reference values for TSH may be inadequate to define hypothyroidism in persons with morbid obesity: Di@bet.es study. Obesity 2017, 25, 788–793. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; Jørgensen, T. Small Differences in Thyroid Function May Be Important for Body Mass Index and the Occurrence of Obesity in the Population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Åsvold, B.O.; Bjøro, T.; Vatten, L.J. Association of Serum TSH with High Body Mass Differs between Smokers and Never-Smokers. J. Clin. Endocrinol. Metab. 2009, 94, 5023–5027. [Google Scholar] [CrossRef]
- Dvořáková, M.; Hill, M.; Čeřovská, J.; Pobišová, Z.; Bílek, R.; Hoskovcová, P.; Zamrazil, V.; Hainer, V. Relationship between Pituitary-Thyroid Axis Hormones and Anthropometric Parameters in Czech Adult Population. Physiol. Res. 2008, 57, S127–S134. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Pencina, M.J.; D’Agostino, R.B.; Murabito, J.; Seely, E.W.; Pearce, E.N.; Vasan, R.S. Relations of Thyroid Function to Body WeightCross-sectional and Longitudinal Observations in a Community-Based Sample. Arch. Intern. Med. 2008, 168, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Liew, G.; Flood, V.M.; Wang, J.J.; Kifley, A.; Leeder, S.R.; Mitchell, P. The Association Between Weight Gain and Thyroid Function in an Older Population. Arch. Intern. Med. 2008, 168, 2283–2284. [Google Scholar] [CrossRef][Green Version]
- Nyrnes, A.; Jorde, R.; Sundsfjord, J. Serum TSH is positively associated with BMI. Int. J. Obes. 2005, 30, 100–105. [Google Scholar] [CrossRef]
- Svare, A.; Nilsen, T.I.L.; Bjøro, T.; Åsvold, B.O.; Langhammer, A. Serum TSH related to measures of body mass: Longitudinal data from the HUNT Study, Norway. Clin. Endocrinol. 2011, 74, 769–775. [Google Scholar] [CrossRef]
- Ambrosi, B.; Masserini, B.; Iorio, L.; Delnevo, A.; Malavazos, A.E.; Morricone, L.; Sburlati, L.F.; Orsi, E. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J. Endocrinol. Investig. 2010, 33, 640–643. [Google Scholar] [CrossRef]
- Bastemir, M.; Akin, F.; Alkis, E.; Kaptanoglu, E.B. Obesity is associated with increased serum TSH level, independent of thy-roid function. Swiss Med. Wkly. 2007, 137, 431–434. [Google Scholar] [PubMed]
- Bétry, C.; Challan-Belval, M.; Bernard, A.; Charrié, A.; Drai, J.; Laville, M.; Thivolet, C.; Disse, E. Increased TSH in obesity: Evidence for a BMI-independent association with leptin. Diabetes Metab. 2014, 41, 248–251. [Google Scholar] [CrossRef]
- Chikunguwo, S.; Brethauer, S.; Nirujogi, V.; Pitt, T.; Udomsawaengsup, S.; Chand, B.; Schauer, P. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg. Obes. Relat. Dis. 2007, 3, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Galofré, J.C.; Pujante, P.; Abreu, C.; Santos, S.; Guillen-Grima, F.; Frühbeck, G.; Salvador, J. Relationship between Thyroid-Stimulating Hormone and Insulin in Euthyroid Obese Men. Ann. Nutr. Metab. 2008, 53, 188–194. [Google Scholar] [CrossRef]
- Iacobellis, G.; Ribaudo, M.C.; Zappaterreno, A.; Iannucci, C.V.; Leonetti, F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin. Endocrinol. 2005, 62, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, S.; Berhouma, R.; Ammar, M.; Rouissi, K.; Jarboui, S.; Clerget-Froidevaux, M.-S.; Seugnet, I.; Abid, H.; Bchir, F.; Demeneix, B.; et al. Relationship of Thyroid Function with Obesity and Type 2 Diabetes in Euthyroid Tunisian Subjects. Endocr. Res. 2012, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, Y.J.; Park, Y.J.; Kim, K.W.; Lee, E.J.; Lim, S.; Park, D.J.; Kim, S.E.; Park, K.S.; Jang, H.C.; et al. Retinol Binding Protein-4 Elevation Is Associated with Serum Thyroid-Stimulating Hormone Level Independently of Obesity in Elderly Subjects with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 2313–2318. [Google Scholar] [CrossRef][Green Version]
- Makepeace, A.E.; Bremner, A.; O’Leary, P.; Leedman, P.J.; Feddema, P.; Michelangeli, V.; Walsh, J.P. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: Differences between smokers and nonsmokers. Clin. Endocrinol. 2008, 69, 648–652. [Google Scholar] [CrossRef]
- Shon, H.S.; Jung, E.D.; Kim, S.H.; Lee, J.H. Free T4 is negatively correlated with body mass index in euthyroid women. Korean J. Intern. Med. 2008, 23, 53–57. [Google Scholar] [CrossRef]
- Bakiner, O.; Bozkirli, E.; Cavlak, G.; Ozsahin, K.; Ertorer, E. Are Plasma Thyroid-Stimulating Hormone Levels Associated with Degree of Obesity and Metabolic Syndrome in Euthyroid Obese Patients? A Turkish Cohort Study. ISRN Endocrinol. 2014, 2014, 803028. [Google Scholar] [CrossRef]
- De Pergola, G.; Ciampolillo, A.; Paolotti, S.; Trerotoli, P.; Giorgino, R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin. Endocrinol. 2007, 67, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Manji, N.; Boelaert, K.; Sheppard, M.C.; Holder, R.L.; Gough, S.C.; Franklyn, J.A. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin. Endocrinol. 2006, 64, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, M.A.; Vagenakis, A.G.; Leonardou, A.S.; Argentou, M.N.; Habeos, I.G.; Makri, M.G.; Psyrogiannis, A.I.; Kalfarentzos, F.E.; Kyriazopoulou, V.E. Thyroid Function in Humans with Morbid Obesity. Thyroid 2006, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Sorice, G.P.; Mezza, T.; Prioletta, A.; Lassandro, A.P.; Pirronti, T.; Della Casa, S.; Pontecorvi, A.; Giaccari, A. High-normal tsh values in obesity: Is it insulin resistance or adipose tissue’s guilt? Obesity 2013, 21, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Leporati, P.; La Manna, A.; Pirali, B.; Mondello, T.; Fonte, R.; Magri, F.; Chiovato, L. Raised serum TSH levels in patients with morbid obesity: Is it enough to diagnose subclinical hypothyroidism? Eur. J. Endocrinol. 2009, 160, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.C.; Feitosa, M.M.; Severo, J.S.; Freitas, T.E.C.; Morais, J.B.S.; Torres-Leal, F.L.; Henriques, G.S.; Marreiro, D.D.N. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm. Metab. Res. 2016, 48, 787–794. [Google Scholar] [CrossRef]
- Bjergved, L.; Jørgensen, T.; Perrild, H.; Laurberg, P.; Krejbjerg, A.; Ovesen, L.; Rasmussen, L.B.; Knudsen, N. Thyroid Function and Body Weight: A Community-Based Longitudinal Study. PLoS ONE 2014, 9, e93515. [Google Scholar] [CrossRef]
- Juiz-Valiña, P.; Outeiriño-Blanco, E.; Pértega, S.; Varela-Rodríguez, B.M.; García-Brao, M.J.; Mena, E.; Pena-Bello, L.; Cordido, M.; Sangiao-Alvarellos, S.; Cordido, F. Effect of Weight Loss after Bariatric Surgery on Thyroid-Stimulating Hormone Levels in Euthyroid Patients with Morbid Obesity. Nutrients 2019, 11, 1121. [Google Scholar] [CrossRef]
- Juiz-Valiña, P.; Cordido, M.; Outeiriño-Blanco, E.; Pértega, S.; Varela-Rodríguez, B.M.; García-Brao, M.J.; Mena, E.; Pena-Bello, L.; Sangiao-Alvarellos, S.; Cordido, F. Central Resistance to Thyroid Hormones in Morbidly Obese Subjects Is Reversed after Bariatric Surgery-Induced Weight Loss. J. Clin. Med. 2020, 9, 359. [Google Scholar] [CrossRef]
- Cordido, M.; Juiz-Valiña, P.; Urones, P.; Sangiao-Alvarellos, S.; Cordido, F. Thyroid Function Alteration in Obesity and the Effect of Bariatric Surgery. J. Clin. Med. 2022, 11, 1340. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.; Chen, Y.; Wang, N.; Li, Q.; Han, B.; Zhao, L.; Chen, C.; Zhai, H.; Zhang, L.; et al. Are Thyroid Autoimmune Diseases Associated with Cardiometabolic Risks in a Population with Normal Thyroid-Stimulating Hormone? Mediat. Inflamm. 2018, 2018, 1856137. [Google Scholar] [CrossRef]
- Jurado-Flores, M.; Warda, F.; Mooradian, A. Pathophysiology and Clinical Features of Neuropsychiatric Manifestations of Thyroid Disease. J. Endocr. Soc. 2022, 6, bvab194. [Google Scholar] [CrossRef] [PubMed]
- Pinna, F.; Sardu, C.; Orrù, W.; Velluzzi, F.; Loviselli, A.; Contu, P.; Carpiniello, B. Psychopathology, psychosocial factors and obesity. Riv. Psichiatr. 2016, 51, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Minocci, A.; Mele, C.; Fessehatsion, R.; Tagliaferri, M.; Pagano, L.; Scacchi, M.; Aimaretti, G.; Sartorio, A. The relationship between resting energy expenditure and thyroid hormones in response to short-term weight loss in severe obesity. PLoS ONE 2018, 13, e0205293. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, E.M.; Beale, E.; Chan, L.S. Thyroid Hormone Therapy for Obesity and Nonthyroidal Illnesses: A Systematic Review. J. Clin. Endocrinol. Metab. 2009, 94, 3663–3675. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. 2008, 32, S52–S54. [Google Scholar] [CrossRef]
- Taddei, S.; Ghiadoni, L.; Salvetti, G.; Virdis, A.; Salvetti, A. Obesity and endothelial dysfunction. G Ital. Cardiol. 2006, 7, 715–723. [Google Scholar]
- Gómez-Zamudio, J.H.; Mendoza-Zubieta, V.; Ferreira-Hermosillo, A.; Molina-Ayala, M.A.; Valladares-Sálgado, A.; Suárez-Sánchez, F.; Peralta-Romero, J.D.J.; Cruz, M. High Thyroid-stimulating Hormone Levels Increase Proinflammatory and Cardiovascular Markers in Patients with Extreme Obesity. Arch. Med. Res. 2016, 47, 476–482. [Google Scholar] [CrossRef]
- Kvetny, J.; Heldgaard, P.E.; Bladbjerg, E.M.; Gram, J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin. Endocrinol. 2004, 61, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Caraccio, N.; Virdis, A.; Dardano, A.; Versari, D.; Ghiadoni, L.; Ferrannini, E.; Salvetti, A.; Monzani, F. Low-Grade Systemic Inflammation Causes Endothelial Dysfunction in Patients with Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 2006, 91, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Biondi, B. The Interconnections Between Obesity, Thyroid Function, and Autoimmunity: The Multifold Role of Leptin. Thyroid 2013, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- A Exley, M.; Hand, L.; O’Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48. [Google Scholar] [CrossRef]
- Gerriets, V.A.; Danzaki, K.; Kishton, R.J.; Eisner, W.; Nichols, A.G.; Saucillo, D.C.; Shinohara, M.L.; MacIver, N.J. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 2016, 46, 1970–1983. [Google Scholar] [CrossRef]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; A Nohr, E.; Linneberg, A.; Jess, T. Body mass index and risk of autoimmune diseases: A study within the Danish National Birth Cohort. Int. J. Epidemiology 2014, 43, 843–855. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Song, R.-H.; Wang, B.; Yao, Q.-M.; Li, Q.; Jia, X.; Zhang, J.-A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Altay, S.; Onat, A.; Can, G.; Tusun, E.; Şimşek, B.; Kaya, A. High-normal thyroid-stimulating hormone in euthyroid subjects is associated with risk of mortality and composite disease endpoint only in women. Arch. Med Sci. 2018, 14, 1394–1403. [Google Scholar] [CrossRef]
- Santini, F.; Marzullo, P.; Rotondi, M.; Ceccarini, G.; Pagano, L.; Ippolito, S.; Chiovato, L.; Biondi, B. MECHANISMS IN ENDOCRINOLOGY: The crosstalk between thyroid gland and adipose tissue: Signal integration in health and disease. Eur. J. Endocrinol. 2014, 171, R137–R152. [Google Scholar] [CrossRef]
- Rotondi, M.; Cappelli, C.; Leporati, P.; Chytiris, S.; Zerbini, F.; Fonte, R.; Magri, F.; Castellano, M.; Chiovato, L. A hypoechoic pattern of the thyroid at ultrasound does not indicate autoimmune thyroid diseases in patients with morbid obesity. Eur. J. Endocrinol. 2010, 163, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rago, T.; Chiovato, L.; Grasso, L.; Pinchera, A.; Vitti, P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J. Endocrinol. Investig. 2001, 24, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.J.; Iglesias, P. Relationship Between Thyrotropin and Body Mass Index in Euthyroid Subjects. Exp. Clin. Endocrinol. Diabetes 2010, 119, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; De González, A.B. Body Fatness and Markers of Thyroid Function among U.S. Men and Women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef]
- Mahdavi, M.; Amouzegar, A.; Mehran, L.; Madreseh, E.; Tohidi, M.; Azizi, F. Investigating the prevalence of primary thyroid dysfunction in obese and overweight individuals: Tehran thyroid study. BMC Endocr. Disord. 2021, 21, 89. [Google Scholar] [CrossRef]
- Bocchetta, A.; Ambrosiani, L.; Baggiani, G.; Pisanu, C.; Chillotti, C.; Ardau, R.; Velluzzi, F.; Piras, D.; Loviselli, A.; Pani, A. Circulating antithyroid antibodies contribute to the decrease of glomerular filtration rate in lithium-treated patients: A longitudinal study. Int. J. Bipolar Disord. 2018, 6, 3. [Google Scholar] [CrossRef]
- Delitala, A.P.; Pilia, M.G.; Ferreli, L.; Loi, F.; Curreli, N.; Balaci, L.; Schlessinger, D.; Cucca, F. Prevalence of unknown thyroid disorders in a Sardinian cohort. Eur. J. Endocrinol. 2014, 171, 143–149. [Google Scholar] [CrossRef]
- Lampis, R.; Morelli, L.; Congia, M.; Macis, M.D.; Mulargia, A.; Loddo, M.; De Virgiliis, S.; Marrosu, M.G.; Todd, J.A.; Cucca, F. The inter-regional distribution of HLA class II haplotypes indicates the suitability of the Sardinian population for case-control association studies in complex diseases. Hum. Mol. Genet. 2000, 9, 2959–2965. [Google Scholar] [CrossRef]
- Loviselli, A.; Velluzzi, F.; Mossa, P.; Cambosu, M.; Secci, G.; Atzeni, F.; Taberlet, A.; Balestrieri, A.; Martino, E.; Grasso, L.; et al. The Sardinian Autoimmunity Study: 3. Studies on Circulating Antithyroid Antibodies in Sardinian Schoolchildren: Relationship to Goiter Prevalence and Thyroid Function. Thyroid 2001, 11, 849–857. [Google Scholar] [CrossRef]
- Sardu, C.; Cocco, E.; Mereu, A.; Massa, R.; Cuccu, A.; Marrosu, M.G.; Contu, P. Population Based Study of 12 Autoimmune Diseases in Sardinia, Italy: Prevalence and Comorbidity. PLoS ONE 2012, 7, e32487. [Google Scholar] [CrossRef]
- Songini, M.; Loche, M.; Muntoni, S.; Stabilini, M.; Coppola, A.; Dessi, G.; Green, A.; Bottazzo, G.F. Increasing prevalence of juvenile onset Type 1 (insulin-dependent) diabetes mellitus in Sardinia: The military service approach. Diabetologia 1993, 36, 547–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velluzzi, F.; The Sardinian Autoimmunity Study Group; Secci, G.; Sepe, V.; Klersy, C.; Shattock, M.; Foxon, R.; Songini, M.; Mariotti, S.; Locatelli, M.; et al. Prediction of type 1 diabetes in Sardinian schoolchildren using islet cell autoantibodies: 10-year follow-up of the Sardinian schoolchildren type 1 diabetes prediction study. Geol. Rundsch. 2015, 53, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Loviselli, A.; Ghiani, M.E.; Velluzzi, F.; Piras, I.S.; Minerba, L.; Vona, G.; Calò, C.M. Prevalence and Trend of Overweight and Obesity among Sardinian Conscripts (Italy) of 1969 and 1998. J. Biosoc. Sci. 2009, 42, 201–211. [Google Scholar] [CrossRef]
- Surks, M.I.; Ortiz, E.; Daniels, G.H.; Sawin, C.T.; Col, N.F.; Cobin, R.H.; Franklyn, J.A.; Hershman, J.M.; Burman, K.D.; Denke, M.A.; et al. Subclinical Thyroid Disease. JAMA 2004, 291, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Surks, M.I.; Chopra, I.J.; Mariash, C.N.; Nicoloff, J.T.; Solomon, E.D.H. American Thyroid Association guidelines for use of laboratory tests in thyroid disorders. JAMA 1990, 263, 1529–1532. [Google Scholar] [CrossRef]
- E Croker, E.; A McGrath, S.; Rowe, C.W. Thyroid disease: Using diagnostic tools effectively. Aust. J. Gen. Pract. 2021, 50, 16–21. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011; 39p. [Google Scholar]
- Yarnold, P.R.; Bryant, F.B.; Soltysik, E.R.C. Maximizing the Accuracy of Multiple Regression Models using UniODA: Regression away from the Mean. Optim. Data Anal. 2013, 2, 19–25. [Google Scholar]
- Bland, J.M.; Altman, D.G. Correlation in restricted ranges of data. BMJ 2011, 342, d556. [Google Scholar] [CrossRef]
- Jonklaas, J.; Razvi, S. Reference intervals in the diagnosis of thyroid dysfunction: Treating patients not numbers. Lancet Diabetes Endocrinol. 2019, 7, 473–483. [Google Scholar] [CrossRef]
- Spencer, C.A.; Hollowell, J.G.; Kazarosyan, M.; Braverman, L. National Health and Nutrition Examination Survey III Thyroid-Stimulating Hormone (TSH)-Thyroperoxidase Antibody Relationships Demonstrate That TSH Upper Reference Limits May Be Skewed by Occult Thyroid Dysfunction. J. Clin. Endocrinol. Metab. 2007, 92, 4236–4240. [Google Scholar] [CrossRef]
- Chiovato, L.; Lapi, P.; Fiore, E.; Tonacchera, M.; Pinchera, A. Thyroid autoimmunity and female gender. J. Endocrinol. Investig. 1993, 16, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, S.; Celis-Morales, C.A.; Lyall, D.M.; Anderson, J.; Gray, S.R.; Mackay, D.F.; Nelson, S.M.; Welsh, P.; Pell, J.P.; Gill, J.M.R.; et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: A cohort study of 296 535 adults of white European descent. Eur. Heart J. 2018, 39, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Delitala, A.P.; Scuteri, A.; Fiorillo, E.; Lakatta, E.G.; Schlessinger, D.; Cucca, F. Role of Adipokines in the Association between Thyroid Hormone and Components of the Metabolic Syndrome. J. Clin. Med. 2019, 8, 764. [Google Scholar] [CrossRef]
- Diniz, M.D.F.H.S.; Beleigoli, A.; Benseñor, I.M.; Lotufo, P.A.; Goulart, A.C.; Barreto, S.M. Association between TSH levels within the reference range and adiposity markers at the baseline of the ELSA–Brasil study. PLoS ONE 2020, 15, e0228801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Wang, N.; Chen, C.; Nie, X.; Li, Q.; Han, B.; Lu, Y. Thyroid Stimulating Hormone Within the Reference Range is Associated with Visceral Adiposity Index and Lipid Accumulation Product: A Population-Based Study of SPECT-China. Horm. Metab. Res. 2017, 50, 29–36. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Meng, Z.; Li, X.; Liu, M.; Ren, X.; Zhu, M.; He, Q.; Zhang, Q.; Song, K.; et al. Waist Circumference and Subclinical Thyroid Dysfunction in a Large Cohort of Chinese Men and Women. Endocr. Pract. 2018, 24, 733–739. [Google Scholar] [CrossRef]
- Sakurai, M.; Nakamura, K.; Miura, K.; Yoshita, K.; Takamura, T.; Nagasawa, S.-Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Association between a Serum Thyroid-stimulating Hormone Concentration within the Normal Range and Indices of Obesity in Japanese Men and Women. Intern. Med. 2014, 53, 669–674. [Google Scholar] [CrossRef]
- Lukens, J.R.; Dixit, V.D.; Kanneganti, E.T.-D. Inflammasome activation in obesity-related inflammatory diseases and auto-immunity. Discov. Med. 2011, 12, 65–74. [Google Scholar]
- Versini, M.; Aljadeff, G.; Jeandel, P.-Y.; Shoenfeld, Y. Obesity: An additional piece in the mosaic of autoimmunity. Isr. Med Assoc. J. IMAJ 2014, 16, 619–621. [Google Scholar]
- Loviselli, A.; Secci, G.; Lai, A.; Velluzzi, F. Mechanisms of regulation of the food intake: Recent advances. Recenti. Prog. Med. 2007, 98, 1–6. [Google Scholar]
- Onat, A.; Aydın, M.; Can, G.; Çelik, E.; Altay, S.; Karagöz, A.; Ademoğlu, E. Normal thyroid-stimulating hormone levels, autoimmune activation, and coronary heart disease risk. Endocrine 2014, 48, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; He, Z.; Shao, S.; Fu, Y.; Zheng, D.; Liu, L.; Gao, L.; Guan, L.; Zhao, M.; Zhao, J. Interaction effect of obesity and thyroid autoimmunity on the prevalence of hyperthyrotropinaemia. Endocrine 2020, 68, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, A.G.; Palacios, S.S.; Guillen-Grima, F.; Galofré, J.C. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.A.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.J.; Tunbrldge, W.M.G.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Evans, J.G.; Hasan, D.M.; Rodgers, H.; Tunbridge, F.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V. The Abdominal Obesity Phenotype and Insulin Resistance are Associated with Abnormalities of the Hypothalamic-Pituitary-Adrenal Axis in Humans. Horm. Metab. Res. 2000, 32, 521–525. [Google Scholar] [CrossRef]
- Guan, B.; Chen, Y.; Yang, J.; Yang, W.; Wang, C. Effect of Bariatric Surgery on Thyroid Function in Obese Patients: A Systematic Review and Meta-Analysis. Obes. Surg. 2017, 27, 3292–3305. [Google Scholar] [CrossRef] [PubMed]
| Class (BMI) | Number | TSH (μUI/L) |
|---|---|---|
| Overweight (25.0–29.9) | 154 | 1.9 (0.4–4.0) |
| Class I Obesity (30.0–34.9) | 290 | 1.9 (0.5–4.1) |
| Class II Obesity (35.0–39.9) | 259 | 1.8 (0.4–4.1) |
| Class III Obesity (≥40) | 199 | 1.9 (0.5–4.1) |
| ATAs+ Patients (N = 236) | ATAs- Patients (N = 516) | ||||
|---|---|---|---|---|---|
| Variable | Median (Range) | M ± SD | Median (Range) | M ± SD | p-Value |
| TSH (μUI/L) | 1.85 (0.7–3.7) | 1.86 (0.7–3.5) | 0.504 | ||
| BMI (kg/m2) | 36.0 ± 6.8 | 35.8 ± 6.4 | 0.749 | ||
| WC (cm) | 112.4 ± 14 | 112.2 ± 14.2 | 0.907 | ||
| ATAs+ Patients (N = 191) | ATAs- Patients (N = 375) | ||||
|---|---|---|---|---|---|
| Variable | Median (Range) | M ± SD | Median (Range) | M ± SD | p-Value |
| TSH (μUI/L) | 1.91 (0.7–3.7) | 1.90 (0.8–3.6) | 0.412 | ||
| BMI (kg/m2) | 36.0 ± 6.9 | 35.1 ± 6.1 | 0.241 | ||
| WC (cm) | 111 ± 14.1 | 109.5 ± 13.2 | 0.194 | ||
| Author | People Assessed | Results |
|---|---|---|
| Kitahara [66]—USA | 3.114 euthyroid adults | TSH positively associated with BMI only when ATAs-positive patients were considered |
| Chen [42]—Japan | 9.082 euthyroid adults | TSH positively associated with BMI in ATAs-positive women |
| Diez [65]—Spain | 412 euthyroid obese adults | TSH positively associated with BMI only when ATAs-positive patients were considered |
| De Pergola [32]–Italy | 201 euthyroid women | TSH positively associated with WC |
| Diniz [87]—Brazil | 11.224 euthyroid adults | TSH associated with BMI and WC only in women |
| Delitala [86]—Italy | 4.733 euthyroid adults | TSH not associated with anthropometric measures |
| Liu [89]—China | 13.505 euthyroid adults | TSH not associated with WC |
| Sakurai [90]—Japan | 2.037 euthyroid adults | TSH not associated with WC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velluzzi, F.; Pisanu, S.; Galletta, M.; Fosci, M.; Secci, G.; Deledda, A.; Boi, F.; Rodia, R.; Fanciulli, G.; Delitala, A.P.; et al. Association between High Normal TSH Levels and Obesity in Women with Anti-Thyroid Autoantibodies (ATAs). J. Clin. Med. 2022, 11, 5125. https://doi.org/10.3390/jcm11175125
Velluzzi F, Pisanu S, Galletta M, Fosci M, Secci G, Deledda A, Boi F, Rodia R, Fanciulli G, Delitala AP, et al. Association between High Normal TSH Levels and Obesity in Women with Anti-Thyroid Autoantibodies (ATAs). Journal of Clinical Medicine. 2022; 11(17):5125. https://doi.org/10.3390/jcm11175125
Chicago/Turabian StyleVelluzzi, Fernanda, Silvia Pisanu, Maura Galletta, Michele Fosci, Gianni Secci, Andrea Deledda, Francesco Boi, Rossella Rodia, Giuseppe Fanciulli, Alessandro Palmerio Delitala, and et al. 2022. "Association between High Normal TSH Levels and Obesity in Women with Anti-Thyroid Autoantibodies (ATAs)" Journal of Clinical Medicine 11, no. 17: 5125. https://doi.org/10.3390/jcm11175125
APA StyleVelluzzi, F., Pisanu, S., Galletta, M., Fosci, M., Secci, G., Deledda, A., Boi, F., Rodia, R., Fanciulli, G., Delitala, A. P., Sainas, G., & Loviselli, A. (2022). Association between High Normal TSH Levels and Obesity in Women with Anti-Thyroid Autoantibodies (ATAs). Journal of Clinical Medicine, 11(17), 5125. https://doi.org/10.3390/jcm11175125









