Evaluation of a New Monoclonal Chemiluminescent Immunoassay Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Spanish Multicentre Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Design
2.2. Stool Antigen Test
2.3. Urea Breath Test
2.3.1. Isotope Ratio Mass Spectrometry (IRMS)
2.3.2. Non-Dispersive Isotope-Selective Infrared Spectrometry (NDIRS)
2.4. H. pylori Detection by PCR
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Cubiella, J.; Pérez Aisa, Á.; Cuatrecasas, M.; Díez Redondo, P.; Fernández Esparrach, G.; Marín-Gabriel, J.C.; Moreira, L.; Núñez, H.; Pardo López, M.L.; Rodríguez de Santiago, E.; et al. Gastric cancer screening in low incidence populations: Position statement of AEG, SEED and SEAP. Gastroenterol. Hepatol. 2021, 44, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Godbole, G.; Mégraud, F.; Bessède, E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2020, 25 (Suppl. S1), e12735. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, P.; Mohammadnia-Afrouzi, M.; Javanian, M.; Babazadeh, A.; Koppolu, V.; Vasigala, V.R.; Nouri, H.R.; Ebrahimpour, S. Diagnostic methods for Helicobacter pylori infection: Ideals, options, and limitations. Eur. J. Clin. Microbiol. Infect Dis. 2019, 38, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Pes, G.M. What Is New in Helicobacter pylori Diagnosis. An Overview. J. Clin. Med. 2021, 10, 2091. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Amador, J.; Ricote, M.; Cañones-Garzón, P.J.; Gene, E.; Calvet, X.; Gisbert, J.P. Spanish primary care survey on the management of Helicobacter pylori infection and dyspepsia: Information, attitudes, and decisions. Helicobacter 2019, 24, e12593. [Google Scholar] [CrossRef]
- Ford, A.C.; Qume, M.; Moayyedi, P.; Arents, N.L.; Lassen, A.T.; Logan, R.F.; McColl, K.E.; Myres, P.; Delaney, B.C. Helicobacter pylori “test and treat” or endoscopy for managing dyspepsia: An individual patient data meta-analysis. Gastroenterology 2005, 128, 1838–1844. [Google Scholar] [CrossRef]
- Beresniak, A.; Malfertheiner, P.; Franceschi, F.; Liebaert, F.; Salhi, H.; Gisbert, J.P. Helicobacter pylori “Test-and-Treat” strategy with urea breath test: A cost-effective strategy for the management of dyspepsia and the prevention of ulcer and gastric cancer in Spain-Results of the Hp-Breath initiative. Helicobacter 2020, 25, e12693. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Helicobacter pylori “Test-and-Treat” Strategy for Management of Dyspepsia: A Comprehensive Review. Clin. Transl. Gastroenterol. 2013, 4, e32. [Google Scholar] [CrossRef]
- Best, L.M.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018, 3, Cd012080. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Pajares, J.M. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection—A critical review. Aliment. Pharmacol. Ther. 2004, 20, 1001–1017. [Google Scholar] [CrossRef]
- Shimoyama, T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol 2013, 19, 8188–8191. [Google Scholar] [CrossRef]
- Leal, Y.A.; Cedillo-Rivera, R.; Simón, J.A.; Velázquez, J.R.; Flores, L.L.; Torres, J. Utility of stool sample-based tests for the diagnosis of Helicobacter pylori infection in children. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 718–728. [Google Scholar] [CrossRef]
- Qiu, E.; Li, Z.; Han, S. Methods for detection of Helicobacter pylori from stool sample: Current options and developments. Braz. J. Microbiol. 2021, 52, 2057–2062. [Google Scholar] [CrossRef]
- Elwyn, G.; Taubert, M.; Davies, S.; Brown, G.; Allison, M.; Phillips, C. Which test is best for Helicobacter pylori? A cost-effectiveness model using decision analysis. Br. J. Gen. Pract. 2007, 57, 401–403. [Google Scholar]
- Schulz, T.R.; McBryde, E.S.; Leder, K.; Biggs, B.A. Using stool antigen to screen for Helicobacter pylori in immigrants and refugees from high prevalence countries is relatively cost effective in reducing the burden of gastric cancer and peptic ulceration. PLoS ONE 2014, 9, e108610. [Google Scholar] [CrossRef]
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 1921–1930. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X.; Bermejo, F.; Boixeda, D.; Bory, F.; Bujanda, L.; Castro-Fernández, M.; Dominguez-Muñoz, E.; Elizalde, J.I.; Forné, M.; et al. III Spanish Consensus Conference on Helicobacter pylori infection. Gastroenterol. Hepatol. 2013, 36, 340–374. [Google Scholar] [CrossRef]
- Zhou, X.; Su, J.; Xu, G.; Zhang, G. Accuracy of stool antigen test for the diagnosis of Helicobacter pylori infection in children: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 629–638. [Google Scholar] [CrossRef] [PubMed]
- CADTH. Stool Antigen Tests for Helicobacter pylori Infection: A Review of Clinical and Cost-Effectiveness and Guidelines. In Rapid Response Reports: Summary with Critical Appraisal; Canadian Agency for Drugs and Technologies in Health (CADTH): Ottawa, ON, Canada, 2015. [Google Scholar]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Ramírez-Lázaro, M.J.; Lite, J.; Lario, S.; Pérez-Jové, P.; Montserrat, A.; Quílez, M.E.; Martínez-Bauer, E.; Calvet, X. Good diagnostic accuracy of a chemiluminescent immunoassay in stool samples for diagnosis of Helicobacter pylori infection in patients with dyspepsia. J. Investig. Med. 2016, 64, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin. Chem. 2003, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R.; Carley, S.; Harrison, M. An introduction to power and sample size estimation. Emerg. Med. J. 2003, 20, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Gomollón, F.; Domínguez-Muñoz, J.E.; Borda, F.; Jiménez, I.; Vázquez, M.A.; Gallego, S.; Iglesias, J.; Pastor, G.; Pajares, J.M. Comparison between two 13C-urea breath tests for the diagnosis of Helicobacter pylori infection: Isotope ratio mass spectrometer versus infrared spectrometer. Gastroenterol. Hepatol. 2003, 26, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Saito, M.; Fukuda, S.; Kato, C.; Ohara, S.; Hamada, S.; Nagashima, R.; Obara, K.; Suzuki, M.; Honda, H.; et al. 13C-Urea breath test, using a new compact nondispersive isotope-selective infrared spectrophotometer: Comparison with mass spectrometry. J. Gastroenterol. 2004, 39, 629–634. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kim, N.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Effect of Citric Acid on Accuracy of (13)C-Urea Breath Test after Helicobacter pylori Eradication Therapy in a Region with a High Prevalence of Atrophic Gastritis. Gut Liver 2019, 13, 506–514. [Google Scholar] [CrossRef]
- Calvet, X.; Sánchez-Delgado, J.; Montserrat, A.; Lario, S.; Ramírez-Lázaro, M.J.; Quesada, M.; Casalots, A.; Suárez, D.; Campo, R.; Brullet, E.; et al. Accuracy of diagnostic tests for Helicobacter pylori: A reappraisal. Clin. Infect Dis. 2009, 48, 1385–1391. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kim, N.; Lee, J.Y.; Choi, Y.J.; Yoon, K.; Hwang, J.J.; Lee, H.J.; Lee, A.; Jeong, Y.S.; Oh, S.; et al. The Diagnostic Validity of Citric Acid-Free, High Dose (13)C-Urea Breath Test After Helicobacter pylori Eradication in Korea. Helicobacter 2015, 20, 159–168. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Olivares, D.; Jimenez, I.; Pajares, J.M. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: Frequency and significance of borderline delta13CO2 values. Aliment. Pharmacol. Ther. 2006, 23, 275–280. [Google Scholar] [CrossRef]
- Keller, J.; Hammer, H.F.; Afolabi, P.R.; Benninga, M.; Borrelli, O.; Dominguez-Munoz, E.; Dumitrascu, D.; Goetze, O.; Haas, S.L.; Hauser, B.; et al. European guideline on indications, performance and clinical impact of (13) C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC. United Eur. Gastroenterol. J. 2021, 9, 598–625. [Google Scholar] [CrossRef]
- Yan, J.; Yamaguchi, T.; Odaka, T.; Suzuki, T.; Ohyama, N.; Hara, T.; Sudo, K.; Nakamura, K.; Denda, T.; Takiguchi, N.; et al. Stool antigen test is a reliable method to detect Helicobacter pylori in the gastric remnant after distal gastrectomy for gastric cancer. J. Clin. Gastroenterol. 2010, 44, 73–74. [Google Scholar] [CrossRef]
- Kodama, M.; Murakami, K.; Okimoto, T.; Fukuda, Y.; Shimoyama, T.; Okuda, M.; Kato, C.; Kobayashi, I.; Fujioka, T. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J. Gastroenterol. 2012, 18, 44–48. [Google Scholar] [CrossRef]
- Roda, A.; Pasini, P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Chemiluminescent enzyme immunoassays: A review of bioanalytical applications. Bioanalysis 2009, 1, 1259–1269. [Google Scholar] [CrossRef]
- Xiao, S.; Shang, K.; Zhang, L.; Li, W.; Wang, X. A rapid anti-Helicobacter pylori biofilm drug screening biosensor based on AlpB outer membrane protein and colloidal gold/nanoporous gold framework. Biosens. Bioelectron. 2022, 215, 114599. [Google Scholar] [CrossRef]
- Xiao, S.; Shang, K.; Li, W.; Wang, X. An efficient biosensor based on the synergistic catalysis of Helicobacter pylori urease b subunit and nanoplatinum for urease inhibitors screening and antagonistic mechanism analyzing. Sens. Actuators B Chem. 2022, 355, 131284. [Google Scholar] [CrossRef]
- Opekun, A.R.; Zierold, C.; Rode, A.; Blocki, F.A.; Fiorini, G.; Saracino, I.M.; Vaira, D.; Sutton, F.M. Clinical Performance of the Automated LIAISON® Meridian H. pylori SA Stool Antigen Test. Biomed. Res. Int. 2020, 2020, 7189519. [Google Scholar] [CrossRef]
- Vargas, J.; López-Sánchez, M.; Pabón, M.; Lamas, E.; Parra, M.; CastroFernández, M. Evaluation of a novel chemiluminiscent inmunoassay for the detection of Helicobacter pylori antigen and their correlation with immunochromatographic rapid test in stools samples from adults patients. Helicobacter 2013, 18, 121. [Google Scholar] [CrossRef]
- Sánchez Delgado, J.; García-Iglesias, P.; Titó, L.; Puig, I.; Planella, M.; Gené, E.; Saló, J.; Martínez-Cerezo, F.; Molina-Infante, J.; Gisbert, J.P.; et al. Update on the management of Helicobacter pylori infection. Position paper from the Catalan Society of Digestology. Gastroenterol. Hepatol. 2018, 41, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, B. Diagnosis of Helicobacter pylori Infection and Recent Advances. Diagnostics 2021, 11, 1305. [Google Scholar] [CrossRef]
- Shuber, A.P.; Ascaño, J.J.; Boynton, K.A.; Mitchell, A.; Frierson, H.F., Jr.; El-Rifai, W.; Powell, S.M. Accurate, noninvasive detection of Helicobacter pylori DNA from stool samples: Potential usefulness for monitoring treatment. J. Clin. Microbiol. 2002, 40, 262–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khadangi, F.; Yassi, M.; Kerachian, M.A. Review: Diagnostic accuracy of PCR-based detection tests for Helicobacter Pylori in stool samples. Helicobacter 2017, 22, e12444. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, F.; Hu, B.; Fang, Y.; Miao, Y.; Huang, Y.; Ji, D.; Zhang, J.; Xu, L.; Zhang, Y.; et al. A Creative Helicobacter pylori Diagnosis Scheme Based on Multiple Genetic Analysis System: Qualification and Quantitation. Helicobacter 2015, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylor infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [PubMed]
- Brown, L.M. Helicobacter pylor: Epidemiology and routes of transmission. Epidemiol. Rev. 2000, 22, 283–297. [Google Scholar]
- Megraud, F. Transmission of Helicobacter pylor: Faecal-oral versus oral-oral route. Aliment. Pharmacol. Ther. 1995, 9 (Suppl. S2), 85–91. [Google Scholar]
- Amieva, M.R.; EI-Omar, E.M. Host-bacterial interactions in Helicobacter pylor infection. Gastroenterology 2008, 134, 306–323. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Statistical Quality Control for Quantitiative Measurements: Priciples and Definition: Approved Guideline, 3rd ed.; CLSI Document C24-A3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2006; Volume 26, ISBN 1-56238-613-1. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Precision Performance of Quantitative Measure Methods; Approved Guideline, 3rd ed.; EP5-A3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2014; Volume 34. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). User Verification of Precision and Estimation of Bias; Approved Guideline, 3rd ed.; EP15-A3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2014; Volume 28. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Interference Testing in Clinical Chemistry; Approved Guideline, 2nd ed.; EP7-A2; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2005; Volume 28. [Google Scholar]
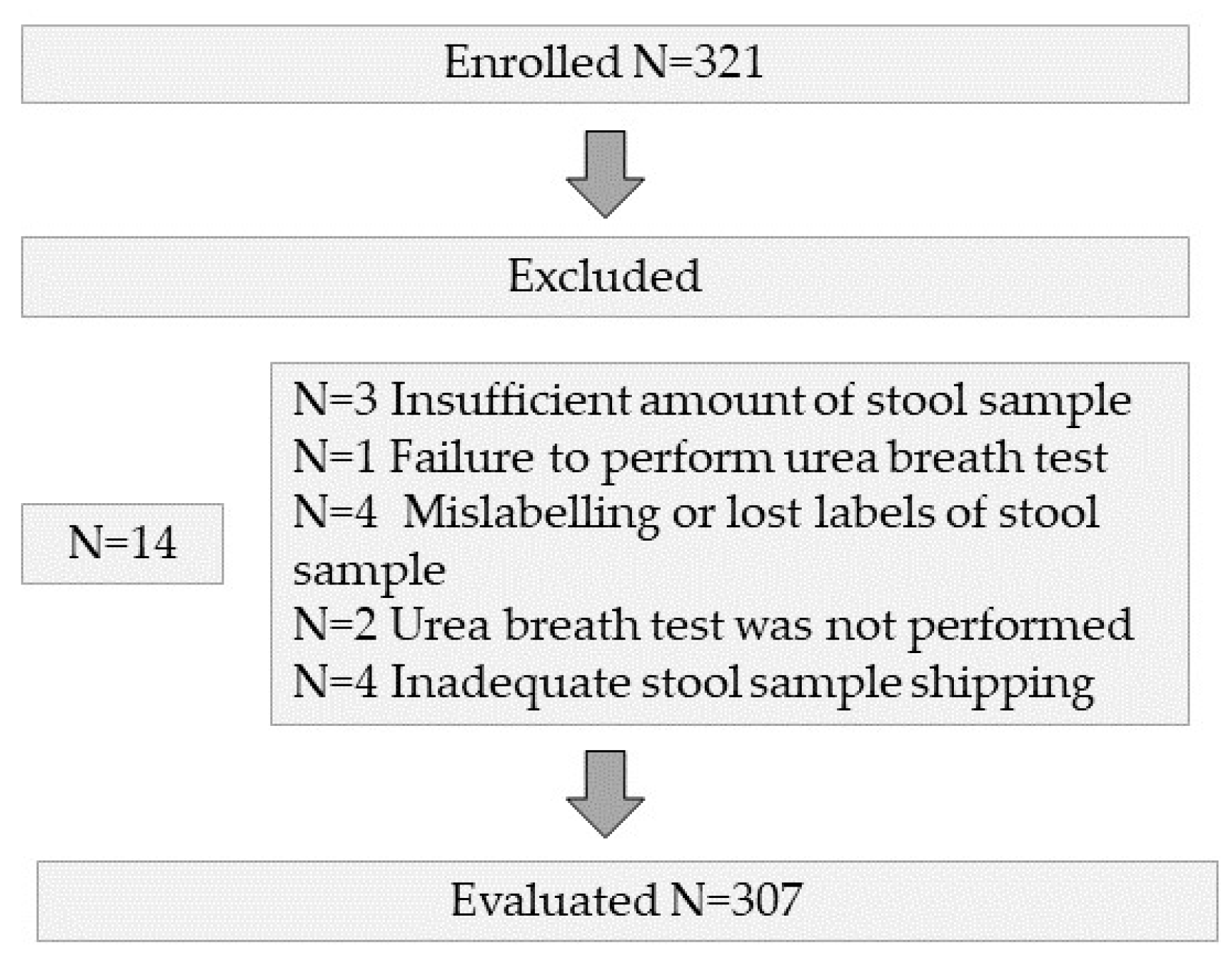
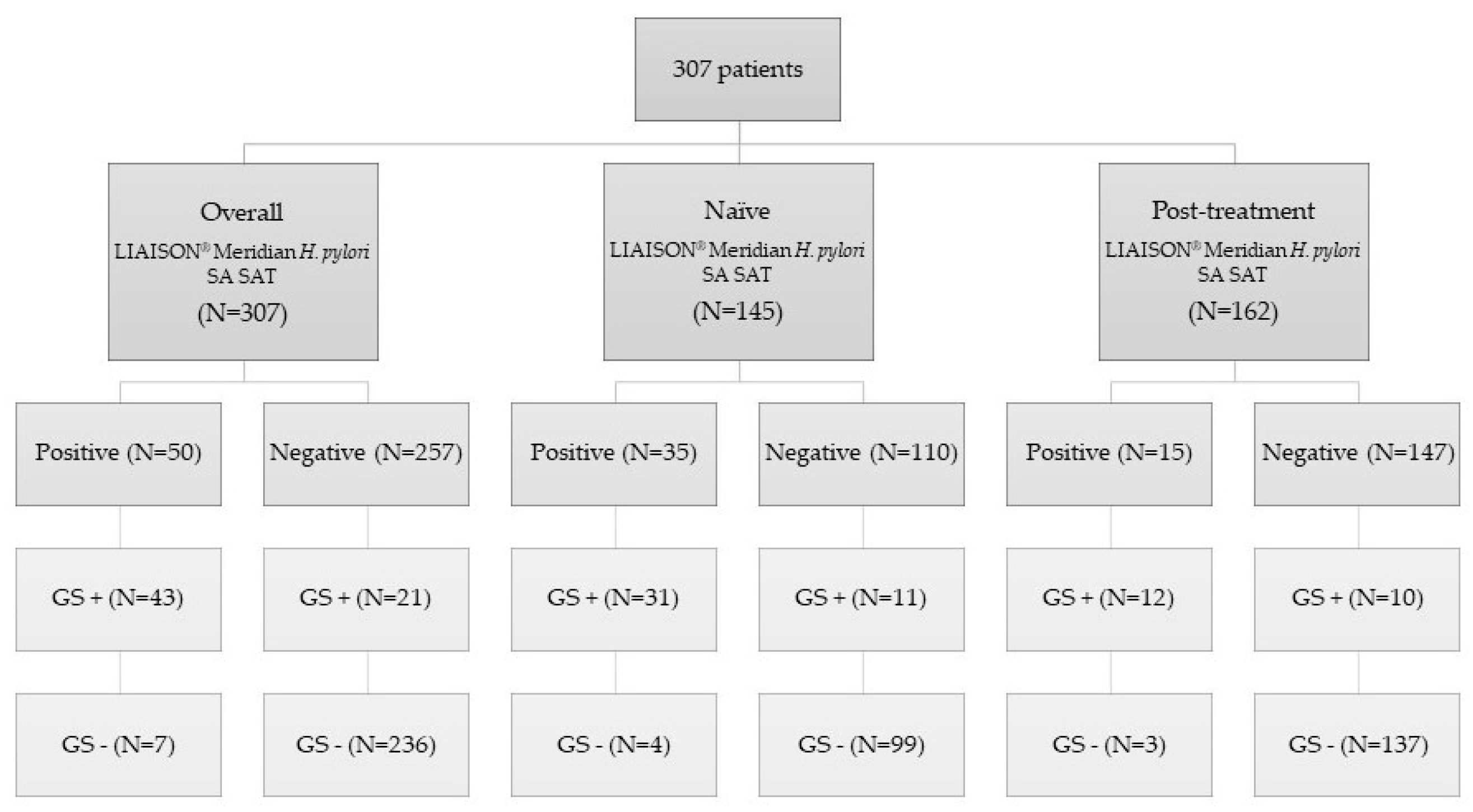
| Variables | |
|---|---|
| Age (mean ± SD) | 47.1 ± 14.4 |
| N (%) | |
| Gender (female) | 207 (67) |
| Clinical indication | |
| 145 (47) |
| 162 (53) |
| UBT method | |
| 118 (38) |
| 189 (62) |
| History of peptic ulcer | 14 (5) |
| H. pylori infection prevalence (by UBT) | % (95% CI) |
| 21 (16–26%) |
| 29 (21–37%) |
| 14 (8–19%) |
| N = 307 |
| Comparison SAT vs. UBT | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) | Global Accuracy (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Naïve | 74% (59–88) | 96% (92–100) | 89% (77–100) | 90% (84–96) | 19 (7–51) | 0.27 (0.16–0.45) | 90% (84–95) | 0.88 (0.80–0.96) |
| NDIRS naïve | 73% (54–92) | 93% (86–100) | 83% (65–100) | 89% (80–97) | 11 (4–29) | 0.29 (0.15–0.55) | 87% (80–95) | 0.86 (0.75–0.96) |
| IRMS naïve | 75% (51–99) | 100% (99–100) | 100% (96–100) | 92% (82–100) | - | 0.25 (0.11–0.58) | 93% (86–100) | 0.91 (0.79–1.0) |
| Comparison SAT vs. UBT | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) | Global Accuracy (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 55% (31–78) | 98% (95–100) | 80% (56–100) | 93% (89–98) | 25 (8–82) | 0.46 (0.29–0.73) | 92% (87–96) | 0.79 (0.65–0.93) |
| NDIRS post-treatment | 53 % (26–80) | 99% (96–100) | 90% (66–100) | 91% (85–98) | 46 (6–336) | 0.48 (0.29–0.79) | 91% (85–97) | 0.81 (0.67–0.95) |
| IRMS post-treatment | 60% (7–100) | 96% (90–100) | 60% (7–100) | 96% (90–100) | 16 (3–75) | 0.42 (0.14–1.22) | 93% (86–100) | 0.78 (0.44–1.0) |
| Comparison SAT vs. UBT | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) | Global Accuracy (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Overall | 94% (85–100) | 97% (95–99) | 86% (75–97) | 99% (97–100) | 35 (17–73) | 0.07 (0.02–0.20) | 97% (95–99) | 0.96 (0.91–1.0) |
| Naïve | 91% (80–100) | 96% (93–100) | 89% (77–100) | 97% (94–100) | 25 (10–67) | 0.09 (0.03–0.27) | 95% (91–99) | 0.996 (0.99–1.0) |
| Post-treatment | 100% (96–100) | 98% (95–100) | 80% (56–100) | 100% (99–100) | 50 (16–153) | 0.00 | 98% (96–100) | 0.94 (0.88–1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resina, E.; Donday, M.G.; Martínez-Domínguez, S.J.; Laserna-Mendieta, E.J.; Lanas, Á.; Lucendo, A.J.; Sánchez-Luengo, M.; Alcaide, N.; Fernández-Salazar, L.; De La Peña-Negro, L.; et al. Evaluation of a New Monoclonal Chemiluminescent Immunoassay Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Spanish Multicentre Study. J. Clin. Med. 2022, 11, 5077. https://doi.org/10.3390/jcm11175077
Resina E, Donday MG, Martínez-Domínguez SJ, Laserna-Mendieta EJ, Lanas Á, Lucendo AJ, Sánchez-Luengo M, Alcaide N, Fernández-Salazar L, De La Peña-Negro L, et al. Evaluation of a New Monoclonal Chemiluminescent Immunoassay Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Spanish Multicentre Study. Journal of Clinical Medicine. 2022; 11(17):5077. https://doi.org/10.3390/jcm11175077
Chicago/Turabian StyleResina, Elena, María G. Donday, Samuel J. Martínez-Domínguez, Emilio José Laserna-Mendieta, Ángel Lanas, Alfredo J. Lucendo, Marta Sánchez-Luengo, Noelia Alcaide, Luis Fernández-Salazar, Luisa De La Peña-Negro, and et al. 2022. "Evaluation of a New Monoclonal Chemiluminescent Immunoassay Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Spanish Multicentre Study" Journal of Clinical Medicine 11, no. 17: 5077. https://doi.org/10.3390/jcm11175077
APA StyleResina, E., Donday, M. G., Martínez-Domínguez, S. J., Laserna-Mendieta, E. J., Lanas, Á., Lucendo, A. J., Sánchez-Luengo, M., Alcaide, N., Fernández-Salazar, L., De La Peña-Negro, L., Bujanda, L., Arbulo, M. G.-R. d., Alcedo, J., Pérez-Aísa, Á., Rodríguez, R., Hermida, S., Brenes, Y., Nyssen, O. P., & Gisbert, J. P. (2022). Evaluation of a New Monoclonal Chemiluminescent Immunoassay Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Spanish Multicentre Study. Journal of Clinical Medicine, 11(17), 5077. https://doi.org/10.3390/jcm11175077









