Real-Life Value of the Odysight® Application in At-Home Screening for Exudative Recurrence of Macular Edema
Abstract
:1. Introduction
2. Materials and Methods
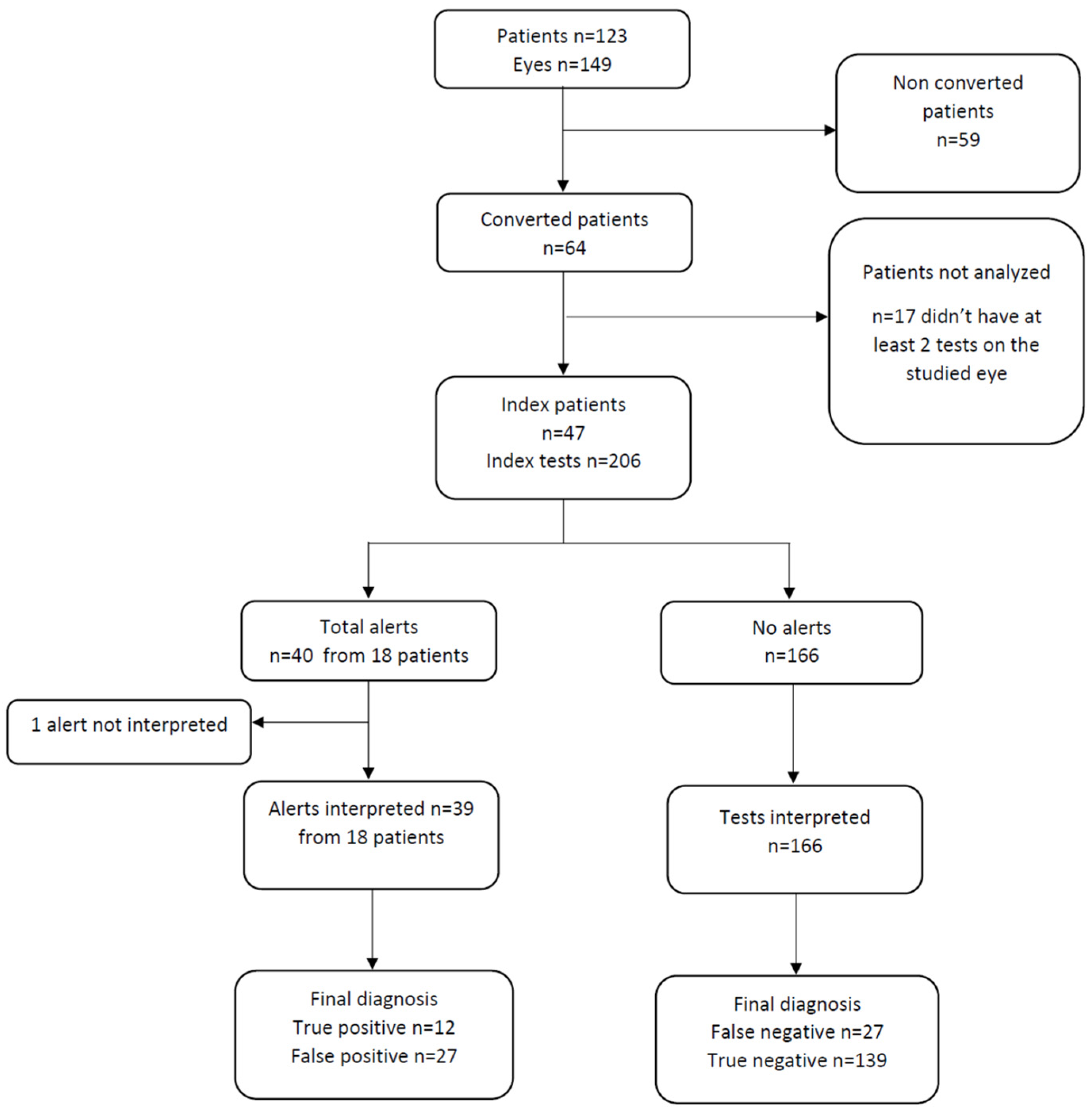
3. Results
3.1. Patients
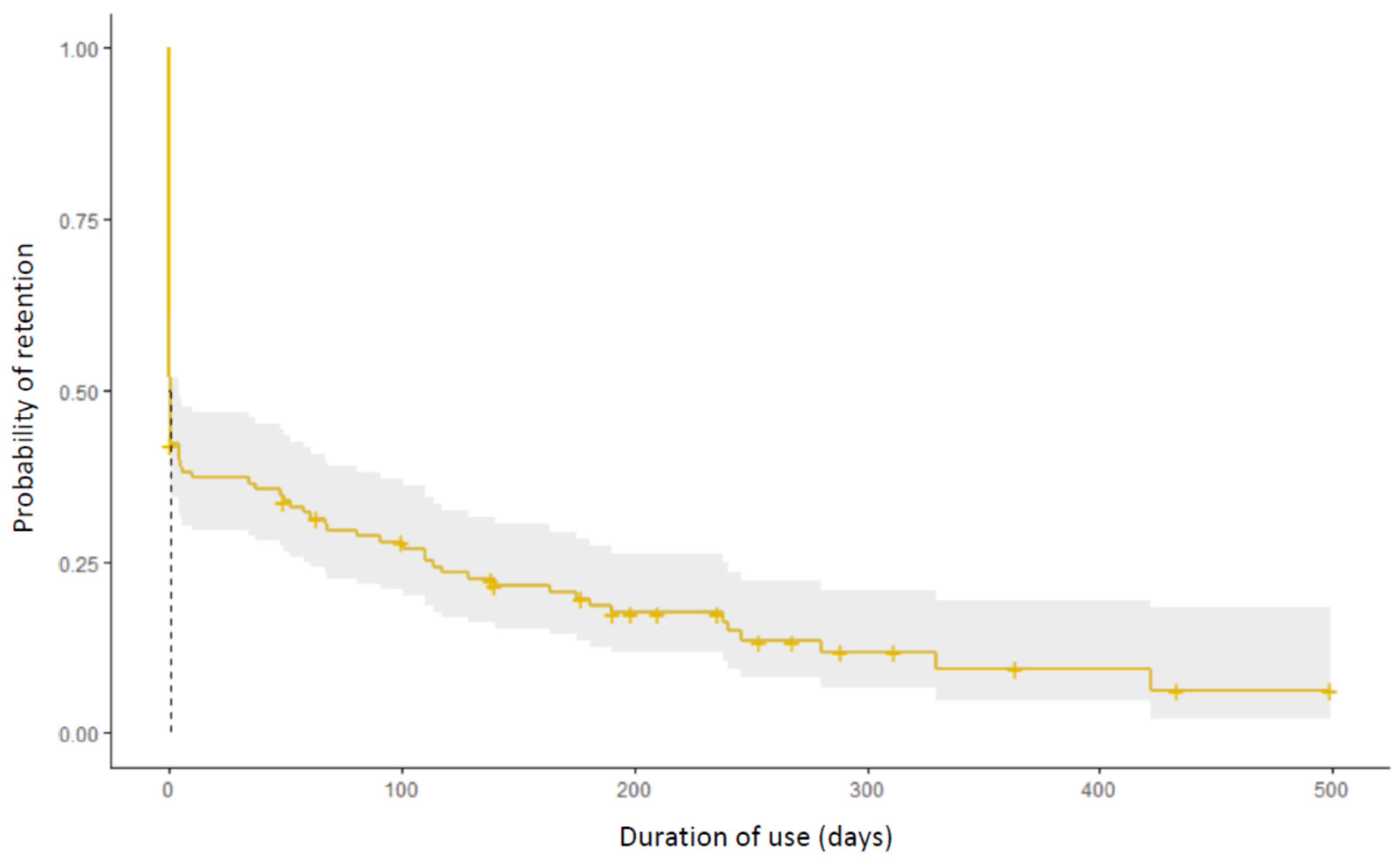
3.2. Conversion and Retention Rate
3.3. Alerts
3.4. Analysis of Patients Who Retained the Application for More Than 3 Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloch, S.B.; Larsen, M.; Munch, I.C. Incidence of Legal Blindness from Age-Related Macular Degeneration in Denmark: Year 2000 to 2010. Am. J. Ophthalmol. 2012, 153, 209–213.e2. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Kim, L.N.; Mathis, T.; Zalmay, P.; Ghanchi, F.; Amoaku, W.M.; Kodjikian, L. Trends in Real-World Neovascular AMD Treatment Outcomes in the UK. Clin. Ophthalmol. 2020, 14, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Bellocq, D.; Mathis, T. Pharmacological Management of Diabetic Macular Edema in Real-Life Observational Studies. BioMed Res. Int. 2018, 28, 8289253. [Google Scholar] [CrossRef]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.T.; Chen, T.C.; Yang, C.H.; Yang, C.M.; Ho, T.C.; Hsieh, Y.T. Treat-and-Extend vs. Pro Re Nata Regimen of Ranibizumab for Diabetic Macular Edema—A Two-Year Matched Comparative Study. Front. Med. 2022, 8, 781421. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Bodmer, N.S.; Bachmann, L.M.; Thiel, M.A.; Schmid, M.K. Diagnostic accuracy of the Amsler grid and the preferential hyperacuity perimetry in the screening of patients with age-related macular degeneration: Systematic review and meta-analysis. Eye 2014, 28, 788–796. [Google Scholar] [CrossRef]
- Zaidi, F.H.; Cheong-Leen, R.; Gair, E.J.; Weir, R.; Sharkawi, E.; Lee, N.; Gregory-Evans, K. The Amsler chart is of doubtful value in retinal screening for early laser therapy of subretinal membranes. The West London Survey. Eye 2004, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Chamard, C.; Lacombe, S.; Navarre, S.; Rohart, C.; Daures, J.P.; Allieu, S. Is current age related macular degeneration self-monitoring a good tool for detecting exudative recurrence? J. Fr. Ophtalmol. 2019, 42, 1049–1055. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinkel, R.A. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design—HOME Study report number 1. Contemp. Clin. Trials 2014, 37, 294–300. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Wang, Y.Z.; He, Y.G.; Weisberger, A.; Wolf, S.; Smith, C.H. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: Results of a Pilot Study. Retina 2013, 33, 1863–1870. [Google Scholar] [CrossRef]
- Schmid, M.K.; Thiel, M.A.; Lienhard, K.; Schlingemann, R.O.; Faes, L.; Bachmann, L.M. Reliability and diagnostic performance of a novel mobile app for hyperacuity self-monitoring in patients with age-related macular degeneration. Eye 2019, 33, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Brucker, J.; Bhatia, V.; Sahel, J.-A.; Girmens, J.-F.; Mohand-Saïd, S. Odysight: A Mobile Medical Application Designed for Remote Monitoring—A Prospective Study Comparison with Standard Clinical Eye Tests. Ophthalmol. Ther. 2019, 8, 461–476. [Google Scholar] [CrossRef]
- Guigou, S.; Michel, T.; Mérité, P.-Y.; Coupier, L.; Meyer, F. Home vision monitoring in patients with maculopathy: Real-life study of the OdySight application. J. Fr. Ophtalmol. 2021, 44, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, D.; Akesbi, J.; Matonti, F.; Vartin, C.; Despreaux, R.; Comet, A.; Voirin, N.; Denis, P.; Mathis, T.; Kodjikian, L. The Pattern of Recurrence in Diabetic Macular Edema Treated by Dexamethasone Implant: The PREDIAMEX Study. Ophthalmol. Retina 2018, 2, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Kuo, B.L.; Chen, A.X.; Wang, K.; Greenlee, T.E.; Conti, T.F.; Singh, R.P. Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents. Eye 2021, 36, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.N.; Reeves, B.C.; Maguire, M.G.; Martin, D.F.; Muldrew, A.; Peto, T.; Rogers, C.; Chakravarthy, U. Associations of Variation in Retinal Thickness with Visual Acuity and Anatomic Outcomes in Eyes with Neovascular Age-Related Macular Degeneration Lesions Treated with Anti–Vascular Endothelial Growth Factor Agents. JAMA Ophthalmol. 2020, 138, 1043. [Google Scholar] [CrossRef]
- Nicolas, C.; Debellemanière, G.; Boissier, F.; Girard, C.; Schwartz, C.; Delbosc, B.; Saleh, M. Reproductibilité des mesures de l’acuité visuelle par échelle ETDRS en pratique quotidienne. J. Fr. Ophtalmol. 2016, 39, 700–705. [Google Scholar] [CrossRef]
- Siderov, J.; Tiu, A.L. Variability of measurements of visual acuity in a large eye clinic: Variability of measurements of visual acuity in a large eye clinic. Acta Ophthalmol. Scand. 1999, 77, 673–676. [Google Scholar] [CrossRef]
- Rosser, D.A.; Cousens, S.N.; Murdoch, I.E.; Fitzke, F.W.; Laidlaw, D.A.H. How Sensitive to Clinical Change are ETDRS logMAR Visual Acuity Measurements? Investig. Opthalmol. Vis. Sci. 2003, 44, 3278. [Google Scholar] [CrossRef]
- Keane, P.A.; De Salvo, G.; Sim, D.A.; Goverdhan, S.; Agrawal, R.; Tufail, A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Teo, K.Y.C.; Bachmann, L.M.; Sim, D.; Lee, S.Y.; Tan, A.; Wong, T.Y.; Cheung, C.M.G.; Tan, G.S.W. Patterns and Characteristics of a Clinical Implementation of a Self-Monitoring Program for Retina Diseases during the COVID-19 Pandemic. Ophthalmol. Retina 2021, 5, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sansome, S.; Das, R.; Lukic, M.; Teo, K.Y.C.; Tan, G.; Balaskas, K.; Thomas, P.B.M.; Bachmann, L.M.; Schimel, A.M.; et al. Smartphone-based remote monitoring of vision in macular disease enables early detection of worsening pathology and need for intravitreal therapy. BMJ Health Care Inform. 2021, 28, e100310. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Islam, M.; Bachmann, L.M.; Lienhard, K.R.; Schmid, M.K.; Sim, D.A. False alarms and the positive predictive value of smartphone-based hyperacuity home monitoring for the progression of macular disease: A prospective cohort study. Eye 2021, 35, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Bachmann, L.M.; Islam, M.; Faes, L.; Schmid, M.K.; Thiel, M.; Schimel, A.; Sim, D. Visual outcomes and treatment adherence of patients with macular pathology using a mobile hyperacuity home-monitoring app: A matched-pair analysis. BMJ Open 2021, 11, e056940. [Google Scholar] [CrossRef]
- Ward, E.; Wickens, R.A.; O’Connell, A.; Culliford, L.A.; Rogers, C.A.; Gidman, E.A.; Peto, T.; Knox, P.C.; Burton, B.J.L.; Lotery, A.J.; et al. Monitoring for neovascular age-related macular degeneration (AMD) reactivation at home: The MONARCH study. Eye 2021, 35, 592–600. [Google Scholar] [CrossRef]
- Nikam, A.; Lefumat, H.; Grondin, E.; Bhatia, V. OdySight: Real-World Data Analysis of a Mobile Medical Application for Ophthalmology. J. Ophthalmol. Sci. 2020, 2, 6–12. [Google Scholar]

| n = 123 | |
|---|---|
| Sex, n (%) | |
| Female | 72 (58.5) |
| Male | 51 (41.5) |
| Age, years, n (%) | |
| <50 | 21 (17.1) |
| 50–64 | 29 (23.6) |
| 65–79 | 56 (45.5) |
| ≤80 | 17 (13.8) |
| Disease, (n = 149) n (%) | |
| Exudative AMD | 69 (46.3) |
| RVO–DME | 31 (20.8) |
| Other | 49 (32.9) |
| Follow-up, n (%) | |
| Reactive regimen | 68 (55.3) |
| Proactive regimen | 55 (44.7) |
| Interval between diagnosis and application prescription, n (%) | |
| <1 year | 13 (10.6) |
| >1 year | 110 (89.4) |
| Mean, years [95% CI] | 3.0 [0.40; 16.1] |
| Baseline VA LogMAR, (n = 149) | |
| <0.2 | 96 (64.4) |
| 0.2–0.4 | 34 (22.8) |
| ≥0.5 | 19 (12.8) |
| Mean (SD) | 0.2 (0.2) |
| Sensitivity [95% CI] | 30.8 [17.6; 44.0] |
| Specificity [95% CI] | 83.7 [73.2; 94.3] |
| Positive predictive value [95% CI] | 30.8 [17.6; 44.0] |
| Negative predictive value [95% CI] | 83.7 [73.2; 94.3] |
| Sensitivity [95% CI] | 47.8 [28.6; 67.0] |
| Specificity [95% CI] | 78.0 [62.1; 93.9] |
| Positive predictive value [95% CI] | 35.5 [17.1; 53.9] |
| Negative predictive value [95% CI] | 85.5 [72.0; 99.0] |
| Retention > 3 Months | Analysis | |||
|---|---|---|---|---|
| No (n = 90) | Yes (n = 33) | Univariate OR [95% CI] | Multivariate OR [95% CI] | |
| Sex, n (%) | p = 0.78 | |||
| Female | 52 (57.7) | 20 (60.6) | ref | ref |
| Male | 38 (42.3) | 13 (39.4) | OR = 0.89 [0.39; 1.99] | p = 0.73 OR = 0.86 [0.35; 2.07] |
| Age, years mean (min, max) | 68.0 (23.0, 89.0) | 69.0 (40.0, 87.0) | p = 0.59 OR = 1.01 [0.98; 1.04] | p = 0.65 OR = 1.01 [0.97; 1.05] |
| Disease, n (%) | p = 0.43 | |||
| Exudative AMD | 41 (45.6) | 16 (48.5) | ref | ref |
| RVO–DME | 20 (22.2) | 10 (30.3) | p = 0.61 OR = 1.28 [0.48; 3.31] | p = 0.86 OR = 1.10 [0.35; 3.48] |
| Other | 29 (32.2) | 7 (21.2) | p = 0.35 OR = 0.62 [0.21; 1.65] | p = 0.49 OR = 0.62 [0.15; 2.39] |
| Follow-up regimen n (%) | p = 0.47 | |||
| Reactive | 48 (53.3) | 20 (60.6) | ref | ref |
| Proactive | 42 (46.7) | 13 (39.4) | OR = 0.74 [0.32; 1.66] | p = 0.36 OR = 0.62 [0.23; 1.58] |
| Interval from diagnosis to application use n (%) | p = 0.74 | |||
| ≤1 year | 9 (10.0) | 4 (12.1) | ref | ref |
| >1 year | 81 (90.0) | 29 (87.9) | OR = 0.81 [0.24; 3.2] | p = 0.95 OR = 0.95 [0.25; 4.22] |
| Visual acuity, LogMAR mean (SD) | 0.2 (0.3) | 0.1 (0.1) | p = 0.03 OR = 0.04 [0.001; 0.53] | p = 0.04 OR = 0.05 [0.002; 0.62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielwasser, G.; Kodjikian, L.; Dot, C.; Burillon, C.; Denis, P.; Mathis, T. Real-Life Value of the Odysight® Application in At-Home Screening for Exudative Recurrence of Macular Edema. J. Clin. Med. 2022, 11, 5010. https://doi.org/10.3390/jcm11175010
Kielwasser G, Kodjikian L, Dot C, Burillon C, Denis P, Mathis T. Real-Life Value of the Odysight® Application in At-Home Screening for Exudative Recurrence of Macular Edema. Journal of Clinical Medicine. 2022; 11(17):5010. https://doi.org/10.3390/jcm11175010
Chicago/Turabian StyleKielwasser, Gauthier, Laurent Kodjikian, Corinne Dot, Carole Burillon, Philippe Denis, and Thibaud Mathis. 2022. "Real-Life Value of the Odysight® Application in At-Home Screening for Exudative Recurrence of Macular Edema" Journal of Clinical Medicine 11, no. 17: 5010. https://doi.org/10.3390/jcm11175010
APA StyleKielwasser, G., Kodjikian, L., Dot, C., Burillon, C., Denis, P., & Mathis, T. (2022). Real-Life Value of the Odysight® Application in At-Home Screening for Exudative Recurrence of Macular Edema. Journal of Clinical Medicine, 11(17), 5010. https://doi.org/10.3390/jcm11175010







