Evaluation of Glycosylated Ferritin in Adult-Onset Still’s Disease and Differential Diagnoses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Glycosylated Ferritin Assays
2.3. Data Collection and Classification
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population and Comparison
3.2. Causes According to the Level of Glycosylated Ferritin
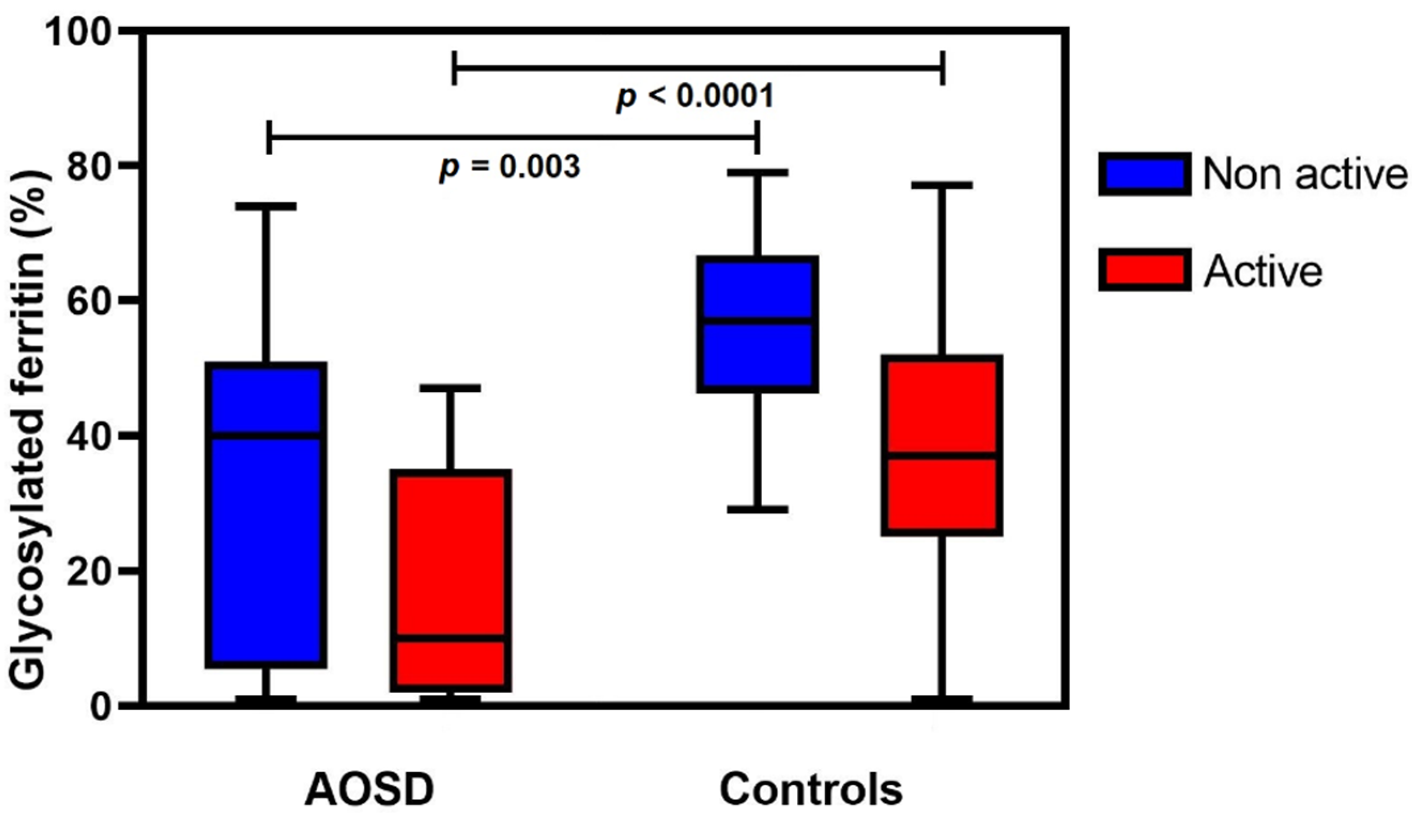
3.3. Glycosylated Ferritin Level as a Function of Disease Activity
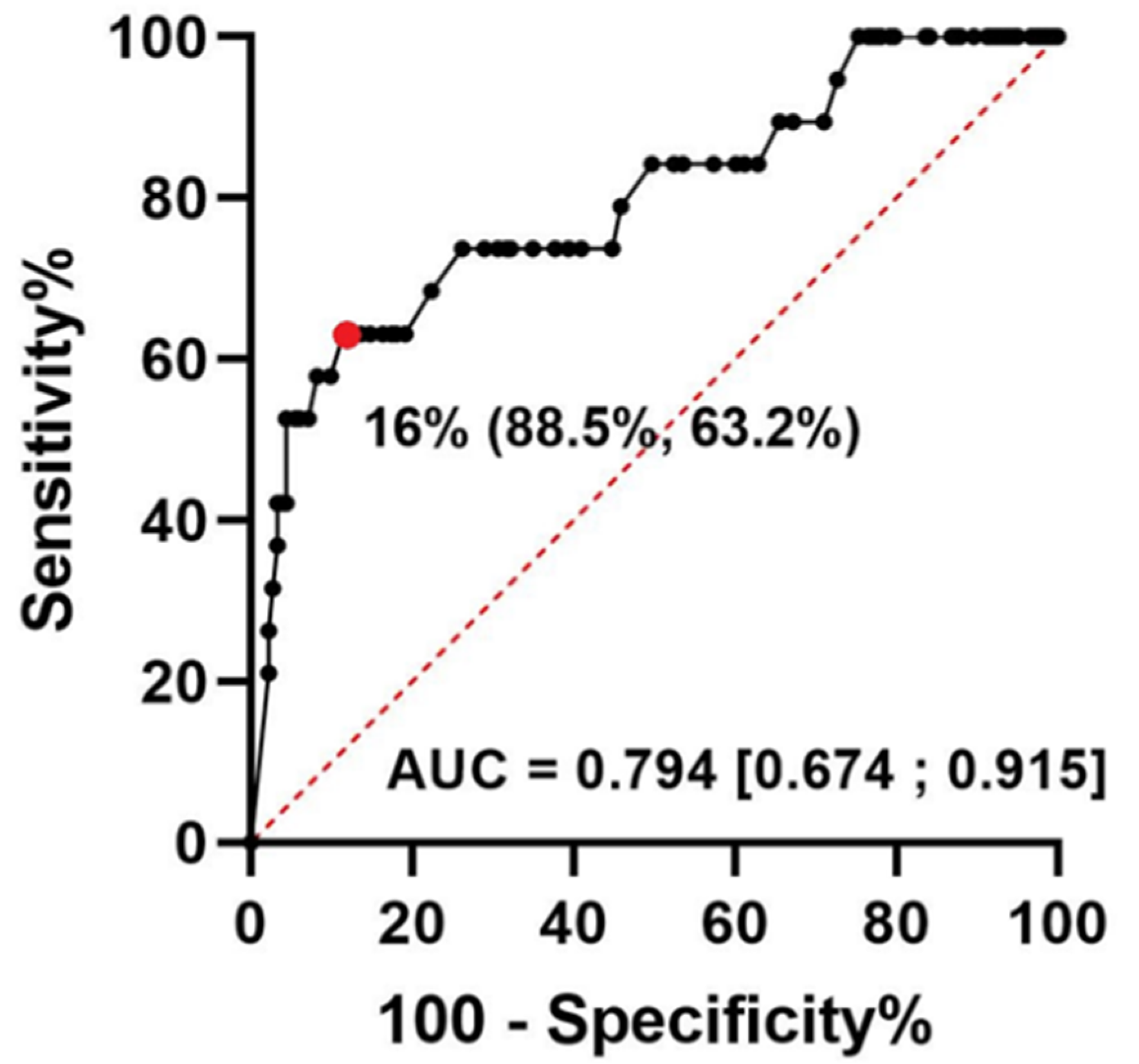
3.4. Glycosylated Ferritin Performance for the Identification of AOSD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, M.; Ohta, A.; Tsunematsu, T.; Kasukawa, R.; Mizushima, Y.; Kashiwagi, H.; Kashiwazaki, S.; Tanimoto, K.; Matsumoto, Y.; Ota, T. Preliminary criteria for classification of adult Still’s disease. J. Rheumatol. 1992, 19, 424–430. [Google Scholar] [PubMed]
- Fautrel, B.; Zing, E.; Golmard, J.-L.; Le Moel, G.; Bissery, A.; Rioux, C.; Rozenberg, S.; Piette, J.-C.; Bourgeois, P. Proposal for a New Set of Classification Criteria for Adult-Onset Still Disease. Medicine 2002, 81, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, D.; Mestrallet, S.; Dehoux, M.; Golmard, J.L.; Granger, B.; Georgin-Lavialle, S.; Arnaud, L.; Grateau, G.; Pouchot, J.; Fautrel, B. Validation of the Fautrel classification criteria for adult-onset Still’s disease. Semin. Arthritis Rheum. 2018, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Fauter, M.; Viel, S.; Zaepfel, S.; Pradat, P.; Fiscus, J.; Villard, M.; Garnier, L.; Walzer, T.; Sève, P.; Henry, T.; et al. Low glycosylated ferritin is a sensitive biomarker of severe COVID-19. Cell Mol. Immunol. 2020, 17, 1183–1185. [Google Scholar] [CrossRef]
- Fardet, L.; Coppo, P.; Kettaneh, A.; Dehoux, M.; Cabane, J.; Lambotte, O. Low glycosylated ferritin, a good marker for the diagnosis of hemophagocytic syndrome. Arthritis Rheum. 2008, 58, 1521–1527. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, J.; Feng, C.; Tian, L.; Wu, L. Early diagnostic value of low percentage of glycosylated ferritin in secondary hemophagocytic lymphohistiocytosis. Int. J. Hematol. 2009, 90, 501–505. [Google Scholar] [CrossRef]
- Javaux, C.; El-Jammal, T.; Neau, P.-A.; Fournier, N.; Gerfaud-Valentin, M.; Perard, L.; Fouillet-Desjonqueres, M.; Le Scanff, J.; Vignot, E.; Durupt, S.; et al. Detection and Prediction of Macrophage Activation Syndrome in Still’s Disease. J. Clin. Med. 2021, 11, 206. [Google Scholar] [CrossRef]
- Hot, A.; Toh, M.-L.; Coppéré, B.; Perard, L.; Madoux, M.H.G.; Mausservey, C.; Desmurs-Clavel, H.; Ffrench, M.; Ninent, J. Reactive hemophagocytic syndrome in adult-onset Still disease: Clinical features and long-term outcome: A case-control study of 8 patients. Medicine 2010, 89, 37–46. [Google Scholar] [CrossRef]
- Arlet, J.-B.; Huong, D.L.T.; Marinho, A.; Amoura, Z.; Wechsler, B.; Papo, T.; Piette, J.-C. Reactive haemophagocytic syndrome in adult-onset Still’s disease: A report of six patients and a review of the literature. Ann. Rheum. Dis. 2006, 65, 1596–1601. [Google Scholar] [CrossRef]
- Lenert, A.; Oh, G.; Ombrello, M.J.; Kim, S. Clinical characteristics and comorbidities in adult-onset Still’s disease using a large US administrative claims database. Rheumatology 2020, 59, 1725–1733. [Google Scholar] [CrossRef]
- Mitrovic, S.; Fautrel, B. Complications of adult-onset Still’s disease and their management. Expert. Rev. Clin. Immunol. 2018, 14, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-K.; Cho, C.-S.; Kim, H.-Y.; Oh, E.-J. Bone marrow findings in patients with adult Still’s disease. Scand. J. Rheumatol. 2003, 32, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Grom, A.A.; Behrens, E.M.; Cron, R.Q. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: Diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012, 13, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.M.; Beukelman, T.; Paessler, M.; Cron, R.Q. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 2007, 34, 1133–1138. [Google Scholar]
- Gerfaud-Valentin, M.; Maucort-Boulch, D.; Hot, A.; Iwaz, J.; Ninet, J.; Durieu, I.; Broussolle, C.; Sève, P. Adult-Onset Still Disease: Manifestations, Treatment, Outcome, and Prognostic Factors in 57 Patients. Medicine 2014, 93, 91–99. [Google Scholar] [CrossRef]
- Schwarz-Eywill, M.; Heilig, B.; Bauer, H.; Breitbart, A.; Pezzutto, A. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann. Rheum. Dis. 1992, 51, 683–685. [Google Scholar] [CrossRef]
- Ota, T.; Higashi, S.; Suzuki, H.; Eto, S. Increased serum ferritin levels in adult Still’s disease. Lancet 1987, 1, 562–563. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, T.; Martin-Mola, E.; Fernandez-Zamorano, A.; Balsa-Criado, A.; de Miguel-Mendieta, E. Serum ferritin can be useful for diagnosis in adult onset Still’s disease. J. Rheumatol. 1989, 16, 412–413. [Google Scholar]
- Shiga, T.; Nozaki, Y.; Tomita, D.; Kishimoto, K.; Hirooka, Y.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Usefulness of Interleukin-18 as a Diagnostic Biomarker to Differentiate Adult-Onset Still’s Disease With/Without Macrophage Activation Syndrome From Other Secondary Hemophagocytic Lymphohistiocytosis in Adults. Front. Immunol. 2021, 12, 750114. [Google Scholar] [CrossRef]
- Worwood, M.; Cragg, S.J.; Wagstaff, M.; Jacobs, A. Binding of human serum ferritin to concanavalin A. Clin. Sci. 1979, 56, 83–87. [Google Scholar] [CrossRef]
- Raynor, A.; Peoc’h, K.; Boutten, A. Measurement of glycosylated ferritin with Concanavalin A: Assay design, optimization and validation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1194, 123184. [Google Scholar] [CrossRef]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome: Score for Reactive Hemophagocytic Syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Van Reeth, C.; Le Moel, G.; Lasne, Y.; Revenant, M.C.; Agneray, J.; Kahn, M.F.; Bourgeois, P. Serum ferritin and isoferritins are tools for diagnosis of active adult Still’s disease. J. Rheumatol. 1994, 21, 890–895. [Google Scholar]
- Fautrel, B.; Le Moël, G.; Saint-Marcoux, B.; Taupin, P.; Vignes, S.; Rozenberg, S.; Koeger, A.C.; Meyer, O.; Guillevin, L.; Piette, J.C.; et al. Diagnostic value of ferritin and glycosylated ferritin in adult onset Still’s disease. J. Rheumatol. 2001, 28, 322–329. [Google Scholar]
- Konijn, A.M.; Kaplan, R.; Or, R.; Matzner, Y. Glycosylated serum ferritin in patients with hematological malignancies before and after bone marrow transplantation. Leuk. Lymphoma 1992, 7, 151–156. [Google Scholar] [CrossRef]
- Godeau, B.; Palazzo, E.; Morinet, F.; Deplanche, M.; Deforge, L.; Schaeffer, A.; Kahn, M.-F. Is Still’s disease associated with parvovirus B19 infection? Lancet 1995, 345, 59–60. [Google Scholar] [CrossRef]
- Vignes, S. Percentage of glycosylated serum ferritin remains low throughout the course of adult onset Still’s disease. Ann. Rheum. Dis. 2000, 59, 347–350. [Google Scholar] [CrossRef]
- Jung, K.-H.; Kim, J.-J.; Lee, J.-S.; Park, W.; Kim, T.-H.; Jun, J.-B.; Yoo, D. Interleukin-18 as an efficient marker for remission and follow-up in patients with inactive adult-onset Still’s disease. Scand. J. Rheumatol. 2014, 43, 162–169. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Suh, C.-H.; Jung, J.-Y.; Kim, A.-R.; Yang, J.W.; Kim, H.-A. The neutrophil-to-lymphocyte ratio could be a good diagnostic marker and predictor of relapse in patients with adult-onset Still’s disease: A STROBE-compliant retrospective observational analysis. Medicine 2017, 96, e7546. [Google Scholar] [CrossRef]
- Choi, J.-H.; Suh, C.-H.; Lee, Y.-M.; Suh, Y.-J.; Lee, S.-K.; Kim, S.-S.; Nahm, D.-H.; Park, H.-S. Serum cytokine profiles in patients with adult onset Still’s disease. J. Rheumatol. 2003, 30, 2422–2427. [Google Scholar]
- Dik, W.A.; Heron, M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth. J. Med. 2020, 78, 220–231. [Google Scholar]


| Characteristics (Unit) | AOSD n = 28 | Controls n = 203 | p-Value |
|---|---|---|---|
| Age (year) | 41 (±18) | 53 (±18) | 0.001 |
| BMI (kg/m2) | 23 (±5) | 24 (±5) | 0.369 |
| Male sex | 12 (42.9%) | 119 (58.6%) | 0.154 |
| Body temperature (°C) | 39.1 (36.9–39.6) | 37.9 (36.8–39.0) | 0.017 |
| Adenopathy | 11 (39.3%) | 30 (14.8%) | 0.003 |
| Hepatomegaly | 4 (14.3%) | 19 (9.4%) | 0.496 |
| Splenomegaly | 4 (14.3%) | 21 (10.3%) | 0.518 |
| Arthralgia | 16 (57.1%) | 56 (27.6%) | 0.004 |
| Arthritis | 8 (28.6%) | 31 (15.3%) | 0.103 |
| Pharyngitis | 12 (42.9%) | 16 (7.9%) | <0.001 |
| Maculopapular rash | 12 (42.9%) | 31 (15.3%) | 0.001 |
| Transient erythema | 6 (21.4%) | 1 (0.5%) | <0.001 |
| Glycosylated ferritin (%) | 22.3 (±20.8) | 39.3 (±17.6) | <0.001 |
| Serum ferritin (µg/L) | 1162 (336–3532) | 717 (295–1942) | 0.194 |
| CRP (mg/L) | 103 (19–177) | 71 (19–155) | 0.823 |
| Fibrinogen levels (g/L) | 5.3 (3.4–7.1) | 4.7 (2.8–8.2) | 0.77 |
| ALT (UI/L) | 51 (29–109) | 34 (18–68) | 0.016 |
| AST (UI/L) | 39 (28–85) | 32 (20–67) | 0.061 |
| Total bilirubin (µmol/L) | 7 (6–12) | 8 (6–14) | 0.243 |
| Serum creatinine (µmol/L) | 62 (54–75) | 68 (56–81) | 0.2 |
| White Blood Cell Count (G/L) | 9.8 (6.5–13.6) | 7.9 (4.8–11.1) | 0.038 |
| Neutrophils (G/L) | 7.3 (3.8–10.7) | 4.5 (2.8–7.6) | 0.012 |
| Relative neutrophil count (%) | 76 (63–82) | 67 (55–77) | 0.012 |
| Lymphocytes (G/L) | 1.6 (1.1–1.8) | 1.3 (0.8–2.1) | 0.89 |
| Platelet (G/L) | 241 (184–275) | 242 (146–323) | 0.886 |
| Hemoglobin (g/L) | 117 (100–131) | 109 (88–134) | 0.187 |
| HLH | 3 (10.7%) | 22 (10.8%) | >0.999 |
| Active disease | 19 (67.9%) | 183 (90.1%) | 0.003 |
| Diagnostic Category | Causes |
|---|---|
| Immune-Mediated Inflammatory Diseases 35% (n = 70) | Idiopathic pericarditis (n = 19) |
| Rheumatoid arthritis (n = 13) | |
| Miscellaneous systemic disorders (n = 10): TAFRO syndrome, inflammatory bowel disease, Sjögren’s syndrome, relapsing polychondritis, IgG4-related disease, autoimmune myositis | |
| Systemic lupus erythematosus (n = 9) | |
| Giant cell arteritis or polymyalgia rheumatica (n = 6) | |
| Spondyloarthropathies (n = 5) | |
| Vasculitis (n = 5): Polyarteritis nodosa, Urticarial vasculitis, Cryoglobulinemic vasculitis, Anti-neutrophil cytoplasmic autoantibody-associated vasculitis | |
| Autoinflammatory syndromes (n = 3): NLRP3 mutation, NLRC4 mutation, ROSAH syndrome | |
| Infectious Diseases 25 % (n = 51) | Pyogenic bacteria (n = 22): Enterobacteriaceae, Staphylococcus, Streptococcus, Anaerobic |
| Viral infection (n = 21): Human herpesvirus (EBV, CMV), Influenza, Parvovirus B19, human immunodeficiency viruses (HIV) | |
| Intracellular bacteria (n = 7): Tuberculosis, Coxiella burnetii, Tropheryma whipplei, Legionella | |
| Parasitic disease (n = 1): Blastocystis hominis | |
| Hematologic Malignancies 12% (n = 24) | Lymphoid malignancies (n = 15): Hodgkin lymphoma, B cell non-Hodgkin lymphoma, T cell non-Hodgkin lymphoma, multiple myeloma |
| Myeloid malignancies (n = 9): acute leukemia, myelodysplastic syndrome, VEXAS syndrome, mastocytosis | |
| Solid Cancers 5% (n = 11) | Metastatic cancer (n = 7) |
| Localized cancer (n = 4) | |
| Acute Hepatitis 5% (n = 11) | Drug induced hepatitis (n = 5) |
| Autoimmune hepatitis (n = 3) | |
| Viral hepatitis (n = 3) | |
| Other 18% (n = 36) | Crystal arthropathies (n = 5) |
| Urticaria (n = 8) | |
| Various (n = 23): DRESS syndrome, Osteoarthritis, Dressler syndrome, Thrombotic microangiopathy, Venous Thromboembolic Disease, Fever of unknown origin, Fibromyalgia, Focal and segmental glomerulosclerosis, Myocardial infarction, subacute granulomatous thyroiditis, Alcohol use disorder |
| Diagnostic Criteria | Specificity | Sensitivity | NPV | PPV |
|---|---|---|---|---|
| GF ≤ 20% | 83.6% | 63.2% | 95.6% | 28.6% |
| GF ≤ 16% | 88.5% | 63.2% | 95.9% | 36.4% |
| GF ≤ 10% | 95.6% | 52.6% | 95.1% | 55.6% |
| Fautrel | 96.7% | 68.4% | 96.7% | 68.4% |
| Fautrel-16 | 97.3% | 68.4% | 96.7% | 72.2% |
| Yamaguchi | 99.4% | 68.4% | 96.8% | 92.9% |
| GF ≤ 50% | 23.0% | 100.0% | 100.0% | 11.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerber, A.; Garneret, E.; El Jammal, T.; Zaepfel, S.; Gerfaud-Valentin, M.; Sève, P.; Jamilloux, Y. Evaluation of Glycosylated Ferritin in Adult-Onset Still’s Disease and Differential Diagnoses. J. Clin. Med. 2022, 11, 5012. https://doi.org/10.3390/jcm11175012
Guerber A, Garneret E, El Jammal T, Zaepfel S, Gerfaud-Valentin M, Sève P, Jamilloux Y. Evaluation of Glycosylated Ferritin in Adult-Onset Still’s Disease and Differential Diagnoses. Journal of Clinical Medicine. 2022; 11(17):5012. https://doi.org/10.3390/jcm11175012
Chicago/Turabian StyleGuerber, Arthur, Etienne Garneret, Thomas El Jammal, Sabine Zaepfel, Mathieu Gerfaud-Valentin, Pascal Sève, and Yvan Jamilloux. 2022. "Evaluation of Glycosylated Ferritin in Adult-Onset Still’s Disease and Differential Diagnoses" Journal of Clinical Medicine 11, no. 17: 5012. https://doi.org/10.3390/jcm11175012
APA StyleGuerber, A., Garneret, E., El Jammal, T., Zaepfel, S., Gerfaud-Valentin, M., Sève, P., & Jamilloux, Y. (2022). Evaluation of Glycosylated Ferritin in Adult-Onset Still’s Disease and Differential Diagnoses. Journal of Clinical Medicine, 11(17), 5012. https://doi.org/10.3390/jcm11175012






