A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial
Abstract
:1. Introduction
2. Methods
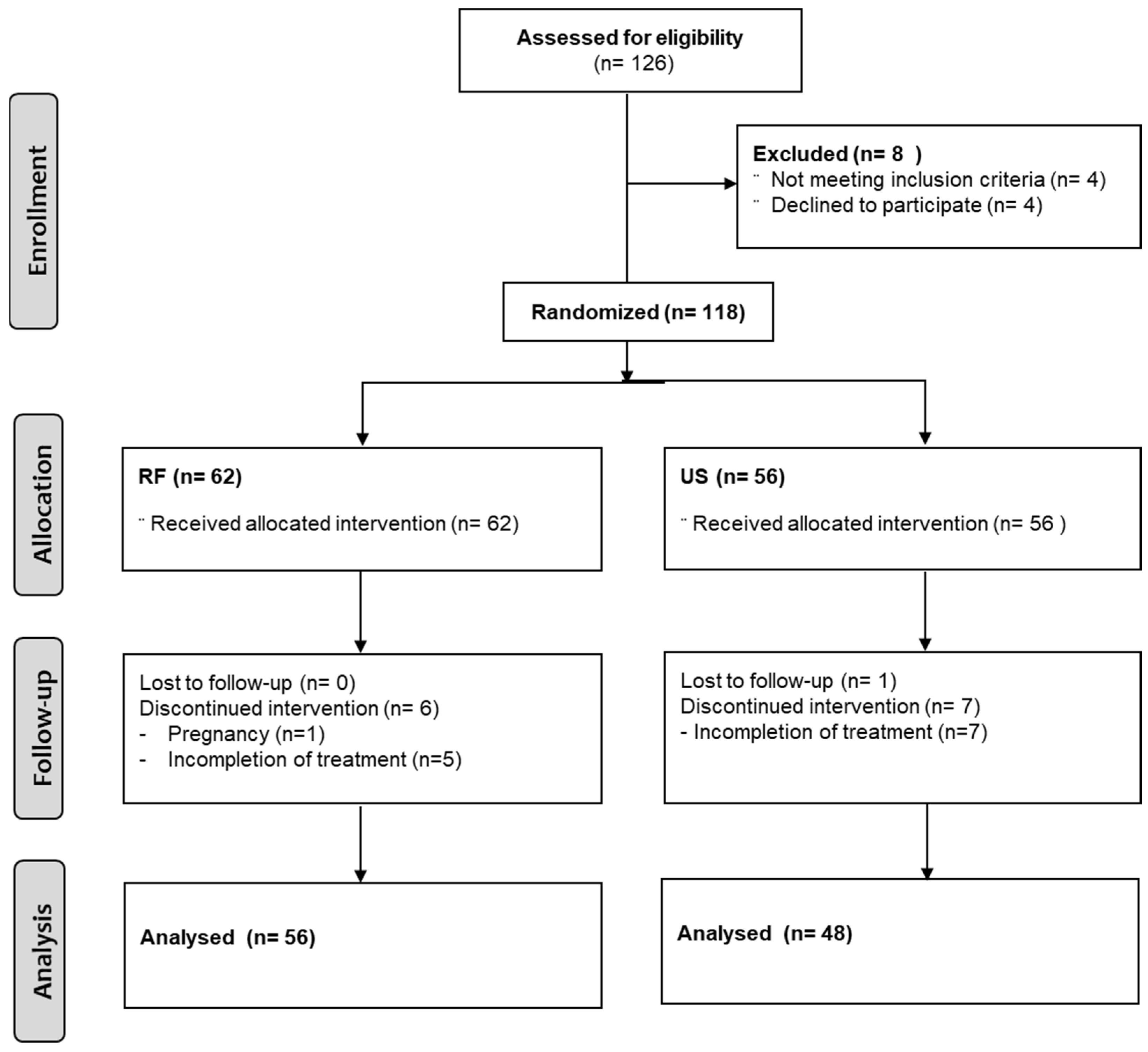
2.1. Study Design and Participants
2.2. Random Assignment and Blinding Technique
2.3. Intervention
2.4. Outcome Measures
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, A.Y.L.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.A.; Rasheed, A.; Aiyer, R.; Jung, B.; Bansal, N.; Chang, K.V.; Ottestad, E.; Gulati, A. Therapeutic Ultrasound for Pain Management in Chronic Low Back Pain and Chronic Neck Pain: A Systematic Review. Pain Med. 2020, 21, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Hashimoto, R.; Weimer, M.; Fu, R.; Dana, T.; Kraegel, P.; Griffin, J.; et al. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 493–505. [Google Scholar] [CrossRef]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Seco, J.; Kovacs, F.M.; Urrutia, G. The efficacy, safety, effectiveness, and cost-effectiveness of ultrasound and shock wave therapies for low back pain: A systematic review. Spine J. 2011, 11, 966–977. [Google Scholar] [CrossRef]
- van der Windt, D.; van der Heijden, G.; van den Berg, S.G.M.; Ter Riet, G.; de Winter, A.F.; Bouter, L.M. Ultrasound therapy for musculoskeletal disorders: A systematic review. Pain 1999, 81, 257–271. [Google Scholar] [CrossRef]
- Clijsen, R.; Stoop, R.; Hohenauer, E.; Aerenhouts, D.; Clarys, P.; Deflorin, C.; Taeymans, J. Local Heat Applications as a Treatment of Physical and Functional Parameters in Acute and Chronic Musculoskeletal Disorders or Pain. Arch. Phys. Med. Rehabil. 2022, 103, 505–522. [Google Scholar] [CrossRef]
- Ebadi, S.; Ansari, N.N.; Naghdi, S.; Jalaei, S.; Sadat, M.; Bagheri, H.; Vantulder, M.W.; Henschke, N.; Fallah, E. The effect of continuous ultrasound on chronic non-specific low back pain: A single blind placebo-controlled randomized trial. BMC Musculoskelet. Disord. 2012, 13, 192. [Google Scholar] [CrossRef]
- Durmus, D.; Durmaz, Y.; Canturk, F. Effects of therapeutic ultrasound and electrical stimulation program on pain, trunk muscle strength, disability, walking performance, quality of life, and depression in patients with low back pain: A randomized-controlled trial. Rheumatol. Int. 2010, 30, 901–910. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Adair, E.R.; Black, D.R. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics 2003, 24 (Suppl. S6), S17–S38. [Google Scholar] [CrossRef]
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and resistive electric transfer therapy in rehabilitation: A systematic review. Int. J. Rehabil. Res. 2020, 43, 291–298. [Google Scholar] [CrossRef]
- Valtonen, E.J.; Alaranta, H. Comparative clinical study of the effect of short-wave and long-wave diathermy on osteo-arthritis of the knee and hip. Scand. J. Rehabil. Med. 1971, 3, 109–112. [Google Scholar]
- Kumar, S.; Negi, M.P.; Sharma, V.P.; Shukla, R.; Dev, R.; Mishra, U.K. Efficacy of two multimodal treatments on physical strength of occupationally subgrouped male with low back pain. J. Back Musculoskelet. Rehabil. 2009, 22, 179–188. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.P.; Negi, M.P. Efficacy of dynamic muscular stabilization techniques (DMST) over conventional techniques in rehabilitation of chronic low back pain. J. Strength Cond. Res. 2009, 23, 2651–2659. [Google Scholar] [CrossRef]
- Zati, A.; Cavazzuti, L.; Colori, B.C.M.; Benedetti, M.G. Deep heating therapy via MF radiowaves versus superficial heating therapy in the treatment of nonspecific chronic low back pain: A double blind randomized trial. J. Back Musculoskelet. Rehabil. 2018, 31, 963–971. [Google Scholar] [CrossRef]
- Karasel, S.; Oncel, S.; Sonmez, I. The Effect of Short-Wave Diathermy and Exercise on Depressive Affect in Chronic Low Back Pain Patients. Med. Arch. 2021, 75, 216–220. [Google Scholar] [CrossRef]
- Durmus, D.; Ulus, Y.; Alayli, G.; Akyol, Y.; Bilgici, A.; Yazicioglu, K.; Kuru, O. Does microwave diathermy have an effect on clinical parameters in chronic low back pain? A randomized-controlled trial. J. Back Musculoskelet. Rehabil. 2014, 27, 435–443. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Cerón Fernández, E.; García Llorent, R.; Ribeiro González, M.; Delgado Martínez, A.D. Microwave diathermy for treating nonspecific chronic neck pain: A randomized controlled trial. Spine J. 2014, 14, 1712–1721. [Google Scholar] [CrossRef]
- Kim, K.A.; Moon, C.W.; Song, D.H.; Kim, S.J. Effect of Transcutaneous High Frequency Wave on the Change of Tissue Temperature and Histology in Sprague-Dawley Rat. Clin. Pain 2016, 15, 92–96. [Google Scholar]
- Song, D.H.; Kim, M.H.; Lee, Y.T.; Lee, J.H.; Kim, K.A.; Kim, S.J. Effect of high frequency electromagnetic wave stimulation on muscle injury in a rat model. Injury 2018, 49, 1032–1037. [Google Scholar] [CrossRef]
- Kim, G.W.; Won, Y.H.; Park, S.H.; Seo, J.H.; Kim, D.H.; Lee, H.N.; Ko, M.H. Effects of a Newly Developed Therapeutic Deep Heating Device Using High Frequency in Patients with Shoulder Pain and Disability: A Pilot Study. Pain Res. Manag. 2019, 2019, 8215371. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.H.; Lee, H.Y.; Lee, H.J.; Chang, S.B.; Chung, S.K.; Kim, H.J. Validation of the Korean version of the oswestry disability index. Spine 2005, 30, E123–E127. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952, discussion 2952. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.; Maher, C.G.; Refshauge, K.; Colaco, I. The reliability and validity of the Biering-Sorensen test in asymptomatic subjects and subjects reporting current or previous nonspecific low back pain. Spine 1999, 24, 2085–2089, discussion 2090. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; Behennah, J.; Fisher, J.; Osborne, N.; Steele, J. Associations between Trunk Extension Endurance and Isolated Lumbar Extension Strength in Both Asymptomatic Participants and Those with Chronic Low Back Pain. Healthcare 2016, 4, 70. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships with Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Cash, K.A.; Pampati, V.; Datta, S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: A randomized, equivalence controlled trial. Pain Physician 2009, 12, E355–E368. [Google Scholar] [CrossRef]
- Daltroy, L.H.; Cats-Baril, W.L.; Katz, J.N.; Fossel, A.H.; Liang, M.H. The North American spine society lumbar spine outcome assessment Instrument: Reliability and validity tests. Spine 1996, 21, 741–749. [Google Scholar] [CrossRef]
- Fiore, P.; Panza, F.; Cassatella, G.; Russo, A.; Frisardi, V.; Solfrizzi, V.; Ranieri, M.; Di Teo, L.; Santamato, A. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of low back pain: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2011, 47, 367–373. [Google Scholar]
- Grubisić, F.; Grazio, S.; Jajić, Z.; Nemcić, T. Therapeutic ultrasound in chronic low back pain treatment. Reumatizam 2006, 53, 18–21. [Google Scholar]
- Ebadi, S.; Henschke, N.; Nakhostin Ansari, N.; Fallah, E.; van Tulder, M.W. Therapeutic ultrasound for chronic low-back pain. Cochrane Database Syst. Rev. 2020, 7, Cd009169. [Google Scholar]
- Koes, B.W.; Bouter, L.M.; van Mameren, H.; Essers, A.H.; Verstegen, G.J.; Hofhuizen, D.M.; Houben, J.P.; Knipschild, P.G. A randomized clinical trial of manual therapy and physiotherapy for persistent back and neck complaints: Subgroup analysis and relationship between outcome measures. J. Manip. Physiol. Ther. 1993, 16, 211–219. [Google Scholar]
- Gibson, T.; Grahame, R.; Harkness, J.; Woo, P.; Blagrave, P.; Hills, R. Controlled comparison of short-wave diathermy treatment with osteopathic treatment in non-specific low back pain. Lancet 1985, 1, 1258–1261. [Google Scholar] [CrossRef]
- Sweetman, B.J.; Heinrich, I.; Anderson, J.A.D. A randomized controlled trial of exercises, short wave diathermy, and traction for low back pain, with evidence of diagnosis-related response to treatment. J. Orthop. Rheumatol. 1993, 6, 159–166. [Google Scholar]
- Kim, K.S.; Hernandez, D.; Lee, S.Y. Time-multiplexed two-channel capacitive radiofrequency hyperthermia with nanoparticle mediation. Biomed. Eng. Online 2015, 14, 95. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, Y.; Hasegawa, S.; Yokota, Y.; Nishiguchi, S.; Fukutani, N.; Shirooka, H.; Tasaka, S.; Matsushita, T.; Matsubara, K.; Nakayama, Y.; et al. Effect of Capacitive and Resistive electric transfer on haemoglobin saturation and tissue temperature. Int. J. Hyperth. 2017, 33, 696–702. [Google Scholar] [CrossRef]
- Lee, J.; Park, S. The relationship between physical capacity and fear avoidance beliefs in patients with chronic low back pain. J. Phys. Ther. Sci. 2017, 29, 1712–1714. [Google Scholar] [CrossRef]
- Hammer, S.M.; Alexander, A.M.; Didier, K.D.; Barstow, T.J. Influence of blood flow occlusion on muscular recruitment and fatigue during maximal-effort small muscle-mass exercise. J. Physiol. 2020, 598, 4293–4306. [Google Scholar] [CrossRef]

| RF | US | Total | p Value | ||
|---|---|---|---|---|---|
| n = 62 (%) | n = 56 (%) | n = 118 | |||
| Gender | Male | 13 (21.0) | 13 (23.2) | 26 (20.0) | 0.7339 |
| Female | 49 (79.0) | 43 (76.8) | 92 (78.0) | ||
| Age (years) | Mean ± SD | 46.5 ± 14.0 | 48.9 ± 12.5 | 47.7 ± 13.3 | 0.3038 |
| 19 to 39 | 22 (34.9) | 15 (23.8) | 37 (31.1) | 0.7738 | |
| 40 to 59 | 30 (47.6) | 31 (49.2) | 61 (51.3) | ||
| 60 to 70 | 11 (17.5) | 10 (15.9) | 21 (17.7) | ||
| Height (cm) | Mean ± SD | 162.9 ± 6.5 | 162.2 ± 7.7 | 162.6 ± 7.1 | 0.2837 |
| Weight (kg) | Mean ± SD | 59.7 ± 8.7 | 60.8 ± 8.2 | 60.2 ± 8.5 | 0.5382 |
| Occupation | Employed | 20 (31.7) | 22 (39.3) | 42 (35.3) | 0.4104 |
| Official | 18 (28.6) | 10 (17.8) | 28 (23.5) | ||
| Homemaker | 22 (34.9) | 23 (41.1) | 45 (37.8) | ||
| Other | 3 (4.8) | 1 (1.8) | 4 (3.4) |
| RF (n = 62) (95% CI) | US (n = 56) (95% CI) | p Value | |
|---|---|---|---|
| ODI (%) | 46.06 ± 13.94 (42.52–49.60) | 44.33 ± 14.68 (40.40–48.26) | 0.5123 |
| NRS | 6.21 ± 1.33 (5.87–6.55) | 5.84 ± 1.29 (5.49–6.18) | 0.1285 |
| Biering–Sorensen test (s) | 16.74 ± 18.17 (12.13–21.36) | 18.53 ± 15.31 (14.39–22.66) | 0.5696 |
| Up-and-go test (s) | 9.07 ± 2.38 (8.47–9.68) | 9.25 ± 2.70 (8.53–9.98) | 0.7024 |
| RF (n = 62) | US (n = 56) | p Value b | p Value c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± SD | Min | Max | 95% CI | p Value a | Mean | ± SD | Min | Max | 95% CI | p Value a | ||||
| ODI (%) | Baseline | 46.06 | 13.94 | 31.11 | 75.56 | 42.52–49.60 | 44.33 | 14.68 | 31.11 | 75.56 | 40.40–48.26 | 0.512 | |||
| At 4 weeks | 20.61 | 11.75 | 0.00 | 53.33 | 17.62–23.59 | <0.001 | 22.42 | 13.56 | 0.00 | 73.33 | 18.79–26.05 | <0.001 | 0.439 | ||
| At 12 weeks | 19.00 | 12.06 | 0.00 | 53.33 | 15.93–22.06 | <0.001 | 20.44 | 15.21 | 0.00 | 73.33 | 16.36–24.51 | <0.001 | 0.568 | 0.367 | |
| NRS | Baseline | 6.21 | 1.33 | 4.00 | 9.00 | 5.87–6.55 | 5.84 | 1.29 | 4.00 | 8.00 | 5.49–6.18 | 0.129 | |||
| At 4 weeks | 3.11 | 2.00 | 0.00 | 9.00 | 2.60–3.62 | <0.001 | 3.25 | 1.75 | 0.00 | 7.00 | 2.78–3.72 | <0.001 | 0.694 | ||
| At 12 weeks | 2.58 | 1.96 | 0.00 | 9.00 | 2.08–3.08 | <0.001 | 2.86 | 1.76 | 0.00 | 7.00 | 2.39–3.33 | <0.001 | 0.424 | 0.118 | |
| Biering–Sorensen test (s) | Baseline | 16.74 | 18.17 | 0.00 | 88.00 | 12.13–21.36 | 18.53 | 15.31 | 0.00 | 74.00 | 14.39–22.66 | 0.570 | |||
| At 4 weeks | 32.02 | 29.69 | 0.20 | 150.89 | 24.48–39.56 | <0.001 | 28.66 | 26.23 | 0.00 | 134.21 | 21.63–35.68 | 0.001 | 0.518 | ||
| At 12 weeks | 33.20 | 30.09 | 0.00 | 179.81 | 25.56–40.84 | <0.001 | 29.66 | 33.37 | 0.00 | 210.00 | 20.72–38.60 | 0.004 | 0.545 | 0.521 | |
| Up-and-go test (s) | Baseline | 9.07 | 2.38 | 5.60 | 17.00 | 8.47–9.68 | 9.25 | 2.70 | 4.12 | 18.00 | 8.53–9.98 | 0.702 | |||
| 4 weeks | 7.90 | 2.18 | 4.60 | 15.00 | 7.35–8.45 | <0.001 | 8.33 | 2.34 | 4.80 | 14.00 | 7.70–8.96 | <0.001 | 0.303 | ||
| 12 weeks | 8.15 | 2.40 | 5.06 | 17.00 | 7.54–8.76 | <0.001 | 8.43 | 2.42 | 4.60 | 16.00 | 7.78–9.08 | <0.001 | 0.528 | 0.669 | |
| RF (n = 62) | US (n = 56) | p Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| NASS at 4 weeks | |||
| 1 | 35 (56.45) | 28 (50.00) | |
| 2 | 20 (32.26) | 20 (35.71) | |
| 3 | 3 (4.84) | 4 (7.14) | |
| 4 | 0 (0.00) | 0 (0.00) | 0.783 |
| NASS at 12 weeks | |||
| 1 | 37 (59.68) | 30 (53.57) | |
| 2 | 17 (27.42) | 16 (28.57) | |
| 3 | 4 (6.45) | 6 (10.71) | |
| 4 | 0 (0.00) | 0 (0.00) | 0.658 |
| Pain medication administered | |||
| Yes | 4 (6.45) | 14 (25.00) | |
| No | 58 (93.55) | 42 (75.00) | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Do, J.G.; Park, H.J.; Lee, Y.-T.; Kim, S.J. A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial. J. Clin. Med. 2022, 11, 5011. https://doi.org/10.3390/jcm11175011
Lee JH, Do JG, Park HJ, Lee Y-T, Kim SJ. A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial. Journal of Clinical Medicine. 2022; 11(17):5011. https://doi.org/10.3390/jcm11175011
Chicago/Turabian StyleLee, Jung Hwan, Jong Geol Do, Hee Jin Park, Yong-Taek Lee, and Sang Jun Kim. 2022. "A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial" Journal of Clinical Medicine 11, no. 17: 5011. https://doi.org/10.3390/jcm11175011
APA StyleLee, J. H., Do, J. G., Park, H. J., Lee, Y.-T., & Kim, S. J. (2022). A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial. Journal of Clinical Medicine, 11(17), 5011. https://doi.org/10.3390/jcm11175011






