Abstract
Type 2 diabetes (T2D) is a complex disease for which an individualised treatment approach is recommended. Once-weekly (OW) semaglutide is a glucagon-like peptide-1 receptor agonist approved for the treatment of insufficiently controlled T2D. The aim of this study was to investigate the use of OW semaglutide in adults with T2D in a real-world context. SURE Spain, from the 10-country SURE programme, was a prospective, multicentre, open-label, observational study, approximately 30 weeks in duration. Adults with T2D and ≥1 documented HbA1c value ≤12 weeks before semaglutide initiation were enrolled. Change in HbA1c from baseline to end of study (EOS) was the primary endpoint, with change in body weight (BW), waist circumference, and patient-reported outcomes as secondary endpoints. Of the 227 patients initiating semaglutide, 196 (86.3%) completed the study on-treatment with semaglutide. The estimated mean changes in HbA1c and body weight between baseline and EOS were −1.3%-points (95% confidence interval (CI) −1.51;−1.18%-points) and −5.7 kg (95% CI −6.36;−4.98 kg). No new safety concerns were identified. Therefore, in routine clinical practice in Spain, OW semaglutide was shown to be associated with statistically significant and clinically relevant reductions in HbA1c and BW in adults with T2D.
1. Introduction
Type 2 diabetes (T2D) places a heavy burden on individuals and healthcare systems across the world. In Spain, an estimated 13.8% of people have T2D, and this is expected to increase in the future [1,2].
The management of T2D is complex. The American Diabetes Association (ADA) Standards of Medical Care in Diabetes 2022 [3] and the 2020 joint consensus statement of the ADA and the European Association for the Study of Diabetes (EASD) [4] recommend that physicians should take an individualised treatment approach when prescribing medications for T2D, and that they consider drug efficacy, risk of hypoglycaemia, cardiorenal benefits, effect on body weight (BW), adverse effects, pricing, and convenience for the patient [4].
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are an established class of antihyperglycaemic drugs used for the treatment of T2D, which have demonstrated improvements in glycaemic control and reductions in BW in patients with T2D [5,6]. In addition to their glucose-dependent function resulting in a low risk for hypoglycaemia [7], some GLP-1RAs (dulaglutide, liraglutide, and semaglutide) have demonstrated cardiovascular (CV) benefits in patients with T2D at high risk of CV disease [8,9,10]. Despite these benefits, access to GLP-1RAs is limited in Spain, and GLP-1RAs are only reimbursed for patients with obesity (body mass index [BMI] ≥ 30 kg/m2) and insufficient glycaemic control as a second-line therapy after metformin [11].
Semaglutide is a human GLP-1 analogue, approved as an add-on to diet and exercise for the treatment of adults with insufficiently controlled T2D, by the European Medicines Agency in February 2018 [12]. It has a long half-life, which makes it suitable for once-weekly (OW) dosing, [13] and is the only GLP-1RA that is available both in a OW subcutaneous (s.c.) injectable formulation and as an oral formulation administered once-daily [14].
The extensive SUSTAIN randomised clinical trial (RCT) programme, which investigated the efficacy and safety of OW s.c. semaglutide, demonstrated that 0.5 mg and 1.0 mg doses were associated with superior, clinically relevant improvements in glycaemic control and weight loss, compared with placebo or active comparators [8,15,16,17,18,19,20,21,22,23]. A safety profile similar to other GLP-1RAs was also observed.
SURE Spain is part of the SURE real-world study programme, which aimed to explore the use of OW semaglutide in a diverse population of adults with T2D in routine, real-world clinical practice across 10 countries (Canada, Denmark/Sweden, France, Germany, Italy, the Netherlands, Spain, Switzerland, and the United Kingdom) and to complement the results of the SUSTAIN RCTs. Unlike RCTs, the SURE studies are non-interventional and observational, allowing the assessment of patient outcomes, as well as product use and performance, in diverse patient populations in routine clinical practice [24].
The aim of this study was to evaluate the real-world use of OW semaglutide in a diverse T2D patient population in Spain.
2. Materials and Methods
2.1. Study Design
SURE Spain was a multicentre, prospective open-label, single-arm, non-interventional study assessing the use of OW semaglutide in adult patients with T2D in routine clinical practice in Spain. Informed consent and treatment initiation took place on the first visit (week 0), followed by an anticipated exposure period of ~30 weeks (range: 28–38 weeks). Intermediate visits scheduled according to local practice and data collection were performed throughout the entire study.
The decision to initiate semaglutide treatment was at the discretion of the treating physician, following requirements stated in the Summary of Product Characteristics (SmPC), therapeutic positioning report and local/regional guidelines, and clearly separated from the decision to include the patient in the SURE Spain study. All parameters collected in the study (except the patient-reported outcomes) were part of routine clinical practice. Patients were treated OW with commercially available s.c. semaglutide (Ozempic®; Novo Nordisk A/S, Bagsværd, Denmark), available in a pre-filled, multidose, pen injector. The treating physician determined the maintenance dose and any subsequent changes to it. Diet and physical activity counselling could be offered in line with routine clinical practice, with modifications to prescribed antihyperglycaemic treatment at the physician’s discretion.
This study was conducted in accordance with the Declaration of Helsinki [25], the Guidelines for Pharmacovigilance Practices Module VI [26], and Good Pharmacoepidemiology Practices [27]. Prior to study initiation, the protocol, protocol amendment, patient information/informed consent form, together with any other written information to be provided to the patient and patient enrolment procedures, were reviewed and approved by the independent ethics committee/institutional review board at each study site (first approved in 2019 by the Ethics Committee of CEIm de EUSkadi, project identifier: NN9535-4368). Written informed consent was obtained from all patients prior to any study-related activities. This study is registered on ClinicalTrials.gov (NCT04067999).
2.2. Study Population
Adult patients (age ≥ 18 years) diagnosed with T2D were included from 34 sites in Spain, with the first participant’s first visit on 5 August 2019, and the last participant’s last visit on 19 July 2021. Inclusion criteria included diagnosis of T2D and availability of one or more documented values of HbA1c within 12 weeks prior to semaglutide treatment initiation. Exclusion criteria included previous participation in a SURE study, mental incapacity, unwillingness, or language barriers precluding adequate understanding or cooperation, prior treatment with any investigational drug (90 days before enrolment), and hypersensitivity to semaglutide or any of the excipients. The study duration of 30 weeks was considered sufficient to initiate and optimise the study treatment regimen and to obtain real-world data for the evaluation of the primary endpoint.
2.3. Endpoints
The primary endpoint was a change from baseline to end of study (EOS) in HbA1c (%-point and mmol/mol). Supportive secondary endpoints included: change from baseline to EOS in BW (kg and %) and waist circumference (cm); proportion of patients achieving HbA1c < 8.0% (64 mmol/mol), <7.5% (59 mmol/mol) and <7.0% (53 mmol/mol) [28]; reduction in HbA1c from baseline to EOS of ≥1.0%-point; weight reduction from baseline to EOS of ≥3.0% [29] and ≥5.0%; HbA1c reduction from baseline to EOS of ≥1.0% and weight reduction from baseline to EOS of ≥3.0% [29]; patient-reported severe or documented hypoglycaemia between baseline and EOS; and change from baseline to EOS in scores for patient-reported outcomes of: the Diabetes Treatment Satisfaction Questionnaire–status (DTSQs; absolute treatment satisfaction) comprising eight questions, of which six questions are combined into a total Treatment Satisfaction score (scale: 0 to 36); the Diabetes Treatment Satisfaction Questionnaire–change (DTSQc; relative treatment satisfaction), total treatment satisfaction (scale: −18.0 to 18.0); and the 36-item Short-Form Health Survey version 2 (SF-36®v2), physical and mental summary component. The proportion of patients who completed the study under treatment with semaglutide was also investigated.
Exploratory assessments included: weekly dose of semaglutide at EOS; proportion of patients who had not added new antihyperglycaemic drug(s) to semaglutide treatment at any time during the study, evaluated at EOS; proportion of patients who had achieved clinical success, in relation to the reason to initiate semaglutide treatment, as assessed by the physician at EOS; patient-reported 8-Item Morisky Medication Adherence Scale (MMAS-8) score at EOS (low, medium, high) [30,31,32]; and the number of severe or documented hypoglycaemic episodes. Post hoc assessments included change from baseline to EOS in BMI (kg/m2). Permission for use of the MMAS-8 was granted prior to the study.
2.4. Safety
Only information on serious adverse drug reactions (SADRs), fatal events, pregnancies in female patients, and adverse events (AEs) in foetuses or newborns were systematically collected during the study. Voluntary reporting of other safety information by the physician followed the same process as for the systematic safety reporting. All episodes of patient-reported documented and/or severe hypoglycaemia were to be recorded.
2.5. Statistical Analyses
Power calculations showed that a sample size of 130 patients was required, based on the criterion of 90% probability of obtaining a 95% confidence interval (CI) for mean change from baseline in HbA1c whose half-width was at most 0.30. The half-width of 0.30 was chosen as a reasonable uncertainty allowing for a robust evaluation of glycaemic efficacy, in line with diabetes guidelines [33]. To ensure sufficient statistical power to evaluate the efficacy of semaglutide on glycaemic control (on the basis of evidence from previous observational studies with GLP-1RA treatment), it was necessary to include at least 217 enrolled patients initiating semaglutide, to ensure that 130 patients completed the study on-treatment [34,35].
The Full Analysis Set (FAS), which included all patients in the study who initiated semaglutide treatment, was used for characterising baseline demographics, analysis of the secondary endpoint related to study completion on-treatment, the selected exploratory assessments, description of AEs, and the sensitivity analyses of the primary and secondary endpoints.
The Effectiveness Analysis Set (EAS) included all patients in the FAS who completed the study and were receiving semaglutide treatment at EOS. The EAS was used for characterising baseline demographics at EOS, the description of antihyperglycaemic medications at baseline and EOS, and the primary, secondary and exploratory endpoint analyses.
Baseline demographic data are summarised using descriptive statistics (mean ± standard deviation [SD] or median and interquartile range for continuous variables and number and proportion for categorical variables). Change in the continuous variables of the primary and secondary endpoints from baseline to EOS were analysed using the Analysis of Covariance (ANCOVA) model. Categorical endpoints were analysed using descriptive statistics.
Sensitivity analyses investigated the robustness of the conclusions from the main analyses and explored the impact of missing data in the primary analysis, for which patients were excluded if they did not complete the study or discontinued treatment, or if HbA1c data were missing at EOS. The prespecified in-study sensitivity analysis of the primary endpoint included all patients in the FAS with at least one post-baseline HbA1c measurement in the in-study period. For this analysis, the primary endpoint was analysed using a Mixed Model for Repeated Measures (MMRM) including all HbA1c assessments in the in-study period. The on-treatment sensitivity analysis included patients in the FAS with at least one post-baseline HbA1c assessment, but it only included HbA1c assessments in the on-treatment period and used the same statistical approach as the in-study sensitivity analysis.
Because of the COVID-19 pandemic, the EOS visit (V6) window was extended beyond 38 weeks to allow participants to complete their EOS assessments. Consequently, an additional post hoc sensitivity analysis was performed to explore the impact of extending the EOS visit (V6) window on the primary endpoint. The sensitivity analysis of the primary endpoint was the same as the primary analysis of the primary endpoint but included only those patients who had an EOS visit between weeks 28 and 38 (the original visit window). An additional post hoc sensitivity analysis was performed to explore the impact of extending the EOS visit on the secondary endpoint of change from baseline to EOS in BW. This sensitivity analysis was the same as the main analysis of this endpoint but included only those patients who had an EOS visit between weeks 28 and 38.
3. Results
3.1. Patient Population and Baseline Characteristics
Of the 228 patients who signed the consent form, one did not meet the eligibility criteria. Therefore, the FAS comprised the 227 patients who were enrolled in the study and who had initiated semaglutide treatment (Figure 1). A total of 210 patients (92.5%) completed the study, and the mean treatment duration was 33.7 weeks. The reasons for non-completion were: death (n = 1; 0.4%), lost to follow-up (n = 3; 1.3%), withdrawal by patient (n = 3; 1.3%), and missed EOS visit within the visit window (n = 10; 4.4%) (Figure 1). The EAS comprised 196 patients (86.3%) who had completed the study on semaglutide treatment (Figure 1). Twelve patients (5.2% of the FAS) had an unknown treatment status at EOS. With regard to discontinuations, 16 patients (7.0%) discontinued treatment due to unacceptable gastrointestinal (GI) intolerability, and a further three patients (1.3%) had ‘other’ recorded as the reason (Figure 1).
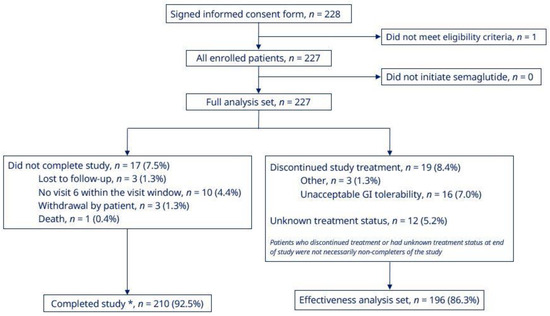
Figure 1.
Patient disposition. * Patients who initiated semaglutide treatment and attended the end of study visit. GI, gastrointestinal.
Baseline characteristics of patients are summarised in Table 1. Hypertension and dyslipidaemia were the most frequent CV comorbidities at baseline, affecting 75.8% and 76.2% of patients, respectively.

Table 1.
Baseline characteristics of patients (FAS).
Most patients initiated semaglutide at a dose of 0.25 mg (83.3%); 13.7% initiated at 0.5 mg and 3.1% at 1.0 mg. The most common reasons for initiating semaglutide as part of T2D treatment were weight reduction (94.3%) and to improve glycaemic control (88.5%).
The most frequent antihyperglycaemic drugs used by patients in the EAS at baseline were metformin (75.5% of patients), sodium–glucose cotransporter-2 inhibitors (SGLT-2is) (42.3%), basal insulin (32.7%), and dipeptidyl peptidase-4 inhibitors (DPP-4is) (20.4%) (Supplementary Table S1).
3.2. HbA1c, BW, BMI, and Waist Circumference Outcomes
For patients in the EAS receiving semaglutide, statistically significant reductions were observed at EOS for mean HbA1c, BW, waist circumference and BMI (Table 2). The mean HbA1c at EOS was 7.1%, and the estimated mean change from baseline was −1.3%-points [95% CI −1.51;−1.18%-points; p < 0.0001] (Table 2, Supplementary Figure S1); mean BW at EOS was 93.2 kg, and the estimated mean change from baseline was −5.7 kg [95% CI −6.36; −4.98 kg; p < 0.0001] (Table 2, Supplementary Figure S1); mean BMI at EOS was 34.4 kg/m2, and the estimated mean change from baseline was −2.1 kg/m2 [95% CI −2.37; −1.86 kg/m2; p < 0.0001]; and mean waist circumference at EOS was 113.4 cm, and the estimated mean change from baseline to EOS was −5.3 cm [95% CI −6.29; −4.41 cm; p < 0.0001] (Table 2, Supplementary Figure S1).

Table 2.
Change from baseline to EOS in HbA1c, body weight, waist circumference, and BMI (EAS).
At EOS, 81.0%, 67.7% and 54.0% of patients in the EAS had an HbA1c of < 8.0%, <7.5% and <7.0%, respectively (Figure 2). The proportion of patients achieving an HbA1c reduction ≥1%-point was 56.6% and the proportions achieving weight reduction of ≥ 3.0% and ≥5.0% were, respectively, 69.2% and 49.7% (Figure 2). The proportion of patients in the EAS achieving the composite endpoint of an HbA1c reduction of ≥ 1.0% and weight reduction ≥3.0% at EOS was 44.3% (Figure 2). In the FAS, 86.3% of patients completed the study on-treatment with semaglutide (Figure 1).
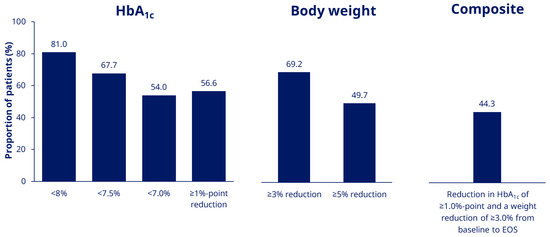
Figure 2.
Proportion of patients achieving HbA1c targets and weight-loss goals (EAS). EAS, Effectiveness Analysis Set; EOS, end of study.
3.3. Sensitivity Analyses
Prespecified sensitivity analyses were used to explore the impact of missing data in the main analysis. The on-treatment sensitivity analysis of the FAS showed that the mean HbA1c decreased over time from initiation of semaglutide to week 30, with an estimated change of −1.4%-points [95% CI −1.59; −1.27%-points] (Supplementary Figure S2). The estimated mean changes from baseline to EOS and associated 95% CIs were similar across sensitivity analyses and showed that the mean changes in HbA1c were statistically significantly different from having no mean change in HbA1c (Supplementary Table S2, Supplementary Figure S2). Moreover, the estimated mean HbA1c and estimated change in HbA1c were similar over the course of the study for both the in-study and on-treatment period.
Additional post hoc sensitivity analyses were performed in patients who had their EOS visit within the original visit window (week 28–38). The post hoc analyses of the mean changes from baseline to EOS for HbA1c and for BW showed similar results to those seen in the primary analysis (Supplementary Table S3).
Collectively, the sensitivity analyses supported the conclusions from the primary analysis, which included assessments for patients who were on-treatment at the EOS visit (V6), also including those completing the study after week 38.
3.4. Semaglutide Dose
The mean ± SD weekly dose of semaglutide at EOS was 0.85 ± 0.24 mg. At EOS, five (2.6%) patients were receiving 0.25 mg OW semaglutide, 50 (25.5%) were receiving 0.5 mg, two (1.0%) were receiving between >0.5 mg and <1.0 mg, and 139 (70.9%) were receiving 1.0 mg.
3.5. Patient-Reported Outcomes
In patients receiving semaglutide at EOS, DTSQs score increased by 4.4 [95% CI 3.66; 5.07; p < 0.0001] from baseline to EOS, representing a significant increase in absolute treatment satisfaction (Figure 3). Patients receiving semaglutide also reported a DTSQc score at EOS of 13.1 (95% CI 12.36; 13.85) out of a maximum score of 18, indicating a significant relative improvement in treatment satisfaction (p < 0.0001) (Figure 3).
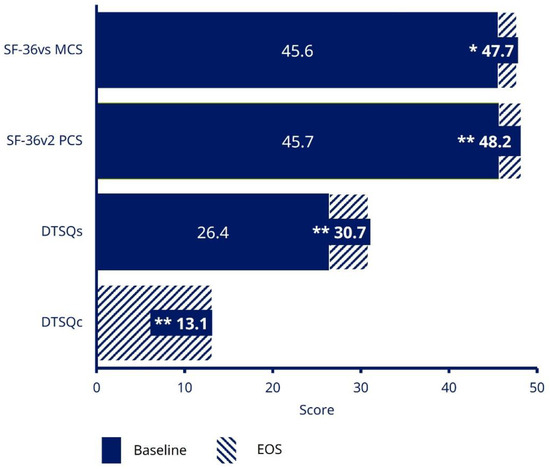
Figure 3.
Treatment satisfaction and HRQoL (EAS). * p = 0.0013; ** p < 0.0001. Data are based on EAS. DTSQ status version (DTSQs) was measured at the informed consent and initiation visit, and the EOS visit; with responses ranging from 0 (very dissatisfied) to 6 (very satisfied) for each item of the questionnaire. The maximum total score is 36. The SF-36®v2 questionnaire has 36 questions grouped into eight domains, which can be combined into two summary component scores (overall mental and physical health); a higher SF-36®v2 score indicates lower disability. DTSQ, Diabetes Treatment Satisfaction Questionnaire; DTSQc, DTSQ change version; DTSQs, DTSQ status version; EAS, Effectiveness Analysis Set; EOS, end of study; HRQoL, health-related quality of life; MCS, mental component summary; PCS, physical component summary; SF-36®v2, 36-Item Short-Form Health Survey version 2.
At EOS, significant increases were observed in both the SF-36®v2 health-related quality-of life (HRQoL) questionnaire physical component score (p < 0.0001) and the mental component score (p = 0.0013), indicating an improvement in quality of life from baseline to EOS (Figure 3).
Mean MMAS-8 score was 7.0 at baseline and 7.4 at EOS, indicating a medium level of treatment adherence. The proportion of participants with medium and high adherence was, respectively, 49.5% and 36.6% at baseline and 34.1% and 57.3% at EOS.
3.6. Adverse Events and Hypoglycaemia
AEs and severe or documented hypoglycaemic episodes in patients receiving semaglutide are summarised in Table 3. In the FAS, 15 patients (6.6%) reported 26 treatment-emergent AEs: 88.5% of AEs were non-serious (13 [5.7%] patients; 23 events) and 46.2% were moderate in intensity (7 [3.1%] patients; 12 events). A total of 13 patients reported 22 GI AEs, which accounted for the highest number of AEs by system organ class. Three serious AEs (SAEs) were reported (Medical Dictionary for Regulatory Activities preferred terms: atrial fibrillation, left ventricular failure, and myocardial infarction) by two patients (0.9%), which were all judged as unlikely to be related to semaglutide treatment by the investigators. One severe SAE (preferred term: myocardial infarction) was reported, which had a fatal outcome.

Table 3.
AEs and severe or documented hypoglycaemic episodes in patients receiving semaglutide (FAS).
Eight patients (4.1%) in the EAS reported severe or documented hypoglycaemia episodes between baseline and EOS, with similar results in the FAS (12 patients; 5.3%). At EOS, 20 severe or documented hypoglycaemic episodes were reported in the EAS, and 26 were reported in the FAS. Of these 26 events, 22 were reported by patients while using insulin and 3 occurred while using sulphonylureas. The date of one hypoglycaemic episode was unrecorded, which prevented an assessment of concurrent medication use. No severe hypoglycaemic episodes were reported during the study.
4. Discussion
The SURE Spain study is part of the SURE study programme, which consists of nine observational studies in ten countries and was conducted to assess the real-world use of OW semaglutide.
The data reported indicate that when OW s.c. semaglutide was taken according to local clinical practice by adult patients with T2D in Spain, a clinically relevant and statistically significant reduction, compared with baseline, was observed for HbA1c at EOS (p < 0.0001) [36]. This was observed despite 14.5% of the study population switching from another GLP-1RA to semaglutide at baseline. While previous treatment may be expected to influence outcomes, improvements have been reported in patients treated with semaglutide who were not naïve to GLP-1RAs [36].
At EOS, patients also experienced statistically significant decreases from baseline in BW and waist circumference. A total of 97 (49.7%) patients achieved a weight reduction from baseline of ≥5%. This weight reduction is a key consideration in terms of reducing CV risk, in view of the beneficial reductions in triglycerides, total cholesterol, and low-density lipoprotein cholesterol that are associated with a weight loss of 5–10% [37].
Furthermore, patients reported substantial improvements in treatment satisfaction and HRQoL, as measured by the DTSQ and the SF-36®v2, respectively. In addition, patients’ adherence to OW semaglutide treatment was good, with 91.4% of patients reporting either high (57.3%) or medium (34.1%) adherence at EOS. Adherence to OW semaglutide in the SURE Spain study compares favourably to the adherence rates of 39.1–64.5% at 1 year reported for GLP-1RAs (including semaglutide) in retrospective, real-world cohort studies [37,38].
Additionally, the patient population in this study had advanced T2D, as indicated by the mean disease duration of 11.8 years from diagnosis and the complex pharmacological treatment at baseline, with 41.9% of patients taking more than two antihyperglycaemic medications and 47.5% taking insulin. These factors are associated with poorer treatment outcomes and make it more difficult to achieve treatment goals.
Drawing comparisons between GLP-1RA RCTs and real-world evidence studies from different countries can be challenging. Local T2D clinical guidelines vary and can restrict clinical access to GLP-1RAs, while local reimbursement policies may impose further barriers to patient access. In Spain, outside of private practice, GLP-1RAs are only reimbursed for patients with a BMI ≥ 30 kg/m2. This is in contrast with Denmark, where the recommendation is independent of BMI, and the UK, where use is recommended in those with a BMI ≥ 35 kg/m2 who show an adequate metabolic response.
Overall, the results of this study support previously reported data on the real-world use of OW semaglutide in Spain [39,40,41]. The reduction in HbA1c and BW observed in Spain align with those observed in the countries that have published results from the SURE programme to date—Canada, Denmark/Sweden, Switzerland, and the UK—for which the mean change in HbA1c from baseline to EOS was between −0.8 and −1.5%-points and the mean change in BW from baseline to EOS was between −4.3 and −5.8 kg [42,43,44,45]. The results are also aligned with real-world evidence from other countries, for example, a study by Marzullo et al., that showed reductions in HbA1c and body weight after 6 and 12 months of OW semaglutide treatment in people with T2D in Italy [46].
Metabolic control in patients with T2D is assessed using multiple factors (e.g., BW, waist circumference), and not only HbA1c. In SURE Spain, the majority (70.9%) of patients were receiving the recommended dose of 1.0 mg OW of semaglutide by EOS, and the significant improvements in primary and secondary endpoints in the study may indicate that this dose is appropriate for the goal of achieving global metabolic control. The safety findings of the real-world T2D population in Spain were also consistent with the safety profile of semaglutide established in the SUSTAIN programme and with that of the GLP-1RA class, with no unexpected safety issues reported.
Study Limitations
The SURE Spain study was non-interventional and single-armed, so the potential impact of other predictive factors cannot be excluded. The fundamental limitation of such a study design is the absence of a randomised comparator, which would otherwise have enabled differentiation of the changes caused by treatment, and the impact of other factors. Data in this study were collected during routine clinical practice, rather than through mandated examinations at predetermined time points, which may have impacted the robustness and completeness of the dataset.
The primary analysis was based on data from patients who completed the study on semaglutide treatment and with their HbA1c levels recorded at EOS. This could have resulted in selection bias, because patients who benefit from the study treatment are more likely to continue than those who do not. To account for this, sensitivity analyses of the primary endpoint included all post-baseline HbA1c assessments as well as evaluations from intermediate visits also including patients who did not complete the study or discontinued semaglutide during the study. In addition, secondary supportive analyses assessed the percentage of patients who had started semaglutide treatment and were receiving it at the EOS.
The inclusion criteria were purposely designed to be broad and reflect a real-world T2D population, which is rarely the case in a standard RCT. However, it is likely that physicians who were highly motivated would have been overrepresented among the participating centres, and that the centres included either highly motivated patients or patients who were difficult to treat with the other therapies available. As a result, the enrolled group may only represent subsets of individuals who are eligible for semaglutide therapy. Nevertheless, study participants were profiled in terms of demographics and clinical data, which allowed for the assessment of the representativeness of the recruited population. Details of medical history (including T2D diagnosis) and concurrent diseases were obtained without further confirmation as provided by the investigators.
A potential limitation of SURE Spain is the study’s geographical location and time of initiation. The study was conducted soon after the launch of OW semaglutide, in a real-world setting, in a diverse T2D population recruited by investigators at 34 sites in Spain. However, the 34 sites that enrolled patients account for approximately half of the communities/regions within Spain, so may not be representative of the entire population. Furthermore, in Spain, GLP-1RAs are only reimbursed for patients who have a BMI ≥ 30 kg/m2, and only approximately 8% of Spanish patients with T2D are prescribed GLP-1RAs [11,47]. Therefore, none of the patients enrolled in the study had a ‘normal’ BMI (≥18.5–<25 kg/m2). These country-specific factors may have influenced the study results; in the future, however, semaglutide will likely be prescribed to a broader range of patients with T2D, including those with less severe disease progression.
The COVID-19 pandemic impacted intermediate and EOS visits in SURE Spain. Because of accessibility issues, several of these visits were instead conducted by telephone, rather than in-person. To further mitigate the challenges raised by the pandemic, changes were made to the study design that allowed patients to postpone their last visit (after the 38-week timepoint). An additional post hoc sensitivity analysis was performed to assess how the primary and secondary endpoints were affected by extending the EOS visit window. Extending the EOS visit window had no impact on the study outcomes.
Evidence has been reported that patients with T2D in Spain may have gained weight during the COVID-19 lockdown, due to their substantial lifestyle changes [48]. Sánchez et al. noted that if another lockdown were to be imposed, there should be greater emphasis on avoiding weight gain, in which case GLP-1RAs might be an effective therapy for these patients. Despite the influence of COVID-19, the data from this study are regarded as robust, and are suitable for further interpretation.
5. Conclusions
In SURE Spain, patients treated with OW semaglutide experienced statistically significant and clinically relevant reductions from baseline to EOS in HbA1c, BW, and waist circumference, and improvements in other clinical parameters such as treatment satisfaction and HRQoL in a real-world setting. These findings were significant, despite the nature of the population (advanced T2D) included in the SURE Spain study and the local limitations on prescribing GLP-1RAs. The reported AEs were consistent with the known safety profile of semaglutide, with no new safety concerns reported. These results support the use of OW semaglutide in routine clinical practice in adults with T2D in Spain.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11174938/s1.
Author Contributions
Conceptualisation, V.B., C.A.P., A.-M.C., A.C., S.B.P. and E.D.; investigation, V.B., C.A.P., A.-M.C., A.C., S.B.P. and E.D.; writing—review and editing, V.B., C.A.P., A.-M.C., A.C., S.B.P. and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Novo Nordisk A/S.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and first approved in 2019 by the Ethics Committee of CEIm de EUSkadi, project identifier: NN9535-4368.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This study was funded by Novo Nordisk A/S. We thank all the participants, investigators, and study-site staff, as well as Kamal Kant Mangla and Sanskruti Jayesh Patel from Novo Nordisk for their review and input to the manuscript, and Julia Peics (AXON Communications) for medical writing and editorial assistance (funded by Novo Nordisk A/S). Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, MMAS Research LLC 14725 NE 20th St. Bellevue WA 98007 or from dmorisky@gmail.com.
Conflicts of Interest
V.B. has received unrestricted research support from Abbot, Novo Nordisk, and Sanofi, and has received speaker/advisory honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, Janssen, Merck, Mundipharma, Novartis, Novo Nordisk, Roche, and Sanofi. E.D. has received unrestricted research support from AstraZeneca, Novo Nordisk, Pfizer, Roche, and Sanofi, and has received consulting fees and/or honoraria for membership on advisory boards and speaker’s bureau from Abbott Laboratories, Almirall, AstraZeneca, Esteve, GlaxoSmithKline, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi.
References
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation Diabetes Atlas 2021, Tenth Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 3 March 2022).
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes–2020. Diabetes Care 2020, 43 (Suppl. 1), S89–S97. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision Medicine in Diabetes: A consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschweitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Fransen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshamanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Reyes-García, R.; Moreno-Pérez, Ó.; Tejera-Pérez, C.; Fernández-García, D.; Bellido-Castañeda, V.; de la Torre Casares, M.L.; Rozas-Moreno, P.; Fernández-García, J.C.; Martínez, A.M.; Escalada-San Martín, J.; et al. Document on a comprehensive approach to type 2 diabetes mellitus. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2019, 66, 443–458. [Google Scholar] [CrossRef]
- Novo Nordisk. Ozempic® (Semaglutide) Summary of Product Characteristics. 2018. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004174/WC500244163.pdf (accessed on 23 March 2022).
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Pioneering oral peptide therapy for patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 500–502. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-week, open-label, randomized clinical trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Masmiquel, L.; Kumar, H.; Sargin, M.; Karsbøl, J.D.; Jacobsen, S.H.; Chow, F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017, 5, 341–354. [Google Scholar] [CrossRef]
- Capehorn, M.S.; Catarig, A.-M.; Furberg, J.K.; Janez, A.; Price, H.C.; Tadayon, S.; Vergès, B.; Marre, M. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020, 46, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Bain, S.C.; Cariou, B.; Piletič, M.; Rose, L.; Axelsen, M.; Rowe, E.; DeVries, J.H. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): A randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 355–366. [Google Scholar] [PubMed]
- Lingvay, I.; Catarig, A.-M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. SUSTAIN 7 investigators; Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Lingvay, I.; Reed, J.; de la Rosa, R.; Rose, L.; Sugimoto, D.; Araki, E.; Chu, P.-L.; Wijayasinghe, N.; Norwood, P. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): A randomized, controlled trial. J. Clin. Endocrinol. Metab. 2018, 103, 2291–2301. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in subjects with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef]
- Zinman, B.; Bhosekar, V.; Bush, R.; Holst, I.; Ludvik, B.; Thielke, D.; Thrasher, J.; Woo, V.; Philis-Tsimikas, A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): A randomised, placebo-controlled trial. 2019. Lancet Diabetes Endocrinol. 2019, 7, 356–367. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, Guideline on Good Pharmacovigilance Practices (GVP)-Module VI. 2017. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 8 March 2022).
- International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol. Drug Saf. 2016, 25, 2–10. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S55–S64. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. Type 2 Diabetes in Adults: Management [NG28]. 2015. Available online: http://www.nice.org.uk/guidance/ng28 (accessed on 8 March 2022).
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef]
- Krousel-Wood, M.; Islam, T.; Webber, L.S.; Re, R.N.; Morisky, D.E.; Muntner, P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am. J. Manag. Care 2009, 15, 59–66. [Google Scholar]
- Morisky, D.E.; DiMatteo, M.R. Improving the measurement of self-reported medication nonadherence: Response to authors. J. Clin. Epidemiol. 2011, 64, 255–257. [Google Scholar] [CrossRef]
- Mody, R.; Grabner, M.; Yu, M.; Turner, R.; Kwan, A.Y.M.; York, W.; Landó, L.F. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr. Med. Res. Opin. 2018, 34, 995–1003. [Google Scholar] [CrossRef]
- Wilke, T.; Mueller, S.; Groth, A.; Berg, B.; Fuchs, A.; Sikirica, M.; Logie, J.; Martin, A.; Maywald, U. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: A retrospective analysis. Diabetes Ther. 2016, 7, 105–124. [Google Scholar] [CrossRef]
- Divino, V.; DeKoven, M.; Hallinan, S.; Varo, N.; Wirta, S.B.; Lee, W.C.; Reaney, M. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014, 5, 499–520. [Google Scholar] [CrossRef] [PubMed]
- Di Loreto, C.; Minarelli, V.; Nasini, G.; Norgiolini, R.; Del Sindaco, P. Effectiveness in real world of once weekly semaglutide in people with type 2 diabetes: Glucagon-like peptide receptor agonist naïve or switchers from other glucagon-like peptide receptor agonists: Results from a retrospective observational study in Umbria. Diabetes Ther. 2022, 13, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Yang, L.; Carr, R.D.; Pal, S.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res. Care 2022, 10, e002517. [Google Scholar] [CrossRef] [PubMed]
- Uzoigwe, C.; Liang, Y.; Whitmire, S.; Paprocki, Y. Semaglutide once-weekly persistence and adherence versus other GLP-1RAs in patients with type 2 diabetes in a US real-world setting. Diabetes Ther. 2021, 12, 1475–1489. [Google Scholar] [CrossRef]
- Ferrer-García, J.C.; Galera, R.A.; Arribas Sr., L.; Torrens, M.T.; Lorente, A.S.; Portilla, A.J.; Artero, A.; Sánchez-Juan, C. Receptor agonist in type 2 diabetes: A study to evaluate real-world effectiveness. In Proceedings of the American Diabetes Association 80th Scientific Sessions, Chicago, IL, USA, 12–16 June 2020. Poster number 947-P. [Google Scholar]
- Cárdenas-Salas, J.J.; Sierra, R.; Luca, B.L.; Sánchez, B.; Modroño, N.; Casado, C.; Sánchez, N.M.; Cruces, E.; Vázquez, C. Semaglutide in patients with type 2 diabetes: Real-world data from Spain. In Proceedings of the American Diabetes Association 81st Scientific Sessions, Washington, DC, USA, 25–29 June 2021. Poster number 690-P. [Google Scholar]
- Garcia De Lucas, M.D.; Avilés Bueno, B.; Pérez Belmonte, L.M.; Jiménez Millán, A.B.; Rivas Ruiz, F. Semaglutide Achieves Better Metabolic and Weight Control than Other GLP-1 RA in Real Life after 12 Months of Follow-up. In Proceedings of the American Diabetes Association 81st Scientific Sessions, Washington, DC, USA, 25–29 June 2021. Poster number 676-P. [Google Scholar]
- Holmes, P.; Bell, H.E.; Bozkurt, K.; Catarig, A.-M.; Clark, A.; Machell, A.; Sathyapalan, T. Real-world use of once-weekly semaglutide in type 2 diabetes: Results from the SURE UK multicentre, prospective, observational study. Diabetes Ther. 2021, 12, 2891–2905. [Google Scholar] [CrossRef]
- Ekberg, N.R.; Bodholt, U.; Catarig, A.-M.; Catrina, S.-B.; Grau, K.; Holmberg, C.N.; Klanger, B.; Knudsen, S.T. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Results from the SURE Denmark/Sweden multicentre, prospective, observational study. Prim. Care Diabetes 2021, 15, 871–878. [Google Scholar] [CrossRef]
- Yale, J.F.; Catarig, A.-M.; Grau, K.; Harris, S.; Klimek-Abercrombie, A.; Rabasa-Lhoret, R.; Reardon, L.; Woo, V.; Liutkus, J. Use of once-weekly semaglutide in patients with type 2 diabetes in routine clinical practice: Results from the SURE Canada multicentre, prospective, observational study. Diabetes Obes. Metab. 2021, 23, 2269–2278. [Google Scholar] [CrossRef]
- Rudofsky, G.; Catarig, A.-M.; Favre, L.; Grau, K.; Häfliger, S.; Thomann, R.; Schultes, B. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res. Clin. Pract. 2021, 178, 108931. [Google Scholar] [CrossRef]
- Instituto Aragonés de Ciencias de la Salud. Un Nuevo Atlas Muestra la Prescripción Recibida Por la Población Diabética de Aragón en 2020. Available online: https://www.iacs.es/un-nuevo-atlas-muestra-la-prescripcion-recibida-por-la-poblacion-diabetica-de-aragon-en-2020/ (accessed on 8 March 2022).
- Marzullo, P.; Daffara, T.; Mele, C.; Zavattaro, M.; Ferrero, A.; Caputo, M.; Prodam, F.; Aimaretti, G. Real-world evaluation of weekly subcutaneous treatment with semaglutide in a cohort of Italian diabetic patients. J. Endocrinol. Invest. 2022, 45, 1587–1598. [Google Scholar]
- Sánchez, E.; Lecube, A.; Bellido, D.; Monereo, S.; Malagón, M.M.; Tinahones, F.J.; on behalf of the Spanish Society for the Study of Obesity. Leading factors for weight gain during COVID-19 lockdown in a Spanish population: A cross-sectional study. Nutrients 2021, 13, 894. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).