The Effect of Roux-en-Y Gastric Bypass on Non-Alcoholic Fatty Liver Disease Fibrosis Assessed by FIB-4 and NFS Scores—An 11.6-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Criteria for Surgery
2.3. Exclusion Criteria
- Missing laboratory tests needed to calculate FIB-4 or NFS
- Excessive alcohol consumption defined as >1 unit/d for women, >2 units/d for men [14] and/or disclosing a problem with alcohol abuse postoperatively.
2.4. Fibrosis Estimation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Change in NFS and FIB-4 Scores during the Observation Period
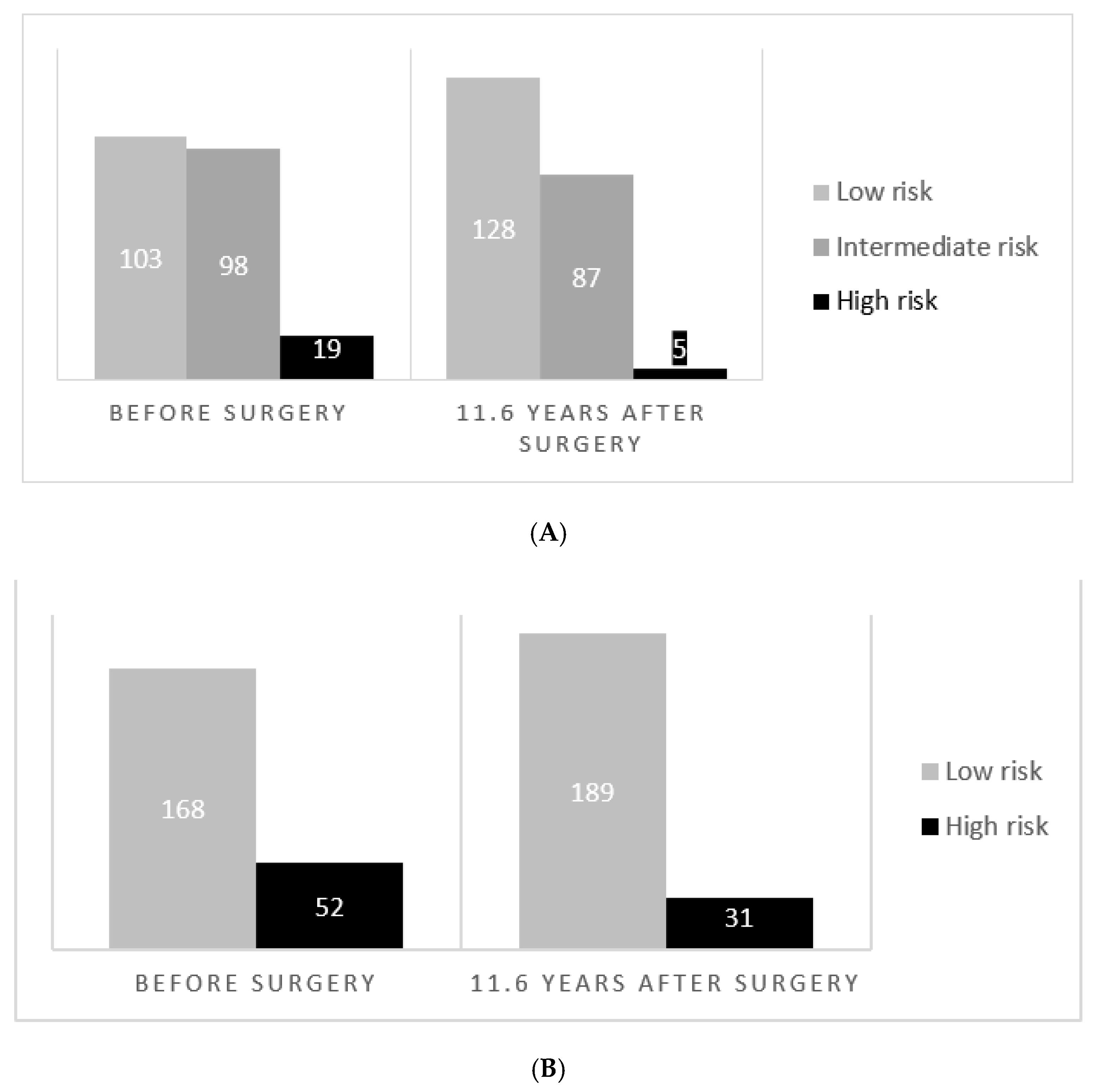
3.3. Reduction of NFS and FIB-4 in High-Risk Patients
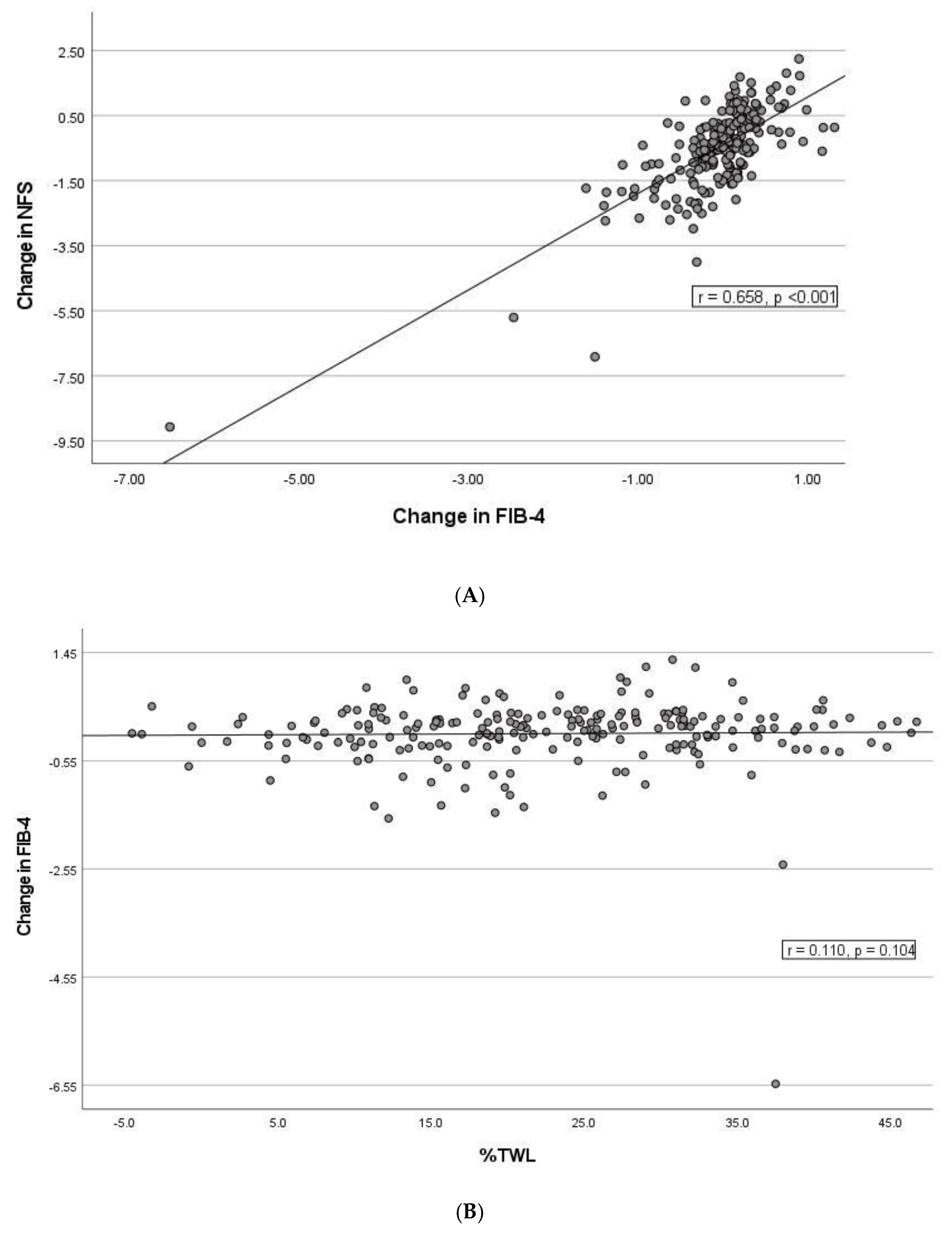
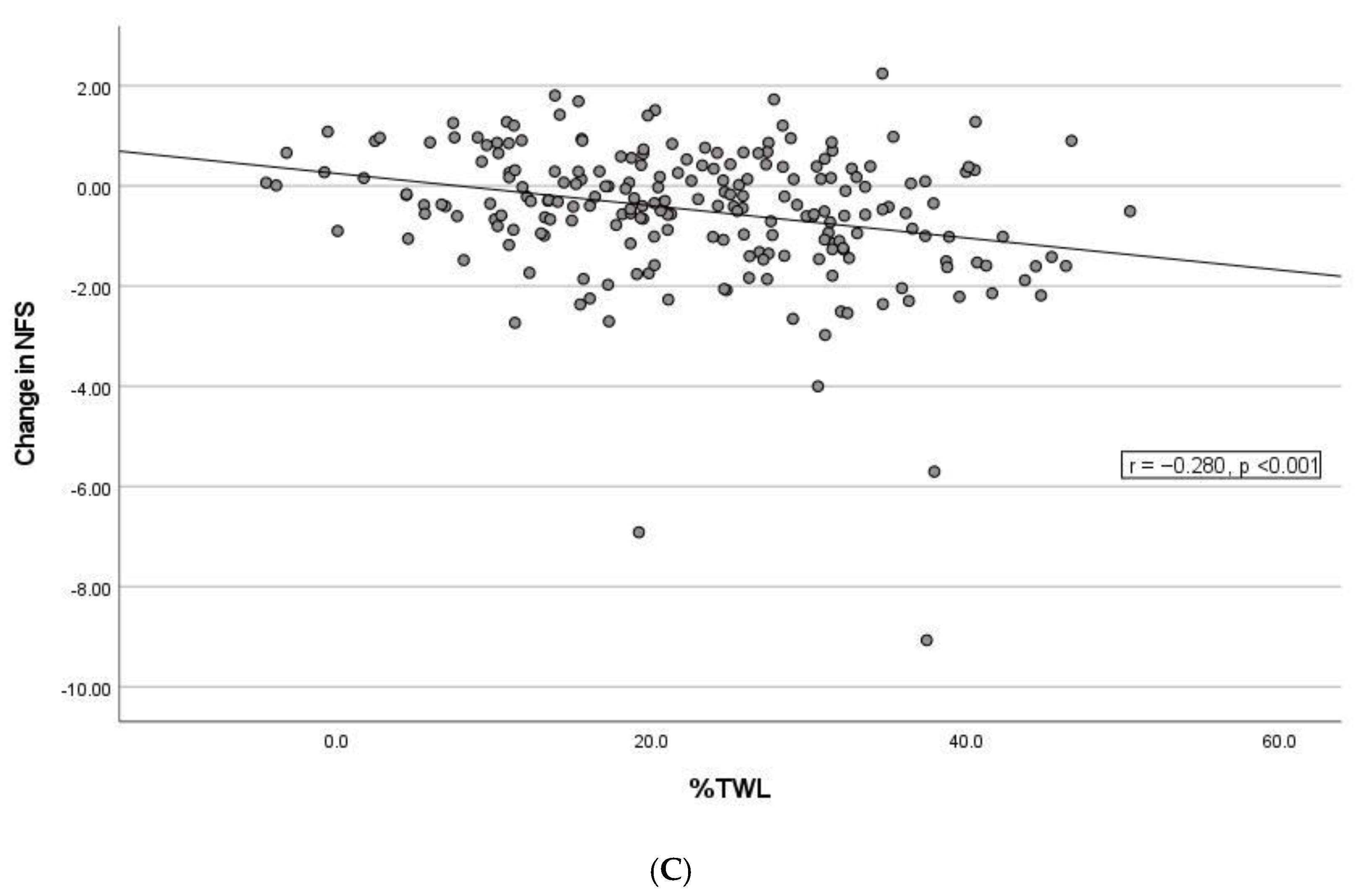
3.4. Correlation between Changes in NFS and FIB-4, Weight Loss and Remission of Type 2 Diabetes Mellitus
3.5. Characteristics of Patients with Elevated Fibrosis Risk at Baseline and Subsequent Improvement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | FIB-4 | NFS | |||||
|---|---|---|---|---|---|---|---|
| Decrease (n = 97) | Increase (n = 123) | p Value | Decrease (n = 137) | Increase (n = 83) | p Value | ||
| Age baseline, years, mean ± SD | 42.0 ± 9.69 | 38.5 ± 9.01 | 0.006 | 40.4 ± 9.37 | 39.4 ± 9.63 | 0.445 | |
| Female n (%) | 77 (79.4) | 95 (77.2) | 0.702 | 110 (80.3) | 62 (74.7) | 0.330 | |
| BMI baseline, kg/m2, median (IQR) | 43.6 (40.2–45.8) | 43.5 (40.5–48.2) | 0.306 | 43.4 (40.6–46.8) | 43.6 (40.2–47.3) | 0.564 | |
| BMI follow-up, kg/m2, median (IQR) | 33.7 (29.6–38.4) | 32.9 (29.5–37.1) | 0.280 | 33.3 (29.4–37.4) | 33.1 (30.5–40.5) | 0.260 | |
| BMI Nadir, kg/m2, median (IQR) | 28.0 (26.0–32.0) | 27.0 (25.0–30.0) | 0.078 | 27.0 (25.0–31.5) | 29.0 (26.0–31.0) | 0.170 | |
| %TWL, mean ± SD | 20.7 ± 11.6 | 24.4 ± 10.6 | 0.016 | 24.3 ± 10.7 | 20.2 ± 11.5 | 0.008 | |
| %EWL, mean ± SD | 50.2 ± 28.0 | 57.8 ± 26.1 | 0.039 | 57.6 ± 25.9 | 49.2 ± 28.5 | 0.026 | |
| T2DM baseline n (%) | 17 (17.5) | 23 (18.7) | 0.823 | 30 (21.9) | 10 (12.0) | 0.073 | |
| T2DM follow-up n (%) | 12 (12.4) | 11 (8.9) | 0.409 | 12 (8.8) | 11 (13.3) | 0.364 | |
| NFS baseline, median (IQR) | FIB-4 baseline, median (IQR) | −0.63 (−1.55–0.22) | −1.76 (−2.49–−0.87) | >0.0001 | 0.96 (0.72–1.42) | 0.65 (0.49–0.85) | >0.0001 |
| NFS follow-up, median (IQR) | FIB-4 follow-up, median (IQR) | −1.85 (−2.65–−0.97) | −1.61 (−2.33–−0.92) | 0.204 | 0.89 (0.69–1.12) | 0.89 (0.69–1.20) | 0.476 |
References
- EASL–EASD–EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Croci, I.; Coombes, J.S.; Bucher Sandbakk, S.; Keating, S.E.; Nauman, J.; Macdonald, G.A.; Wisloff, U. Non-alcoholic fatty liver disease: Prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Prog. Cardiovasc. Dis. 2019, 62, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Holmer, M.; Melum, E.; Isoniemi, H.; Ericzon, B.-G.; Castedal, M.; Nordin, A.; Aagaard Schultz, N.; Rasmussen, A.; Line, P.-D.; Stål, P.; et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. 2018, 38, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Piazzolla, V.A.; Mangia, A. Noninvasive Diagnosis of NAFLD and NASH. Cells 2020, 9, 1005. [Google Scholar] [CrossRef]
- Kupčová, V.; Fedelešová, M.; Bulas, J.; Kozmonová, P.; Turecký, L. Overview of the Pathogenesis, Genetic, and Non-Invasive Clinical, Biochemical, and Scoring Methods in the Assessment of NAFLD. Int. J. Environ. Res. Public Health 2019, 16, 3570. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.-C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e1295. [Google Scholar] [CrossRef]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Brar, K.; Banfield, L.; Gmora, S.; Anvari, M.; Hong, D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1040–1060.e1011. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Luo, P.; Li, P.; Wang, G.; Yi, X.; Fu, Z.; Sun, X.; Cui, B.; Zhu, L.; Zhu, S. Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 1872–1883. [Google Scholar] [CrossRef]
- Rehm, J.; Taylor, B.; Mohapatra, S.; Irving, H.; Baliunas, D.; Patra, J.; Roerecke, M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol. Rev. 2010, 29, 437–445. [Google Scholar] [CrossRef]
- Mellinger, J.L.; Shedden, K.; Winder, G.S.; Fernandez, A.C.; Lee, B.P.; Waljee, J.; Fontana, R.; Volk, M.L.; Blow, F.C.; Lok, A.S.F. Bariatric surgery and the risk of alcohol-related cirrhosis and alcohol misuse. Liver Int. 2021, 41, 1012–1019. [Google Scholar] [CrossRef]
- Azam, H.; Shahrestani, S.; Phan, K. Alcohol use disorders before and after bariatric surgery: A systematic review and meta-analysis. Ann. Transl. Med. 2018, 6, 148. [Google Scholar] [CrossRef]
- Lefere, S.; Onghena, L.; Vanlander, A.; van Nieuwenhove, Y.; Devisscher, L.; Geerts, A. Bariatric surgery and the liver-Mechanisms, benefits, and risks. Obes. Rev. 2021, 22, e13294. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Cheah, M.C.; McCullough, A.J.; Goh, G.B.-B. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2017, 5, 261–271. [Google Scholar] [CrossRef] [Green Version]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [Green Version]
- Losekann, A.; Weston, A.C.; De Mattos, A.A.; Tovo, C.V.; De Carli, L.A.; Espindola, M.B.; Pioner, S.R.; Coral, G.P. Non-Alcoholic Steatohepatitis (NASH): Risk Factors in Morbidly Obese Patients. Int. J. Mol. Sci. 2015, 16, 25552–25559. [Google Scholar]
- Luger, M.; Kruschitz, R.; Kienbacher, C.; Traussnigg, S.; Langer, F.B.; Schindler, K.; Würger, T.; Wrba, F.; Trauner, M.; Prager, G.; et al. Prevalence of Liver Fibrosis and its Association with Non-invasive Fibrosis and Metabolic Markers in Morbidly Obese Patients with Vitamin D Deficiency. Obes. Surg. 2016, 26, 2425–2432. [Google Scholar] [CrossRef] [Green Version]
- Gholam, P.M.; Flancbaum, L.; Machan, J.T.; Charney, D.A.; Kotler, D.P. Nonalcoholic Fatty Liver Disease in Severely Obese Subjects. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 399–408. [Google Scholar]
- Ratziu, V.; Giral, P.; Charlotte, F.; Bruckert, E.; Thibault, V.; Theodorou, I.; Khalil, L.; Turpin, G.; Opolon, P.; Poynard, T. Liver fibrosis in overweight patients. Gastroenterology 2000, 118, 1117–1123. [Google Scholar] [CrossRef]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, T.M.; Alves-Júnior, A.; Nunes, M.A.; de Freitas, T.R.; da Silva, M.A.; Alves, M.R. Comparison of Hepatic Profile in Pre and Postoperative of Bariatric Surgery: Private vs. Public Network. Arq. Bras. Cir. Dig. 2015, 28, 274–277. [Google Scholar] [CrossRef] [Green Version]
- Nickel, F.; Tapking, C.; Benner, L.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; von Frankenberg, M.; Fischer, L.; et al. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up: BariScan Study. Obes. Surg. 2018, 28, 1342–1350. [Google Scholar] [CrossRef]
- Toman, D.; Vávra, P.; Jelinek, P.; Ostruszka, P.; Ihnát, P.; Foltys, A.; Pelikan, A.; Roman, J. Effect of bariatric surgery on fatty liver disease in obese patients: A prospective one year follow-up study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czechoslov. 2021, 166, 195–203. [Google Scholar] [CrossRef]
- Cazzo, E.; Jimenez, L.S.; Pareja, J.C.; Chaim, E.A. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: A prospective study. Obes. Surg. 2015, 25, 982–985. [Google Scholar] [CrossRef]
- Agarwal, L.; Aggarwal, S.; Shalimar; Yadav, R.; Dattagupta, S.; Garg, H.; Agarwal, S. Bariatric Surgery in Nonalcoholic Fatty Liver Disease (NAFLD): Impact Assessment Using Paired Liver Biopsy and Fibroscan. Obes. Surg. 2021, 31, 617–626. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, T.K.; Mhaskar, R.; Schwitalla, T.; Muradova, E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves nonalcoholic fatty liver disease: A contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 502–511. [Google Scholar] [CrossRef]
- Mummadi, R.R.; Kasturi, K.S.; Chennareddygari, S.; Sood, G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2008, 6, 1396–1402. [Google Scholar] [CrossRef]
- Schmid, A.; Arians, M.; Karrasch, T.; Pons-Kühnemann, J.; Schäffler, A.; Roderfeld, M.; Roeb, E. Improvement of Type 2 Diabetes Mellitus and Attenuation of NAFLD Are Associated with the Success of Obesity Therapy. J. Clin. Med. 2022, 11, 1756. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ong, W.M.; Cheng, K.S.A.; Tan, C.H. Weight Loss After Bariatric Surgery Predicts an Improvement in the Non-alcoholic Fatty Liver Disease (NAFLD) Fibrosis Score. Obes. Surg. 2019, 29, 1295–1300. [Google Scholar] [CrossRef]
- Głuszyńska, P.; Lemancewicz, D.; Dzięcioł, J.B.; Razak Hady, H. Non-Alcoholic Fatty Liver Disease (NAFLD) and Bariatric/Metabolic Surgery as Its Treatment Option: A Review. J. Clin. Med. 2021, 10, 5721. [Google Scholar] [CrossRef]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Abu-Gazala, S.; Horwitz, E.; Ben-Haroush Schyr, R.; Bardugo, A.; Israeli, H.; Hija, A.; Schug, J.; Shin, S.; Dor, Y.; Kaestner, K.H.; et al. Sleeve Gastrectomy Improves Glycemia Independent of Weight Loss by Restoring Hepatic Insulin Sensitivity. Diabetes 2018, 67, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Stevenson, M.; Lee, J.; Hall, C.; Palaia, T.; Zhao, C.L.; Lau, R.; Brathwaite, C.; Ragolia, L. Reversal of NAFLD After VSG Is Independent of Weight-Loss but RYGB Offers More Efficacy When Maintained on a High-Fat Diet. Obes. Surg. 2022, 32, 2010–2022. [Google Scholar] [CrossRef]
- Ahmed, S.; Pouwels, S.; Parmar, C.; Kassir, R.; de Luca, M.; Graham, Y.; Mahawar, K. Outcomes of Bariatric Surgery in Patients with Liver Cirrhosis: A Systematic Review. Obes. Surg. 2021, 31, 2255–2267. [Google Scholar] [CrossRef]
- Norwegian Surveillance System for Communicable Diseases (MSIS). Available online: http://www.msis.no/ (accessed on 16 May 2022).
| Variable | Overall (n = 220) | FIB-4 | NFS | |||
|---|---|---|---|---|---|---|
| Characteristics | <1.3 (n = 168) | ≥1.3 (n = 52) | <−1.455 (n = 100) | Intermediate (n = 101) | >0.675 (n = 19) | |
| Age, years, mean ± SD | 40.1 ± 9.5 | 37.7 ± 8.4 | 47.6 ± 9.0 ***,a | 36.8 ± 7.8 | 42.0 ± 9.9 | 46.6 ± 9.4 ***,b |
| Female, n (%) | 172 (78.2) | 135 (80.4) | 37 (71.2) a | 79 (79.0) | 77 (76.2) | 16 (84.2) b |
| BMI, kg/m2, median (IQR) | 43.5 (40.4–46.9) | 43.7 (40.6–47.4) | 43.2 (39.5–45.7) a | 42.7 (40.3–45.5) | 43.8 (40.6–47.4) | 45.7 (41.2–51.2) *,b |
| T2DM n (%) | 40 (18.2) | 28 (16.7) | 12 (23.1) a | 8 (8.0) | 27 (26.7) | 5 (26.3) **,b |
| Diet controlled | 4 (1.8) | 3 (1.8) | 1 (1.9) | 0 (0) | 3 (3.0) | 1 (5.3) |
| Oral diabetics | 23 (10.5) | 14 (8.3) | 9 (17.3) | 6 (6.0) | 14 (13.9) | 3 (15.8) |
| Insulin | 11 (5.0) | 9 (5.4) | 2 (3.8) | 2 (2.0) | 8 (7.9) | 1 (5.3) |
| AST, U/L, median (IQR) | 37.5 (28.0–49.8) | 34.0 (27.0–44.0) | 53.5 (42.0–71.3) ***,a | 36.0 (27.0–46.0) | 38.0 (29.5–47.5) | 54.0 (37.0–69.0) **,b |
| ALT, U/L, median (IQR) | 34.0 (23.3–50.8) | 35.0 (24.5–54.0) | 30.5 (19.0–44.8) a | 38.5 (29.0–60.8) | 32.0 (23.0–46.0) | 20.0 (18.0–34.0) ***,b |
| PLT, ×109/L, median (IQR) | 297 (253–349) | 311 (268–359) | 248 (210–292) ***,a | 341 (301–384) | 271 (239–301) | 225 (188–286) ***,b |
| ALB, g/L, median (IQR) | 42.0 (40.0–44.0) | 42.0 (40.0–44.0) | 42.0 (40.0–44.8) a | 43.0 (41.0–45.0) | 41.0 (39.5–43.5) | 41.0 (39.0–43.0) **,b |
| FIB-4, median (IQR) | 0.81 (0.59–1.25) | 0.72 (0.55–0.89) | 1.61 (1.46–2.07) ***,a | 0.62 (0.47–0.79) | 0.96 (0.77–1.40) | 2.08 (1.56–2.99) ***,b |
| NFS, median (IQR) | −1.32 (−2.33–−0.39) | −1.74 (−2.51–−1.03) | 0.07 (−0.54–0.94) ***,a | −2.40 (−2.81–−1.89) | −0.61 (−1.14–−0.07) | 1.17 (0.91–1.71) ***,b |
| Variable | Overall (n = 220) | FIB-4 | NFS | |||
|---|---|---|---|---|---|---|
| Characteristics | <1.3 (n = 189) | ≥1.3 (n = 31) | <−1.455 (n = 128) | Intermediate (n = 87) | >0.675 (n = 5) | |
| Age, years, mean ± SD | 51.7 ± 9.6 | 50.2 ± 9.0 | 60.5 ± 8.6 ***,a | 48.8 ± 8.7 | 55.0 ± 9.3 | 65.1 ± 10.4 b |
| Female n (%) | 172 (78.2) | 152 (80.4) | 20 (64.5) *,a | 108 (84.4) | 60 (69.0) | 4 (80.0) *,b |
| BMI, kg/m2, median (IQR) | 33.1 (29.5–38.1) | 33.1 (29.6–38.4) | 32.9 (29.0–36.1) a | 31.7 (28.5–35.3) | 35.8 (31.9–41.0) | 36.7 (29.9–49.2) ***,b |
| BMI nadir, kg/m2, median (IQR) | 28.0 (26.0–31.0) | 28.0 (26.0–32.0) | 28.0 (26.0–30.0) a | 27.0 (24.3–29.0) | 29.0 (27.0–33.0) | 35.0 (30.5–42.0) ***,b |
| %TWL, mean ± SD | 22.8 ± 11.2 | 22.4 ± 11.4 | 25.2 ± 9.32 a | 25.5 ± 10.6 | 19.0 ± 11.1 | 19.3 ± 10.3 ***,b |
| %EWL, mean ± SD | 54.5 ± 27.1 | 53.2 ± 27.5 | 62.0 ± 24.0 a | 61.9 ± 26.1 | 44.0 ± 25.2 | 47.1 ± 28.2 ***,b |
| T2DM n (%) | 23 (10.5) | 18 (9.5) | 5 (16.1) a | 3 (2.3) | 17 (19.5) | 3 (60.0) ***,b |
| AST, U/L, median (IQR) | 21.0 (17.0–25.0) | 20.0 (17.0–24.0) | 26.0 (23.0–31.0) ***,a | 21.0 (17.0–25.0) | 20.0 (18.0–25.0) | 21.0 (17.5–31.0) b |
| ALT, U/L, median (IQR) | 21.0 (17.0–27.0) | 21.0 (17.0–27.0) | 22.0 (16.0–31.0) a | 21.0 (17.0–27.8) | 20.0 (16.0–28.0) | 21.0 (19.5–25.0) b |
| PLT, ×109/L, median (IQR) | 252 (220–301) | 265 (225–307) | 188 (175–231) ***,a | 283 (239–318) | 229 (199–252) | 176 (149–209) ***,b |
| ALB, g/L, median (IQR) | 44.0 (42.0–45.0) | 44.0 (42.0–45.0) | 44.0 (42.0–45.0) a | 44.0 (43.0–45.8) | 43.0 (42.0–45.0) | 41.0 (39.5–42.5) **,b |
| FIB-4, median (IQR) | 0.89 (0.69–1.16) | 0.81 (0.66–1.06) | 1.59 (1.48–1.81) ***,a | 0.77 (0.60–0.93) | 1.14 (0.89–1.35) | 2.32 (1.04–2.69) ***,b |
| NFS, median (IQR) | −1.71 (−2.49–−0.95) | −1.83 (−2.65–−1.23) | −0.50 (−0.99–0.10) ***,a | −2.31 (−2.89–−1.83) | −0.91 (−1.17–−0.50) | 1.07 (0.85–1.40) ***,b |
| Characteristics (n = 220) | Before Surgery | 11.6 Years after Surgery | p Value |
|---|---|---|---|
| NFS, median (IQR) | −1.32 (−2.33–−0.39) | −1.71 (−2.49–−0.95) | <0.001 |
| FIB-4, median (IQR) | 0.81 (0.59–1.25) | 0.89 (0.69–1.16) | 0.556 |
| BMI, kg/m2, median (IQR) | 43.5 (40.4–46.9) | 33.1 (29.5–38.1) | <0.001 |
| T2DM n (%) | 40 (18.2) | 23 (10.5) | <0.001 |
| AST, U/L, median (IQR) | 37.5 (28.0–49.8) | 21.0 (17.0–25.0) | <0.001 |
| ALT, U/L, median (IQR) | 34.0 (23.3–50.8) | 21.0 (17.0–27.0) | <0.001 |
| PLT, ×109/L, median (IQR) | 297 (253–349) | 252 (220–301) | <0.001 |
| ALB, g/L, median (IQR) | 42.0 (40.0–44.0) | 44.0 (42.0–45.0) | <0.001 |
| Variable | FIB-4 < 1.3 (n = 32) | FIB-4 ≥ 1.3 (n = 20) | p Value |
|---|---|---|---|
| Age baseline, years, mean ± SD | 45.9 ± 9.33 | 50.2 ± 7.84 | 0.099 |
| Female n (%) | 25 (78.1) | 12 (60.0) | 0.213 |
| BMI baseline, kg/m2, median (IQR) | 43.1 (39.5–45.8) | 44.1 (39.3–45.6) | 0.821 |
| BMI follow-up, kg/m2, median (IQR) | 33.1 (28.8–37.0) | 31.9 (28.2–36.6) | 0.529 |
| BMI nadir, kg/m2, median (IQR) | 28.0 (25.0–32.0) | 28.0 (25.5–33.0) | 0.741 |
| %TWL, mean ± SD | 21.7 ± 11.8 | 26.8 ± 9.55 | 0.108 |
| %EWL, mean ± SD | 53.1 ± 28.7 | 66.1 ± 24.9 | 0.101 |
| T2DM baseline, n (%) | 9 (28.1) | 3 (15.0) | 0.330 |
| T2DM follow-up, n (%) | 6 (18.8) | 2 (10.0) | 0.463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandvik, E.C.S.; Aasarød, K.M.; Johnsen, G.; Hoff, D.A.L.; Kulseng, B.; Hyldmo, Å.A.; Græslie, H.; Nymo, S.; Sandvik, J.; Fossmark, R. The Effect of Roux-en-Y Gastric Bypass on Non-Alcoholic Fatty Liver Disease Fibrosis Assessed by FIB-4 and NFS Scores—An 11.6-Year Follow-Up Study. J. Clin. Med. 2022, 11, 4910. https://doi.org/10.3390/jcm11164910
Sandvik ECS, Aasarød KM, Johnsen G, Hoff DAL, Kulseng B, Hyldmo ÅA, Græslie H, Nymo S, Sandvik J, Fossmark R. The Effect of Roux-en-Y Gastric Bypass on Non-Alcoholic Fatty Liver Disease Fibrosis Assessed by FIB-4 and NFS Scores—An 11.6-Year Follow-Up Study. Journal of Clinical Medicine. 2022; 11(16):4910. https://doi.org/10.3390/jcm11164910
Chicago/Turabian StyleSandvik, Elfrid Christine Smith, Kristin Matre Aasarød, Gjermund Johnsen, Dag Arne Lihaug Hoff, Bård Kulseng, Åsne Ask Hyldmo, Hallvard Græslie, Siren Nymo, Jorunn Sandvik, and Reidar Fossmark. 2022. "The Effect of Roux-en-Y Gastric Bypass on Non-Alcoholic Fatty Liver Disease Fibrosis Assessed by FIB-4 and NFS Scores—An 11.6-Year Follow-Up Study" Journal of Clinical Medicine 11, no. 16: 4910. https://doi.org/10.3390/jcm11164910
APA StyleSandvik, E. C. S., Aasarød, K. M., Johnsen, G., Hoff, D. A. L., Kulseng, B., Hyldmo, Å. A., Græslie, H., Nymo, S., Sandvik, J., & Fossmark, R. (2022). The Effect of Roux-en-Y Gastric Bypass on Non-Alcoholic Fatty Liver Disease Fibrosis Assessed by FIB-4 and NFS Scores—An 11.6-Year Follow-Up Study. Journal of Clinical Medicine, 11(16), 4910. https://doi.org/10.3390/jcm11164910






