Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
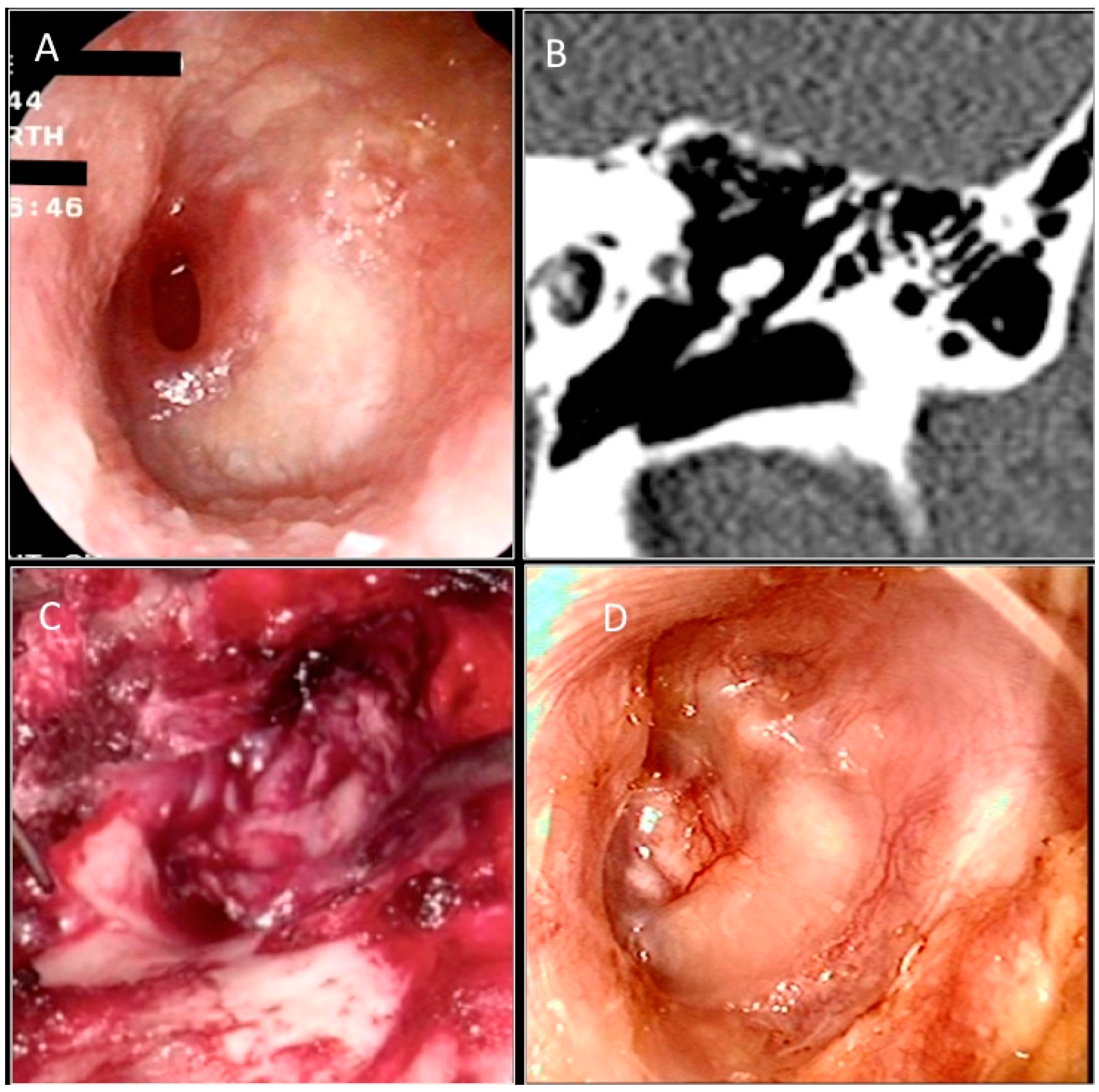
2.1. Operative Techniques and Follow-Up
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zollner, F. The principles of plastic surgery of the sound-conducting apparatus. J. Laryngol. Otol. 1955, 69, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Wullstein, H. Funktionelle operationen im mittelohr mit hilfe des freien spaltlappen-transplantates. Archiv. für. Ohren-Nasen-und Kehlkopfheilkunde 1952, 161, 422–435. [Google Scholar] [CrossRef]
- Cable, H.R. Surface tension and temporalis fascia grafts. J. Laryngol. Otol. 1981, 95, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Iacovou, E.; Vlastarakos, P.V.; Papacharalampous, G.; Kyrodimos, E.; Nikolopoulos, T.P. Is cartilage better than temporalis muscle fascia in type I tympanoplasty? Implications for current surgical practice. Eur. Arch. Otorhinolaryngol. 2013, 270, 2803–2813. [Google Scholar] [CrossRef]
- Sheehy, J.L.; Anderson, R.G. Myringoplasty. A review of 472 cases. Ann. Otol. Rhinol. Laryngol. 1980, 89, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, E.; Nuutinen, J. Success and pitfalls in myringoplasty: Follow-up study of 404 cases. Am. J. Otol. 1993, 14, 301–305. [Google Scholar] [PubMed]
- Kotecha, B.; Fowler, S.; Topham, J. Myringoplasty: A prospectiveaudit study. Clin. Otolaryngol. 1999, 24, 126. [Google Scholar] [CrossRef]
- Vartiainen, E.; Karja, J.; Karjalainen, S.; Harma, R. Failures in myringoplasty. Arch. Otorhinolaryngol. 1985, 242, 27–33. [Google Scholar] [CrossRef]
- Gersdorff, M.; Garin, P.; Decat, M.; Juantegui, M. Myringoplasty: Long-term results in adults and children. Am. J. Otol. 1995, 16, 532–535. [Google Scholar]
- Yung, M.W. Myringoplasty for subtotal perforation. Clin. Otolaryngol. 1995, 20, 241–245. [Google Scholar] [CrossRef]
- Te, G.O.; Rizer, F.M.; Schuring, A.G. Pediatric tympanoplasty of iatrogenic perforations from ventilation tube therapy. Am. J. Otol. 1998, 19, 301–305. [Google Scholar] [PubMed]
- Denoyelle, F.; Roger, G.; Chauvin, P.; Garabedian, E.N. Myringoplasty in children: Predictive factors of outcome. Laryngoscope 1999, 109, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Ophir, D.; Berco, E.; Sade, J. Revision myringoplasty. J. Laryngol. Otol. 1997, 111, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Palva, T.; Virtanen, H. Pitfalls in myringoplasty. Acta Otolaryngol. 1982, 93, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sözen, E.; Orhan Uçal, Y.; Tansuker, H.D.; Uslu Coşkun, B.; Yasemin Korkut, A.; Dadaş, B. Is the tragal cartilage necessary for type 1 tympanoplasties? J. Craniofac. Surg. 2012, 23, e280–e283. [Google Scholar] [CrossRef]
- Cabra, J.; Moñux, A. Efficacy of cartilage palisade tympanoplasty: Randomized controlled trial. Otol. Neurotol. 2010, 31, 589–595. [Google Scholar] [CrossRef]
- Kazikdas, K.C.; Onal, K.; Boyraz, I.; Karabulut, E. Palisade cartilage tympanoplasty for management of subtotal perforations: A comparison with the temporalis fascia technique. Eur. Arch. Otorhinolaryngol. 2007, 264, 985–989. [Google Scholar] [CrossRef]
- Yetiser, S.; Hidir, Y. Temporalis fascia and cartilage-perichondrium composite shield grafts for reconstruction of the tympanic membrane. Ann. Otol. Rhinol. Laryngol. 2009, 118, 570–574. [Google Scholar] [CrossRef]
- Demirpehlivan, I.A.; Onal, K.; Arslanoglu, S.; Songu, M.; Ciger, E.; Can, N. Comparison of different tympanic membrane reconstruction techniques in type I tympanoplasty. Eur. Arch. Otorhinolaryngol. 2011, 268, 471–474. [Google Scholar] [CrossRef]
- Derlacki, E.L. Residual perforations after tympanoplasty: Office technique for closure. Otolaryngol. Clin. N. Am. 1982, 15, 861–867. [Google Scholar] [CrossRef]
- Payne, M.C.; Githler, F.J. Effects of perforations of the tympanic membrane on cochlear potentials. Arch. Otolaryngol. 1951, 54, 666. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Vivekanandan, S.; Smith, P. Randomized study comparing fascia and cartilage grafts in myringoplasty. Ann. Otol. Rhinol. Laryngol. 2011, 120, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.H.; Khan, I.; Hussain, S.S. Is cartilage tympanoplasty more effective than fascia tympanoplasty? A systematic review. Otol. Neurotol. 2012, 33, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Chen, J.H.; Chou, Y.F.; Hsu, L.P.; Chen, P.R.; Liu, T.C. Optimal graft thickness for different sizes of tympanic membrane perforation in cartilage myringoplasty: A finite element analysis. Laryngoscope 2007, 117, 725–730. [Google Scholar] [CrossRef]
- Demirci, S.; Tuzuner, A.; Karadas, H.; Acıkgoz, C.; Caylan, R.; Samim, E.E. Comparison of temporal muscle fascia and cartilage grafts in pediatric tympanoplasties. Am. J. Otolaryngol. 2014, 35, 796–799. [Google Scholar] [CrossRef]
- Khan, M.M.; Parab, S.R. Comparative study of sliced tragal cartilage and temporalis fascia in type I tympanoplasty. J. Laryngol. Otol. 2015, 129, 16–22. [Google Scholar] [CrossRef]
- Lyons, S.A.; Su, T.; Vissers, L.E.; Peters, J.P.; Smit, A.L.; Grolman, W. Fascia compared to one-piece composite cartilage-perichondrium grafting for tympanoplasty. Laryngoscope 2016, 126, 1662–1670. [Google Scholar] [CrossRef]
- Vashishth, A.; Mathur, N.N.; Choudhary, S.R.; Bhardwaj, A. Clinical advantages of cartilage palisades over temporalis fascia in type I tympanoplasty. Auris Nasus Larynx 2014, 41, 422–427. [Google Scholar] [CrossRef]
- Yang, T.; Wu, X.; Peng, X.; Zhang, Y.; Xie, S.; Sun, H. Comparison of cartilage graft and fascia in type 1 tympanoplasty: Systematic review and meta-analysis. Acta Otolaryngol. 2016, 136, 1085–1090. [Google Scholar] [CrossRef]
- Iannella, G.; Marcotullio, D.; Re, M.; Manno, A.; Pasquariello, B.; Angeletti, D.; Falasca, V.; Magliulo, G. Endoscopic vs Microscopic Approach in Stapes Surgery: Advantages in the Middle Ear Structures Visualization and Trainee’s Point of View. J. Int. Adv. Otol. 2017, 13, 14–20. [Google Scholar] [CrossRef]
- Iannella, G.; De Vincentiis, M.; Greco, A.; Vicini, C.; De Vito, A.; Meccariello, G.; Cammaroto, G.; Pelucchi, S.; Magliulo, G. Endoscopic approach in second stage ossicular chain reconstruction. Am. J. Otolaryngol. 2019, 40, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, S.; Cocuzza, S.; Grillo, C.; Luca, M.D.; Maniaci, A. Complications and sequelae following tympanostomy tube placement in children with effusion otitis media: Single center experience and review of literature. Acta Medica Mediterranea 2020, 36, 1905–1912. [Google Scholar]
- Pace, A.; Visconti, I.C.; Iannella, G.; Milani, A.; Rossetti, V.; Cocuzza, S.; Maniaci, A.; Messineo, D.; Magliulo, G. Petrous Bone Cholesteatoma: Facial and Hearing Preservation. Ear. Nose Throat J. 2021, 1455613211056554. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Visconti, I.C.; Pace, A.; Iannella, G.; Rossetti, V.; Mastino, P.; Vicini, C.; Salzano, F.; Artico, M.; Greco, A.; et al. Facial nerve dehiscence and cholesteatoma: Pediatrics vs adults. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110260. [Google Scholar] [CrossRef]
- Ciofalo, A.; Zambetti, G.; Romeo, M.; Vestri, A.R.; Iannella, G.; Re, M.; Magliulo, G. Taste and olfaction in middle ear surgery. Ann Otol. Rhinol. Laryngol. 2015, 124, 312–316. [Google Scholar] [CrossRef]
- Tarumoto, S.; Sugahara, K.; Hashimoto, M.; Hirose, Y.; Tsuda, J.; Takemoto, Y.; Fujii, H.; Matsuura, T.; Shimogori, H.; Ohgi, J.; et al. Effect of preservation on the physical and chemical properties of the temporal fascia. Auris Nasus Larynx 2020, 47, 377–382. [Google Scholar] [CrossRef]
- Trindade, V.; Martins, P.; Parente, M.; Jorge, R.N.; Santos, A.; Santos, L.; Fernandes, J. The influence of regional profiles and senescence on the biomechanical properties of the temporalis muscle. J. Biomech. 2013, 46, 1592–1595. [Google Scholar] [CrossRef]
- Yüksel Aslıer, N.G.; Gürkan, S.; Aslıer, M.; Kirkim, G.; Güneri, E.A.; Ikiz, A.Ö. Sound energy absorbance characteristics of cartilage grafts used in type 1 tympanoplasty. Auris Nasus Larynx 2018, 45, 985–993. [Google Scholar] [CrossRef]
- Onal, K.; Arslanoglu, S.; Songu, M.; Demiray, U.; Demirpehlivan, I.A. Functional results of temporalis fascia versus cartilage tympanoplasty in patients with bilateral chronic otitis media. J. Laryngol. Otol. 2012, 126, 22–25. [Google Scholar] [CrossRef]
- Jalali, M.M.; Motasaddi, M.; Kouhi, A.; Dabiri, S.; Soleimani, R. Comparison of cartilage with temporalis fascia tympanoplasty: A meta-analysis of comparative studies. Laryngoscope 2017, 127, 2139–2148. [Google Scholar] [CrossRef]
- Al lackany, M.; Sarkis, N.N. Functional results after myringoplasty and Type 1 tympanoplasty with the use of different graft materials. J. Med. Res. Inst. 2005, 26, 369–374. [Google Scholar]

| Fascia Group (n = 98) | Cartilage Group (n = 44) | ||||
|---|---|---|---|---|---|
| n/SD | % | n/SD | % | p-Value | |
| Age (year) | 52.5 ± 18.9 | 55.3 ± 14.5 | 0.38 | ||
| Mean follow-up (months) | 67.1 ± 3.2 | 66.6 ± 2.5 | 0.36 | ||
| Gender | |||||
| Male | 46/98 | 46.93 | 24/44 | 54.54 | 0.40 |
| Female | 52/98 | 53.06 | 20/44 | 45.46 | |
| Side | |||||
| Left | 54/98 | 55.1 | 27/44 | 61.36 | 0.48 |
| Right | 44/98 | 44.9 | 17/44 | 38.64 | |
| Perforation size | |||||
| <50% | 49/98 | 50 | 8/44 | 18.18 | <0.001 |
| >50% | 49/98 | 50 | 36/44 | 81.72 | |
| Anterior | 16/98 | 16.32 | 11/44 | 25 | 0.83 |
| Posterior | 33/98 | 33.67 | 25/44 | 75 | |
| Recurrence rate | 15/98 | 15.30 | 4/44 | 9.09 | 0.37 |
| Fascia Group (n = 98) | Cartilage Group (n = 44) | p-Value | |
|---|---|---|---|
| Air conductive (AC) (dB) | |||
| Pre-operative | 24.6 ± 6.81 | 26.58 ± 7.81 | 0.128 |
| 500 | 35.2 ± 4.47 | 34.24 ± 4.01 | |
| 1000 | 34.73 ± 4.18 | 30.17 ± 3.15 | |
| 2000 | 20.9 ± 3.98 | 25.6 ± 3.74 | |
| 4000 | 6.21 ± 2.87 | 15.61 ± 2.23 | |
| Post-operative 6 months | 11.23 ± 3.92 | 11.54 ± 4.18 | <0.001 |
| 500 | 14.78 ± 3.23 | 15.75 ±2.93 | |
| 1000 | 10.97 ± 3.46 | 9.78 ± 3.64 | |
| 2000 | 11.25 ± 3.49 | 10.18 ± 4.15 | |
| 4000 | 6.7 ± 2.5 | 5.87 ± 3.11 | |
| Post-operative 5 years | 15.32 ± 4.39 | 11.68 ± 4.78 | <0.001 |
| 500 | 21.2 ± 4.68 | 14.56 ± 3.48 | |
| 1000 | 20.3 ± 3.69 | 14.98 ± 4.83 | |
| 2000 | 10.86 ± 3.16 | 11.38 ± 3.81 | |
| 4000 | 10.27 ± 3.95 | 5.13 ± 4.12 | |
| Bone conductive (BC) | 5.73 ± 1.23 | 5.31 ± 0.95 | 0.46 |
| 500 | 5.81 ± 2.9 | 5.35 ± 3.28 | |
| 1000 | 5.32 ± 4.33 | 5.81 ± 3.42 | |
| 2000 | 5.08 ± 1.58 | 5.02 ± 2.85 | |
| 4000 | 4.93 ± 2.58 | 4.71 ± 2.63 | |
| ABG (dB) | |||
| Pre-operative | 20.3 ± 3.0 | 19.3 ± 2.7 | 0.04 |
| Post-operative 6 months | 4.9 ± 0.9 | 5.3 ± 1.2 | 0.04 |
| Post-operative 5 years | 10.0 ± 1.7 | 6.4 ± 2.0 | <0.001 |
| Fascia Group (n = 98) | p-Value | Cartilage Group (n = 44) | p-Value | |||
|---|---|---|---|---|---|---|
| Perforated | Non-Perforated | Perforated | Non-Perforated | |||
| Air conductive (AC) (dB) | 20.9 ± 2.4 | 15.4 ± 1.7 | <0.001 | 15.7 ± 1.3 | 12.3 ± 1.0 | <0.001 |
| Bone conductive (BC) | 10.2 ± 1.8 | 5.2 ± 1.5 | <0.001 | 8.3 ± 2.4 | 5.8 ± 2.2 | <0.001 |
| ABG (dB) | 10.5 ± 2.1 | 10.1 ± 1.7 | 0.002 | 7.3 ± 2 | 5.1 ± 1.5 | 0.01 |
| Source Dependent Variables | Mean Square | F | Sig. | |
|---|---|---|---|---|
| Functional success | Sex | 0.041 | 0.161 | 0.689 |
| Age | 1.107 | 4.591 | 0.036 | |
| Side | 0.029 | 0.118 | 0.733 | |
| Perforation type/size | ||||
| >50% | 1.148 | 4.820 | 0.030 | |
| <50% | 0.452 | 1.821 | 0.179 | |
| Anterior | 0.160 | 1.153 | 0.287 | |
| Posterior | 0.002 | 0.011 | 0.916 | |
| Graft type | 0.257 | 1.239 | 0.269 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferlito, S.; Fadda, G.; Lechien, J.R.; Cammaroto, G.; Bartel, R.; Borello, A.; Cavallo, G.; Piccinini, F.; La Mantia, I.; Cocuzza, S.; et al. Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study. J. Clin. Med. 2022, 11, 7000. https://doi.org/10.3390/jcm11237000
Ferlito S, Fadda G, Lechien JR, Cammaroto G, Bartel R, Borello A, Cavallo G, Piccinini F, La Mantia I, Cocuzza S, et al. Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study. Journal of Clinical Medicine. 2022; 11(23):7000. https://doi.org/10.3390/jcm11237000
Chicago/Turabian StyleFerlito, Salvatore, Gianluca Fadda, Jerome Rene Lechien, Giovanni Cammaroto, Ricardo Bartel, Andrea Borello, Giovanni Cavallo, Francesca Piccinini, Ignazio La Mantia, Salvatore Cocuzza, and et al. 2022. "Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study" Journal of Clinical Medicine 11, no. 23: 7000. https://doi.org/10.3390/jcm11237000
APA StyleFerlito, S., Fadda, G., Lechien, J. R., Cammaroto, G., Bartel, R., Borello, A., Cavallo, G., Piccinini, F., La Mantia, I., Cocuzza, S., Merlino, F., Achena, A., Brucale, C., Mat, Q., Gargula, S., Fakhry, N., & Maniaci, A. (2022). Type 1 Tympanoplasty Outcomes between Cartilage and Temporal Fascia Grafts: A Long-Term Retrospective Study. Journal of Clinical Medicine, 11(23), 7000. https://doi.org/10.3390/jcm11237000















