Immune Checkpoint Inhibitors’ Associated Renal Toxicity: A Series of 12 Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics-Clinical Characteristics
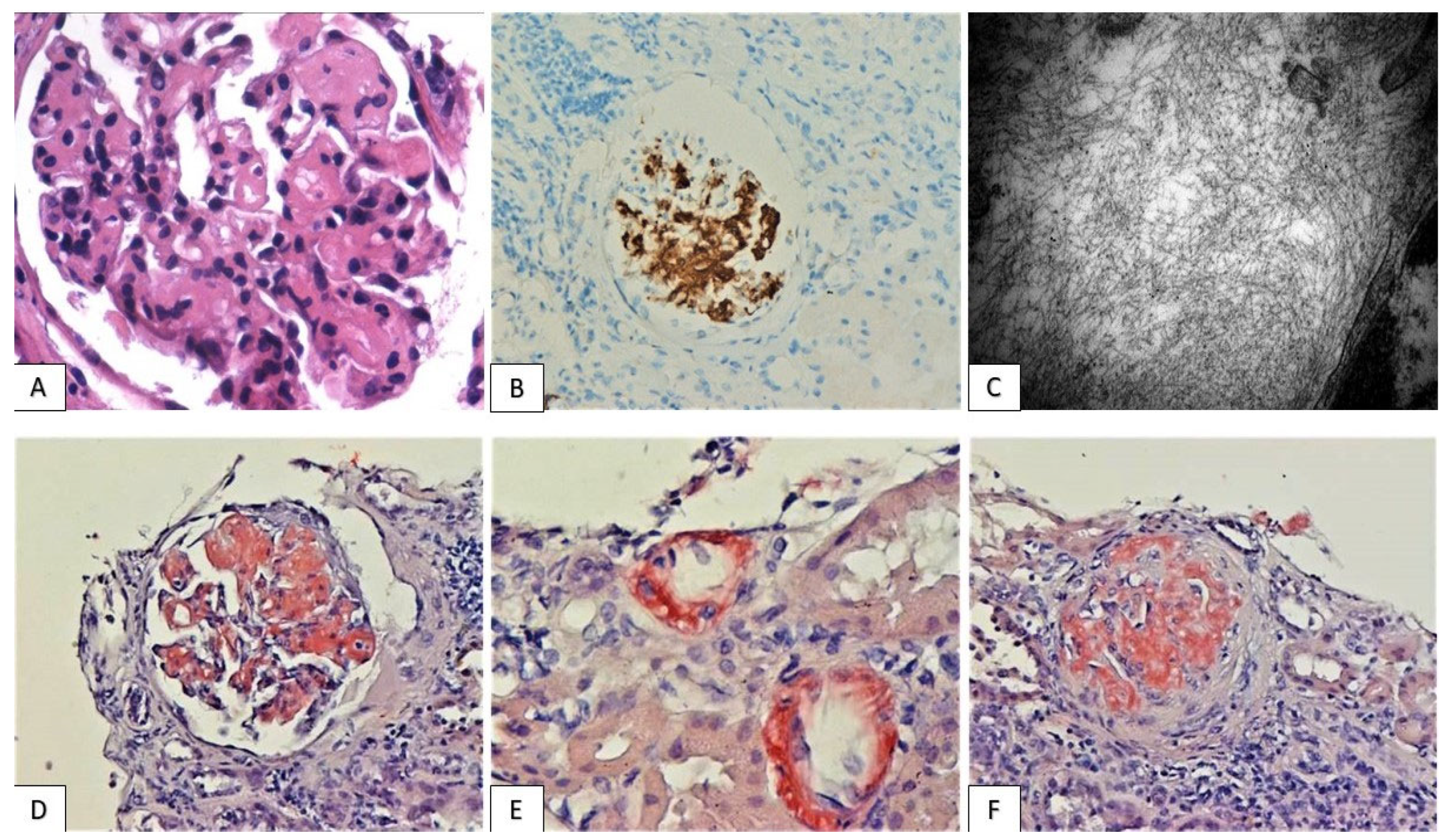
3.2. Histological Findings
3.3. PD-L1 Expression Pattern
3.4. Treatment-Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sury, K.; Perazella, M.A.; Shirali, A.C. Cardiorenal Complications of Immune Checkpoint Inhibitors. Nat. Rev. Nephrol. 2018, 14, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological Features of Acute Kidney Injury Associated with Immune Checkpoint Inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Gueutin, V.; Gharbi, C.; Mateus, C.; Robert, C.; Routier, E.; Thomas, M.; Baumelou, A.; Rouvier, P. Kidney Injuries Related to Ipilimumab. Investig. New Drugs 2014, 32, 769–773. [Google Scholar] [CrossRef]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of Acute Interstitial Nephritis with Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef]
- Izzedine, H.; Mateus, C.; Boutros, C.; Robert, C.; Rouvier, P.; Amoura, Z.; Mathian, A. Renal Effects of Immune Checkpoint Inhibitors. Nephrol. Dial. Transplant. 2017, 32, 936–942. [Google Scholar] [CrossRef]
- Kitchlu, A.; Jhaveri, K.D.; Wadhwani, S.; Deshpande, P.; Harel, Z.; Kishibe, T.; Henriksen, K.; Wanchoo, R. A Systematic Review of Immune Checkpoint Inhibitor–Associated Glomerular Disease. Kidney Int. Rep. 2021, 6, 66–77. [Google Scholar] [CrossRef]
- Wanchoo, R.; Karam, S.; Uppal, N.N.; Barta, V.S.; Deray, G.; Devoe, C.; Launay-Vacher, V.; Jhaveri, K.D. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am. J. Nephrol. 2017, 45, 160–169. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeon, H.; Kim, S.; Lee, W.; Kim, H.J.; Rhee, H.; Song, S.H.; Seong, E.Y. Pembrolizumab-Induced Focal Segmental Glomerulosclerosis: A Case Report. Medicine 2021, 100, e27546. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Zhong, W.; Zheng, K.; Ye, W.; Wang, M. Case Report: THSD7A-Positive Membranous Nephropathy Caused by Tislelizumab in a Lung Cancer Patient. Front. Immunol. 2021, 12, 619147. [Google Scholar] [CrossRef]
- Gao, B.; Lin, N.; Wang, S.; Wang, Y. Minimal Change Disease Associated with Anti-PD1 Immunotherapy: A Case Report. BMC Nephrol. 2018, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ishibuchi, K.; Iwakura, T.; Kaneko, M.; Fukasawa, H.; Furuya, R. Pembrolizumab-Associated Nephrotic Syndrome Recovered from Transient Hemodialysis in a Patient with Lung Cancer. CEN Case Rep. 2020, 9, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Fadel, F.; el Karoui, K.; Knebelmann, B. Anti-CTLA4 Antibody-Induced Lupus Nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Fingrut, W.; Avila-Casado, C.; Chan, C.T.; Crump, M.; Hogg, D.; Reich, H.N. Nephrotic Syndrome With Cancer Immunotherapies: A Report of 2 Cases. Am. J. Kidney Dis. 2017, 70, 581–585. [Google Scholar] [CrossRef]
- Kidd, J.M.; Gizaw, A.B. Ipilimumab-Associated Minimal-Change Disease. Kidney Int. 2016, 89, 720. [Google Scholar] [CrossRef]
- Kishi, S.; Minato, M.; Saijo, A.; Murakami, N.; Tamaki, M.; Matsuura, M.; Murakami, T.; Nagai, K.; Abe, H.; Nishioka, Y.; et al. IgA Nephropathy after Nivolumab Therapy for Postoperative Recurrence of Lung Squamous Cell Carcinoma. Intern. Med. 2018, 57, 1259–1263. [Google Scholar] [CrossRef]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of Immune Checkpoint Inhibitors beyond Tubulointerstitial Nephritis: Single-Center Experience 11 Medical and Health Sciences 1103 Clinical Sciences. J. ImmunoTherapy Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Cruz-Whitley, J.; Giehl, N.; Jen, K.Y.; Young, B. Membranoproliferative Glomerulonephritis Associated with Nivolumab Therapy. Case Rep. Nephrol. 2020, 2020, 2638283. [Google Scholar] [CrossRef]
- Uner, M.; Alhasson, B.; Obhrai, J.; Bagnasco, S.M. ANCA-Associated Pauci-Immune Necrotizing Glomerulonephritis during the Treatment with Pembrolizumab. Virchows Archiv. 2021, 478, 801–804. [Google Scholar] [CrossRef]
- Tanabe, K.; Kanzaki, H.; Wada, T.; Nakashima, Y.; Sugiyama, H.; Okada, H.; Wada, J. Nivolumab-Induced IgA Nephropathy in a Patient with Advanced Gastric Cancer: A Case Report. Medicine 2020, 99, e20464. [Google Scholar] [CrossRef]
- Gallan, A.J.; Alexander, E.; Reid, P.; Kutuby, F.; Chang, A.; Henriksen, K.J. Renal Vasculitis and Pauci-Immune Glomerulonephritis Associated with Immune Checkpoint Inhibitors. Am. J. Kidney Dis. 2019, 74, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Ville, S.; Kandel-Aznar, C.; Frémeaux-Bacchi, V.; Fakhouri, F. C3 Glomerulonephritis in a Patient Treated with Anti–PD-1 Antibody. Eur. J. Cancer 2020, 125, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Short, S.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, S.M.; Abudayyeh, A.; Anand, S.; et al. ICPi-AKI Consortium Investigators. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Seethapathy, H.; Zhao, S.; Chute, D.F.; Zubiri, L.; Oppong, Y.; Strohbehn, I.; Cortazar, F.B.; Leaf, D.E.; Mooradian, M.J.; Villani, A.C.; et al. The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin. J. Am. Soc. Nephrol. CJASN 2019, 14, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Cassol, C.; Satoskar, A.; Lozanski, G.; Rovin, B.; Hebert, L.; Nadasdy, T.; Brodsky, S.V. Anti−PD-1 Immunotherapy May Induce Interstitial Nephritis with Increased Tubular Epithelial Expression of PD-L1. Kidney Int. Rep. 2019, 4, 1152–1160. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Starke, A.; Wüthrich, R.P. PD-L1 Partially Protects Renal Tubular Epithelial Cells from the Attack of CD8+ Cytotoxic T Cells. Nephrol. Dial. Transplant. 2007, 22, 1527–1536. [Google Scholar] [CrossRef]
- Simpson, I.J.; Marshall, M.R.; Pilmore, H.; Manley, P.; Williams, L.; Thein, H.; Voss, D. Proton Pump Inhibitors and Acute Interstitial Nephritis: Report and Analysis of 15 Cases. Nephrology 2006, 11, 381–385. [Google Scholar] [CrossRef]
- Geevasinga, N.; Coleman, P.L.; Webster, A.C.; Roger, S.D. Proton Pump Inhibitors and Acute Interstitial Nephritis. Clin. Gastroenterol. Hepatol. 2006, 4, 597–604. [Google Scholar] [CrossRef]
- Spanou, Z.; Keller, M.; Britschgi, M.; Yawalkar, N.; Fehr, T.; Neuweiler, J.; Gugger, M.; Mohaupt, M.; Pichler, W.J. Involvement of Drug-Specific T Cells in Acute Drug-Induced Interstitial Nephritis. J. Am. Soc. Nephrol. 2006, 17, 2919–2927. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Curran, C.S.; Gupta, S.; Sanz, I.; Sharon, E. PD-1 Immunobiology in Systemic Lupus Erythematosus. J. Autoimmun. 2019, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Liao, W.; Zheng, H.; Wu, S.; Zhang, Y.; Wang, W.; Zhang, Z.; Zhou, C.; Wu, H.; Min, J. The Systemic Activation of Programmed Death 1-PD-L1 Axis Protects Systemic Lupus Erythematosus Model from Nephritis. Am. J. Nephrol. 2017, 46, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Smarz-Widelska, I.; Krasowska-Zajac, E.; Korona-Glowniak, I.; Zaluska-Patel, K.; Mielnik, M.; Podgajna, M.; Malm, A.; Rolinski, J.; Zaluska, W. The PD-1/PD-L1 Inhibitory Pathway Is Altered in Primary Glomerulonephritides. Arch. Immunol. Ther. Exp. 2018, 66, 133–143. [Google Scholar] [CrossRef]
- Lapman, S.; Whittier, W.L.; Parikh, R.; Khanin, Y.; Bijol, V.; Wanchoo, R.; Jhaveri, K.D. Immune Checkpoint Inhibitor–Associated Renal Amyloid A Amyloidosis: A Case Series and Review of the Literature. J. Onco-Nephrol. 2020, 4, 52–58. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Guarnieri, A.; Tripodi, S.A.; Maccari, M.; Mancianti, N.; Guido, G.; Rossi, G.; Daffinà, M.G.; Calabrò, L.; Valente, M.; et al. Brief Communication PD1-related Nephrotoxicity: Optimizing Its Clinical Management through Histopathologic Features. J. Immunother. 2022, 45, 217–221. [Google Scholar] [CrossRef]
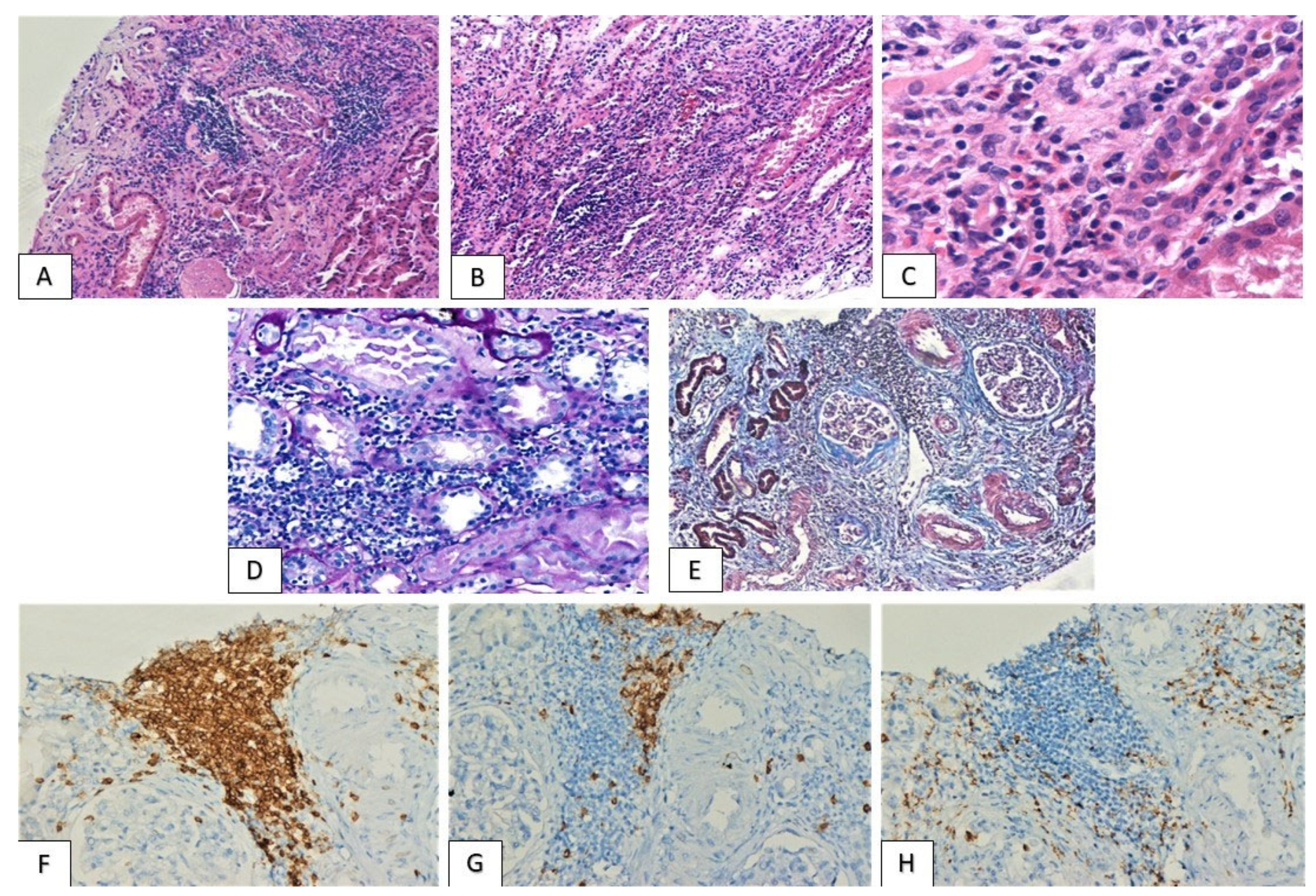
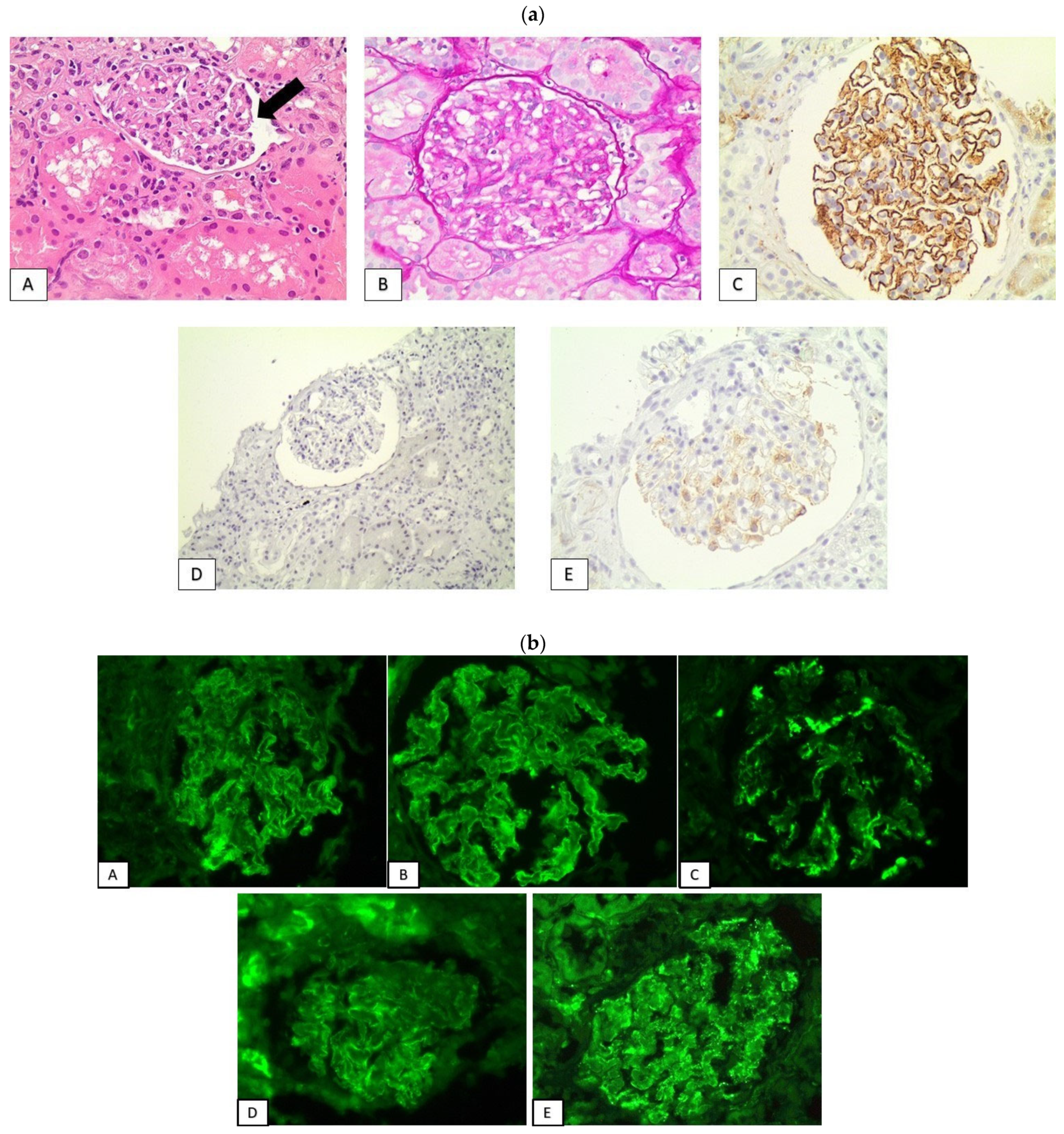
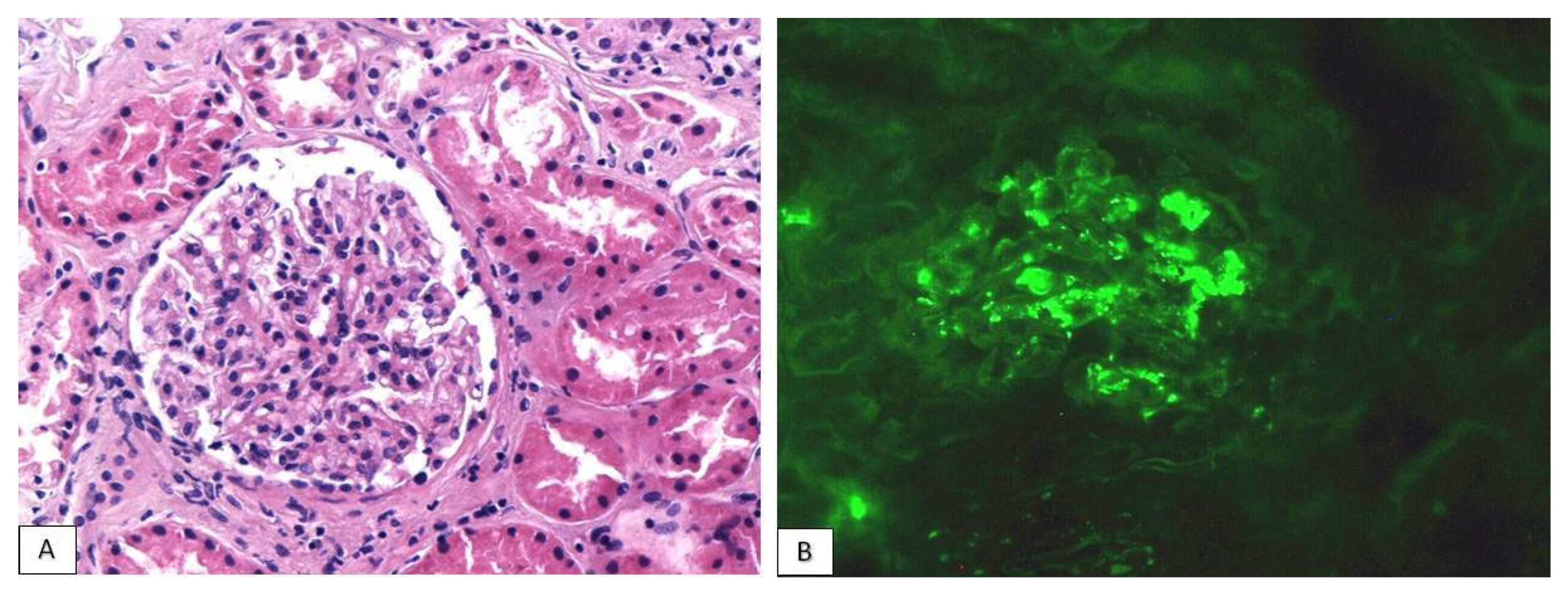
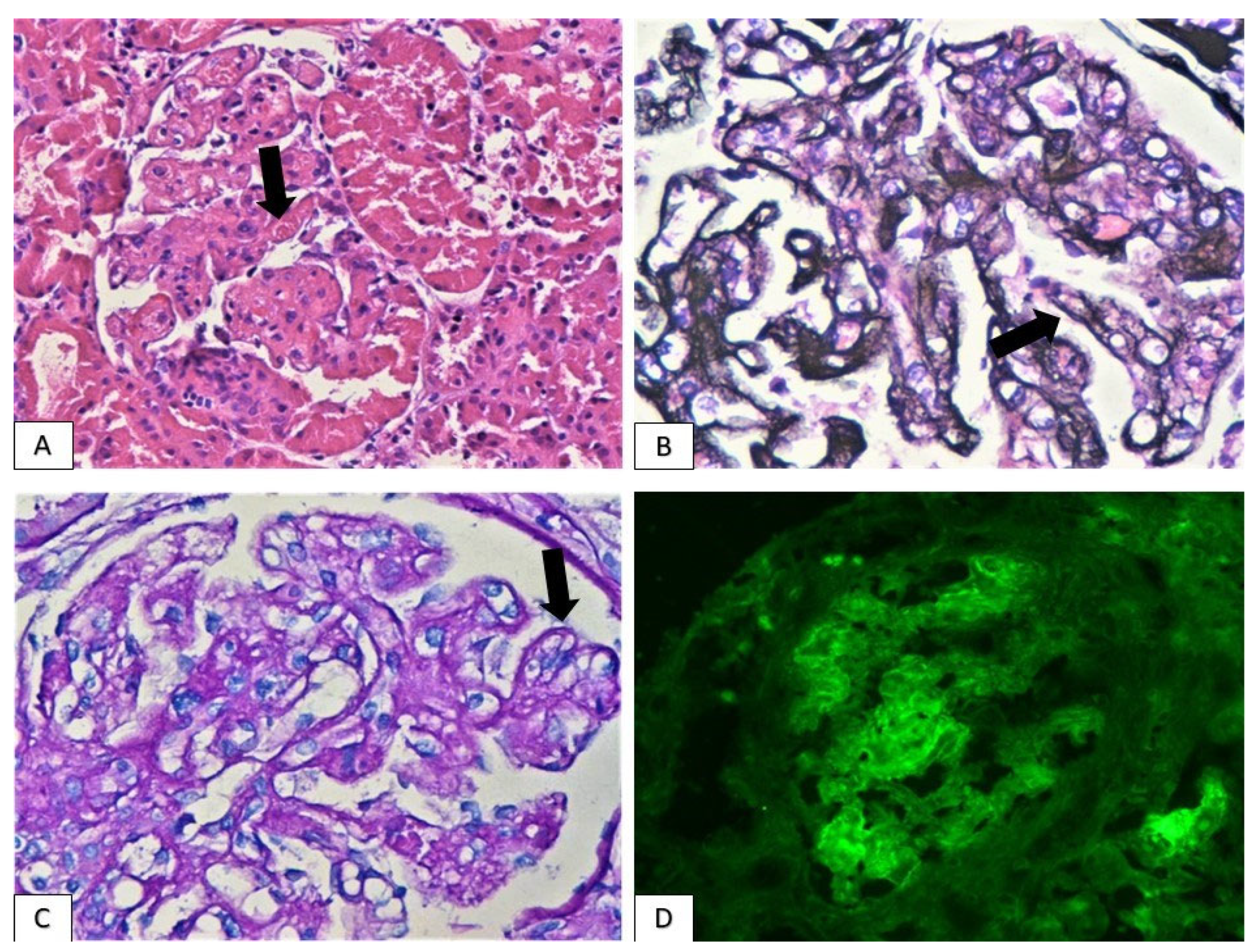

| Patients | Gender | Age | Malignancy | Immunotherapeutic Agents | Latent Period (Months) | Other Adverse Effects | PPIs |
|---|---|---|---|---|---|---|---|
| 1 | Male | 73 | bladder cancer | atezolizumab | 12 | - | - |
| 2 | Male | 69 | bladder cancer | nivolumab | 12 | - | - |
| 3 | Female | 64 | lung cancer | pembrolizumab | 24 | - | - |
| 4 | Male | 73 | lung cancer | nivolumab/ipilimumab | 3 | hepatitis | + |
| 5 | Male | 57 | renal cell carcinoma | pembrolizumab | 14 | - | - |
| 6 | Male | 73 | Merkel cell tumor | avelumab | 3 | - | + |
| 7 | Female | 57 | squamous cell cervical carcinoma | pembrolizumab | 16 | - | - |
| 8 | Male | 66 | melanoma | ipilimumab | 3 | rheumatic polymyalgia | - |
| 9 | Male | 58 | lung cancer | pembrolizumab | 19 | pneumonitis | + |
| 10 | Female | 69 | lung cancer | pembrolizumab | 23 | dermatitis, colitis | + |
| 11 | Male | 68 | lung cancer | nivolumab | 10 | - | - |
| 12 | Female | 70 | lung cancer | pembrolizumab | 28 | rheumatoid arthritis, thyroiditis, pancreatitis | - |
| Patients | Renal Failure | Creatinine (mg/dL) | Hematuria | Proteinuria | Urine Protein (g/24 h) | Nephrotic Syndrome | Tubulointerstitial Nephritis | Glomerulopathy |
|---|---|---|---|---|---|---|---|---|
| 1 | + | 2 | + | - | - | - | moderate | IgA glomerulonephritis |
| 2 | + | 3.9 | - | - | - | - | severe | - |
| 3 | + | 2 | - | + | 0.3 | - | severe | - |
| 4 | + | 2.8 | - | - | - | - | moderate | - |
| 5 | + | 2.2 | + | - | - | - | severe | - |
| 6 | + | 2.5 | + | - | - | - | mild | - |
| 7 | + | 2.2 | - | + | 3.2 | - | mild | Membranoproliferative glomerulonephritis/ thrombotic microangiopathy |
| 8 | + | 1.8 | - | + | 0.254 | - | mild | - |
| 9 | + | 4.26 | - | + | 0.8 | - | mild | N/A (renal medulla) |
| 10 | - | normal | - | + | 16 | + | mild | “lupus-like” secondary membranous glomerulopathy |
| 11 | - | normal | - | + | 11 | + | mild | “lupus-like” secondary membranous glomerulopathy |
| 12 | - | normal | - | + | 10 | + | mild | Secondary AA amyloidosis |
| Patients | Global Glomerulosclerosis (%) | IF/TA | Arterial Fibrous Intimal Thickening | IgG | IgA | IgM | C3 | C1q |
|---|---|---|---|---|---|---|---|---|
| 1 | 41.66 | moderate | moderate | Glomeruli (1+) | Glomeruli (3+) | - | Glomeruli (1+) | - |
| 2 | 12.5 | severe | moderate | - | - | - | - | - |
| 3 | 11.7 | mild | moderate | - | - | - | - | - |
| 4 | 29.4 | moderate | moderate | - | - | Glomeruli (1+) | - | - |
| 5 | NA (renal medulla) | NA (renal medulla) | No arteries | - | - | - | - | - |
| 6 | 33.3 | mild | severe | - | - | Glomeruli (1+) | - | - |
| 7 | 0 | mild | Glomeruli (2+) | Glomeruli (1+) | Glomeruli (2+) | Glomeruli (2+) | - | |
| 8 | 22.22 | mild | severe | - | - | - | - | - |
| 9 | NA (renal medulla) | NA (renal medulla) | No arteries | - | - | - | - | - |
| 10 | 18.18 | moderate | severe | Glomeruli (2+) | Glomeruli (2+) | Glomeruli (2+) | Glomeruli (2+) | Glomeruli (2+) |
| 11 | 25 | moderate | moderate | Glomeruli (3+) | Glomeruli (1+) | Glomeruli (1+) | Glomeruli (3+) | Glomeruli (2+) |
| 12 | 35.48 | mild | moderate | - | Glomeruli (1+) | Glomeruli (1+) | Glomeruli (2+) | Glomeruli (2+) |
| Patients | Tubular Epithelium | Glomeruli (Intensity) | |
|---|---|---|---|
| Cytoplasmic (Intensity) | Membranous (Intensity) | ||
| 1 | +(mild) | - | - |
| 2 | +(moderate) | - | - |
| 3 | +(mild) | - | - |
| 4 | +(severe) | +(severe) | - |
| 5 | +(mild) | - | NA |
| 6 | +(severe) | +(severe) | - |
| 7 | +(moderate) | +(moderate) | - |
| 8 | - | - | - |
| 9 | +(moderate) | - | NA |
| 10 | - | - | +(moderate) |
| 11 | +(moderate) | - | +(mild) |
| 12 | +(mild) | - | +(mild) |
| Patients | Immunotherapy Withdrawal | Immunosuppression | Kidney Response | Hemodialysis |
|---|---|---|---|---|
| 1 | NA | NA | NA | NA |
| 2 | + | prednisolone | No remission | + |
| 3 | + | prednisolone | remission | - |
| 4 | + | prednisolone | remission | - |
| 5 | + | prednisolone | remission | - |
| 6 | + | prednisolone | remission | - |
| 7 | NA | NA | NA | NA |
| 8 | NA | NA | NA | NA |
| 9 | + | prednisolone | remission | - |
| 10 | + | prednisolone | remission | - |
| 11 | NA | NA | NA | NA |
| 12 | + | prednisolone and colchicine | remission | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palamaris, K.; Alexandris, D.; Stylianou, K.; Giatras, I.; Stofas, A.; Kaitatzoglou, C.; Migkou, M.; Goutas, D.; Psimenou, E.; Theodoropoulou, E.; et al. Immune Checkpoint Inhibitors’ Associated Renal Toxicity: A Series of 12 Cases. J. Clin. Med. 2022, 11, 4786. https://doi.org/10.3390/jcm11164786
Palamaris K, Alexandris D, Stylianou K, Giatras I, Stofas A, Kaitatzoglou C, Migkou M, Goutas D, Psimenou E, Theodoropoulou E, et al. Immune Checkpoint Inhibitors’ Associated Renal Toxicity: A Series of 12 Cases. Journal of Clinical Medicine. 2022; 11(16):4786. https://doi.org/10.3390/jcm11164786
Chicago/Turabian StylePalamaris, Kostas, Dimitrios Alexandris, Kostas Stylianou, Ioannis Giatras, Anastasios Stofas, Christina Kaitatzoglou, Magda Migkou, Dimitrios Goutas, Erasmia Psimenou, Eleni Theodoropoulou, and et al. 2022. "Immune Checkpoint Inhibitors’ Associated Renal Toxicity: A Series of 12 Cases" Journal of Clinical Medicine 11, no. 16: 4786. https://doi.org/10.3390/jcm11164786
APA StylePalamaris, K., Alexandris, D., Stylianou, K., Giatras, I., Stofas, A., Kaitatzoglou, C., Migkou, M., Goutas, D., Psimenou, E., Theodoropoulou, E., Theocharis, S., Alevizopoulos, N., Kastritis, E., Gerakis, A., & Gakiopoulou, H. (2022). Immune Checkpoint Inhibitors’ Associated Renal Toxicity: A Series of 12 Cases. Journal of Clinical Medicine, 11(16), 4786. https://doi.org/10.3390/jcm11164786







