Intracranial Solitary Fibrous Tumor: A “New” Challenge for PET Radiopharmaceuticals
Abstract
1. Introduction
2. Solitary Fibrous Tumor
3. Search Strategy
4. Evidence-Based Medicine of PET/CT in Intracranial Solitary Fibrous Tumor/Hemangiopericytoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.J.; Labella, D.A.; Richardson, K.M.; Sheehan, J.P.; Kersh, C.R. Recurrent Solitary Fibrous Tumor (Intracranial Hemangiopericytoma) Treated with a Novel Combined-Modality Radiosurgery Technique: A Case Report and Review of the Literature. Front. Oncol. 2022, 12, 907324. [Google Scholar] [CrossRef] [PubMed]
- Altini, C.; Lavelli, V.; Ruta, R.; Ferrari, C.; Nappi, A.G.; Pisani, A.; Sardaro, A.; Rubini, G. Typical and atypical PET/CT findings in non-cancerous conditions. Hell. J. Nucl. Med. 2020, 23, 48–59. [Google Scholar] [CrossRef]
- Altini, C.; Asabella, A.N.; Lavelli, V.; Bianco, G.; Ungaro, A.; Pisani, A.; Merenda, N.; Ferrari, C.; Rubini, G. Role of 18F-FDG PET/CT in comparison with CECT for whole-body assessment of patients with esophageal cancer. Recenti Prog. Med. 2019, 110, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.P.; Murray, M.R. Hemangiopericytoma: A vascular tumor featuring Zimmermann’s pericytes. Ann. Surg. 1942, 116, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef]
- Sardaro, A.; Ferrari, C.; Mammucci, P.; Piscitelli, D.; Rubini, D.; Maggialetti, N. The significant role of multimodality imaging with 18 Fluorocholine PET/CT in relapsed intracranial hemangiopericytoma. Rev. Esp. Med. Nucl. Imagen Mol. 2021, in press. [Google Scholar] [CrossRef]
- Cheng, K.P.; Wong, W.J.; Hashim, S.; Mun, K.S. Hemangiopericytoma 11 years later: Delayed recurrence of a rare soft tissue sarcoma. J. Thorac. Dis. 2017, 9, E752–E756. [Google Scholar] [CrossRef]
- Ciliberti, M.P.; D’Agostino, R.; Gabrieli, L.; Nikolaou, A.; Sardaro, A. The radiation therapy options of intracranial hemangiopericytoma: An overview and update on a rare vascular mesenchymal tumor. Oncol. Rev. 2018, 12, 63–68. [Google Scholar] [CrossRef]
- Ramsey, H.J. Fine structure of hemangiopericytoma and hemangio-endothelioma. Cancer 1966, 19, 2005–2018. [Google Scholar] [CrossRef]
- d’Amore, E.S.G.; Manivel, J.C.; Sung, J.H. Soft-tissue and meningeal hemangiopericytomas: An immunohistochemical and ultrastructural study. Hum. Pathol. 1990, 21, 414–423. [Google Scholar] [CrossRef]
- Purandare, N.C.; Dua, S.G.; Rekhi, B.; Shah, S.; Sharma, A.R.; Rangarajan, V. Metastatic recurrence of an intracranial hemangiopericytoma 8 years after treatment: Report of a case with emphasis on the role of PET/CT in follow-up. Cancer Imaging 2010, 10, 117–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobs, A.H.; Kracht, L.W.; Gossmann, A.; Rüger, M.A.; Thomas, A.V.; Thiel, A.; Herholz, K. Imaging in neurooncology. NeuroRx 2005, 2, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, H.; Weng, D.; Ding, Y. Rapid recurrence and bilateral lungs, multiple bone metastasis of malignant solitary fibrous tumor of the right occipital lobe: Report of a case and review. Diagn. Pathol. 2015, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.; Lawhn-Heath, C.; Lopez, G.; Vella, M.; Aparici, C.M. Metastatic cervical paravertebral solitary fibrous tumor detected by fluorodeoxyglucose positron emission tomography-computed tomography. Radiol. Case Rep. 2018, 13, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, T.; Sakaguchi, T.; Shibasaki, Y.; Morita, Y.; Suzuki, A.; Inaba, K.; Tokuyama, T.; Baba, S.; Suzuki, S.; Konno, H. Pancreatic metastases of cerebellar hemangiopericytoma occurring 24 years after initial presentation: Report of a case. Surg. Today 2014, 44, 558–563. [Google Scholar] [CrossRef]
- Xiao, L.; Li, L. Bilateral renal metastasis from intracranial solitary fibrous tumor/hemangiopericytoma revealed on 18 F-FDG PET/CT and contrast-enhanced CT. Hell. J. Nucl. Med. 2021, 24, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Hayenga, H.N.; Bishop, A.J.; Wardak, Z.; Sen, C.; Mickey, B. Intraspinal Dissemination and Local Recurrence of an Intracranial Hemangiopericytoma. World Neurosurg. 2019, 123, 68–75. [Google Scholar] [CrossRef]
- Yasen, A.; Ran, B.; Jiang, T.; Maimaitinijiati, Y.; Zhang, R.; Guo, Q.; Shao, Y.; Aji, T.; Wen, H. Liver metastasis and local recurrence of meningeal hemangiopericytoma: A case report. Transl. Cancer Res. 2020, 9, 1278–1283. [Google Scholar] [CrossRef]
- Grünig, H.; Skawran, S.; Stolzmann, P.; Messerli, M.; Huellner, M.W. A Rare Case of Metastatic Solitary Fibrous Tumor (Hemangiopericytoma) of the Dura on 18F-FDG PET/CT. Clin. Nucl. Med. 2021, 46, 768–769. [Google Scholar] [CrossRef]
- Jehanno, N.; Cassou-Mounat, T.; Mammar, H.; Luporsi, M.; Huchet, V. 18F-Choline PET/CT Imaging for Intracranial Hemangiopericytoma Recurrence. Clin. Nucl. Med. 2019, 44, e305–e307. [Google Scholar] [CrossRef]
- Lavacchi, D.; Antonuzzo, L.; Briganti, V.; Berti, V.; Abenavoli, E.M.; Linguanti, F.; Messerini, L.; Giaccone, G. Metastatic intracranial solitary fibrous tumors/hemangiopericytomas: Description of two cases with radically different behaviors and review of the literature. Anti-Cancer Drugs 2020, 31, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Kota, G.; Gupta, P.; Lesser, G.J.; Wilson, J.A.; Mintz, A. Somatostatin receptor molecular imaging for metastatic intracranial hemangiopericytoma. Clin. Nucl. Med. 2013, 38, 984–987. [Google Scholar] [CrossRef]
- Hung, T.J.; MacDonald, W.; Muir, T.; Celliers, L.; Al-Ogaili, Z. 68Ga DOTATATE PET/CT of Non-FDG-Avid pulmonary metastatic hemangiopericytoma. Clin. Nucl. Med. 2016, 41, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Patro, K.C.; Palla, M.; Kashyap, R. Unusual Case of Metastatic Intracranial Hemangiopericytoma and Emphasis on Role of 68Ga-PSMA PET in Imaging. Clin. Nucl. Med. 2018, 43, e331–e333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Wu, Z.; Yao, S.; Miao, W. Intense [ 68 Ga] Ga-FAPI-04 uptake in solitary fibrous tumor/hemangiopericytoma of the central nervous system. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4103–4104. [Google Scholar] [CrossRef] [PubMed]
- Jong, I.; Chen, S.; Leung, Y.L.; Cheung, S.K.; Ho, C.-L. 11C-acetate PET/CT in a case of recurrent hemangiopericytoma. Clin. Nucl. Med. 2014, 39, 478–479. [Google Scholar] [CrossRef]
- Chan, W.S.W.; Zhang, J.; Khong, P.L. 18F-FDG-PET-CT imaging findings of recurrent intracranial haemangiopericytoma with distant metastases. Br. J. Radiol. 2010, 83, e172–e174. [Google Scholar] [CrossRef] [PubMed]
- Kracht, L.W.; Bauer, A.; Herholz, K.; Terstegge, K.; Friese, M.; Schröder, R.; Heiss, W.D. Positron emission tomography in a case of intracranial hemangiopericytoma. J. Comput. Assist. Tomogr. 1999, 23, 365–368. [Google Scholar] [CrossRef]
- Cho, Y.D.; Choi, G.H.; Lee, S.P.; Kim, J.K. 1H-MRS metabolic patterns for distinguishing between meningiomas and other brain tumors. Magn. Reson. Imaging 2003, 21, 663–672. [Google Scholar] [CrossRef]
- Mama, N.; Ben Abdallah, A.; Hasni, I.; Kadri, K.; Arifa, N.; Ladib, M.; Tlili-Graiess, K. MR imaging of intracranial hemangiopericytomas. J. Neuroradiol. 2014, 41, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yokoyama, J.; Yoshimoto, H.; Yazawa, M.; Kazuo, K.; Hanaguri, M.; Ohba, S.; Fujimaki, M.; Ikeda, K. Usefulness of Choline-PET for the detection of residual hemangiopericytoma in the skull base: Comparison with FDG-PET. Head Face Med. 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, V.; Di Stasio, G.D.; Evangelista, L.; Castoria, G.; Mansi, L. Biochemical and Pathophysiological Premises to Positron Emission Tomography with Choline Radiotracers. J. Cell. Physiol. 2017, 232, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Penet, M.F.; Jiang, L.; Jacobs, M.A.; Bhujwalla, Z.M. Choline metabolism-based molecular diagnosis of cancer: An update. Expert Rev. Mol. Diagn. 2015, 15, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Allen, D.D. The transport of choline. Drug Dev. Ind. Pharm. 2002, 28, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Quartuccio, N.; Arnone, A.; Kokomani, A.; Allocca, M.; Nappi, A.G.; Santo, G.; Mantarro, C.; Laudicella, R. Brain PET/CT using prostate cancer radiopharmaceutical agents in the evaluation of gliomas. Clin. Transl. Imaging 2020, 8, 433–448. [Google Scholar] [CrossRef]
- Khan, N.; Oriuchi, N.; Ninomiya, H.; Higuchi, T.; Kamada, H.; Endo, K. Positron emission tomographic imaging with 11C-choline in differential diagnosis of head and neck tumors: Comparison with 18F-FDG PET. Ann. Nucl. Med. 2004, 18, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Dunet, V.; Pomoni, A.; Hottinger, A.; Nicod-Lalonde, M.; Prior, J.O. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: Systematic review and meta-analysis. Neuro Oncol. 2016, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen van Zanten, S.E.M.; Bos, E.M.; Verburg, F.A.; van Doormaal, P.J. Intracranial hemangiopericytoma showing excellent uptake on arterial injection of [68 Ga] DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- Hammes, J.; Kobe, C.; Hilgenberg, U.; Lieb, W.E.; Drzezga, A. Orbital Hemangiopericytoma in 68Ga-Prostate-Specific Membrane Antigen-HBED-CC PET/CT. Clin. Nucl. Med. 2017, 42, 812–814. [Google Scholar] [CrossRef]
- Matsuda, M.; Ishikawa, E.; Yamamoto, T.; Hatano, K.; Joraku, A.; Iizumi, Y.; Masuda, Y.; Nishiyama, H.; Matsumura, A. Potential use of prostate specific membrane antigen (PSMA) for detecting the tumor neovasculature of brain tumors by PET imaging with 89 Zr-Df-IAB2M anti-PSMA minibody. J. Neurooncol. 2018, 138, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either 18 F-AlF or Cold-Kit 68 Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2021, 62, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Nanni, C.; Fanti, S. PET Tracers Beyond FDG in Prostate Cancer. Semin. Nucl. Med. 2016, 46, 507–521. [Google Scholar] [CrossRef] [PubMed]
| Case | Authors | Year | Age, Sex | Intracranial Primitive Site | Metastatic Sites | Radiopharmaceuticals | Qualitative and Semiquantitative Uptake Level |
|---|---|---|---|---|---|---|---|
| 1 | Z. Wu et al. [14] | 2015 | 25, M | Right occipital lobe | Lungs, bones | 18F-FDG | Mild–moderate SUVmean 4.9—SUVmax 8.1 |
| 2 | H. Cheung et al. [15] | 2018 | 67, F | Right posterior occipital calvary | Paravertebral, bones, lymph nodes | 18F-FDG | Mild * |
| 3 | K.P. Cheng et al. [8] | 2017 | 41, F | Intracranial meninges | Bones | 18F-FDG | Intense * |
| 4 | T. Hiraide et al. [16] | 2012 | 41, M | Cerebellum | Kidneys, lungs, pancreas | 18F-FDG | Intense * |
| 5 | X. Liu et al. [17] | 2021 | 40, M | Fronto-parietal | Kidney | 18F-FDG | Mild, SUVmax 3.17 |
| 6 | H. N. Hayenga et al. [18] | 2019 | 34, F | Right cerebellopontine angle | Thoracic spine | 18F-FDG | Low * |
| 7 | A. Yasen et al. [19] | 2020 | 62, F | Frontal cerebral convex, parafalx | Liver | 18F-FDG | Absent |
| 8 | H. Grunig et al. [20] | 2021 | 46, F | Intracranial dura | Liver, muscles | 18F-FDG | High–moderate SUVmax 9.0 |
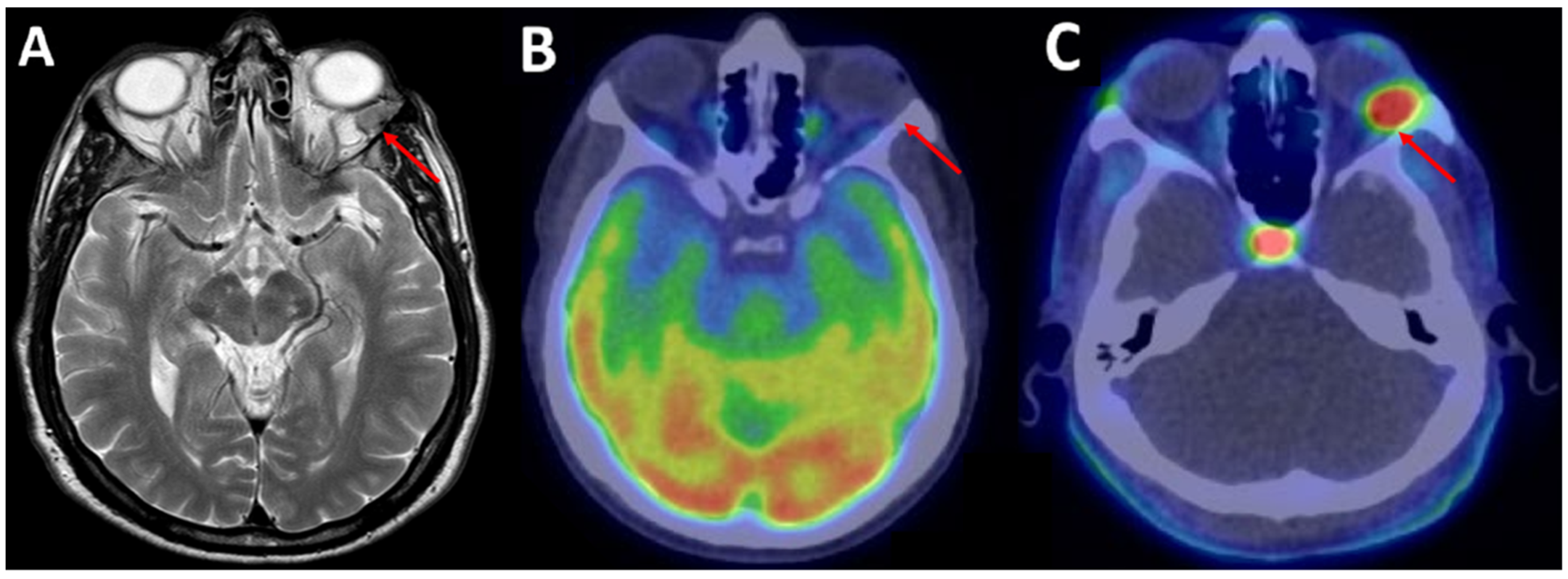
| 9 | Sardaro et al. [7] | 2021 | 69, M | Left orbit | / | 18F-FDG | Absent |
| 18F-FCH | Intense, SUVmax 6.8 | ||||||
| 10 | Jehanno et al. [21] | 2019 | 50, M | Right spheno-orbital region | / | 18F-FDG | Low, SUVmax 3.5 |
| 18F-FCH | Intense, SUVmax 5.9 | ||||||
| 11 | Lavacchi et al. [22] | 2020 | 64, F | Posterior fossa | Liver, kidneys, lungs | 111In-Pentreotide | Intense * |
| 35, M | Falx cerebri | Liver | 18F-FDG | Intense * | |||
| 12 | G. Kota et al. [23] | 2013 | 54, F | Right optic nerve sheath | Bones | 18F-FDG | Low * |
| 111In-Pentreotide | Intense * | ||||||
| 13 | T. Hung et al. [24] | 2016 | 68, F | Not specified | Lungs | 18F-FDG | Minimal * |
| 68GA-DOTATATE | Intense * | ||||||
| 14 | K.C. Patro et al. [25] | 2018 | 53, F | Right posterior cranial fossa | Bones, liver | 18F-FDG | Low * |
| 68Ga-PSMA | Intense * | ||||||
| 15 | Zhang et al. [26] | 2021 | 23, F | Right frontal lobe | / | 18F-FDG | Low, SUVmax 1.6 |
| 68GA-FAPI | Intense, SUVmax 30.9 | ||||||
| 16 | I. Jong et al. [27] | 2013 | 47, M | Not specified | Bones | 18F-FDG | Mild * |
| 11C-Acetate | Intense * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardaro, A.; Mammucci, P.; Pisani, A.R.; Rubini, D.; Nappi, A.G.; Bardoscia, L.; Rubini, G. Intracranial Solitary Fibrous Tumor: A “New” Challenge for PET Radiopharmaceuticals. J. Clin. Med. 2022, 11, 4746. https://doi.org/10.3390/jcm11164746
Sardaro A, Mammucci P, Pisani AR, Rubini D, Nappi AG, Bardoscia L, Rubini G. Intracranial Solitary Fibrous Tumor: A “New” Challenge for PET Radiopharmaceuticals. Journal of Clinical Medicine. 2022; 11(16):4746. https://doi.org/10.3390/jcm11164746
Chicago/Turabian StyleSardaro, Angela, Paolo Mammucci, Antonio Rosario Pisani, Dino Rubini, Anna Giulia Nappi, Lilia Bardoscia, and Giuseppe Rubini. 2022. "Intracranial Solitary Fibrous Tumor: A “New” Challenge for PET Radiopharmaceuticals" Journal of Clinical Medicine 11, no. 16: 4746. https://doi.org/10.3390/jcm11164746
APA StyleSardaro, A., Mammucci, P., Pisani, A. R., Rubini, D., Nappi, A. G., Bardoscia, L., & Rubini, G. (2022). Intracranial Solitary Fibrous Tumor: A “New” Challenge for PET Radiopharmaceuticals. Journal of Clinical Medicine, 11(16), 4746. https://doi.org/10.3390/jcm11164746






