A Proliferation-Inducing Ligand and B-Cell Activating Factor Are Upregulated in Patients with Essential Thrombocythemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Material Isolation
2.3. Immunoassay
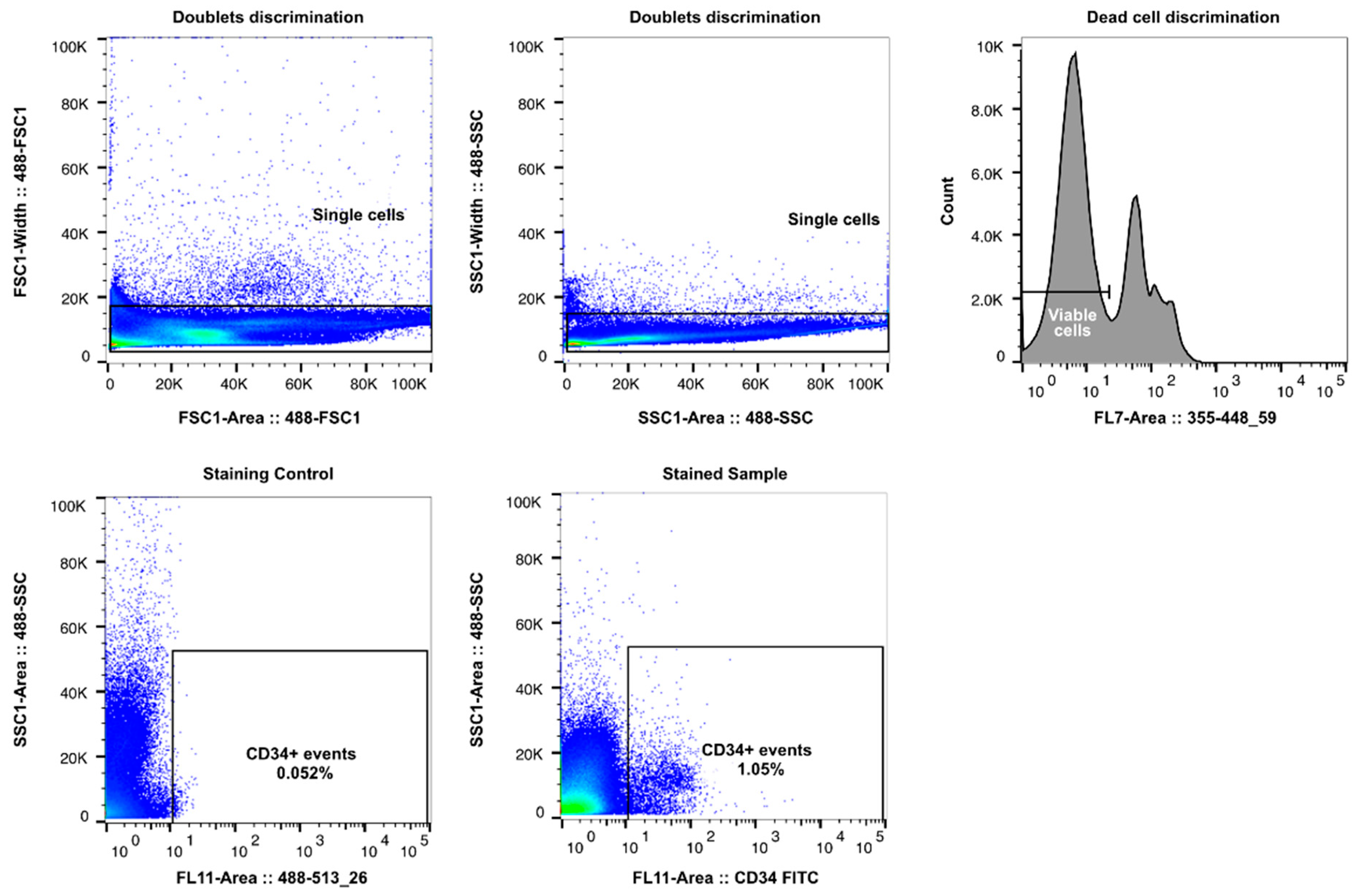
2.4. Cell Sorting
2.5. Hematopoietic Stem Cell Differentiation
2.6. qPCR
2.7. Statistics
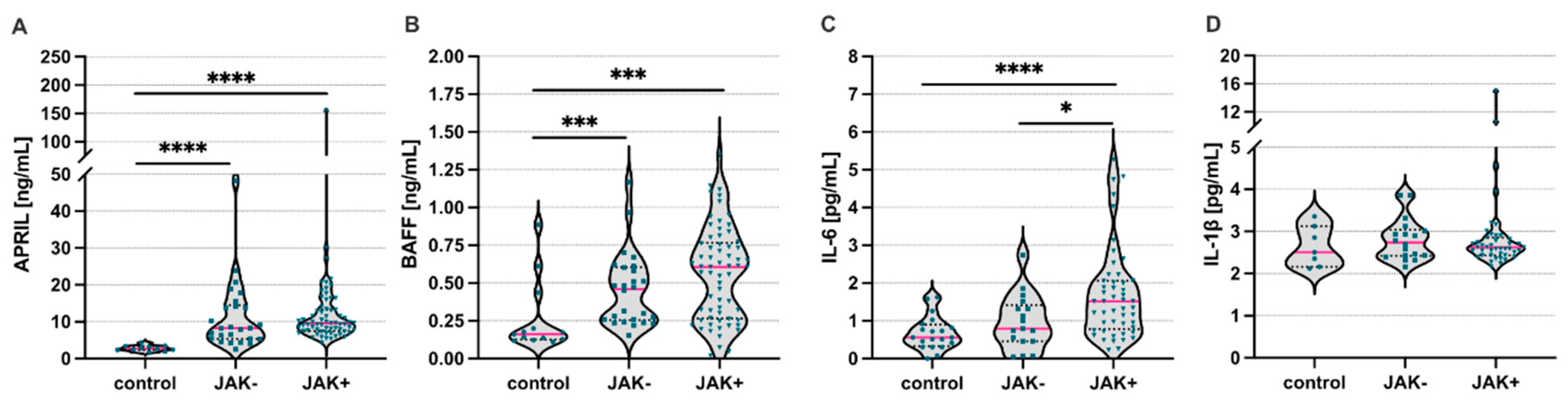
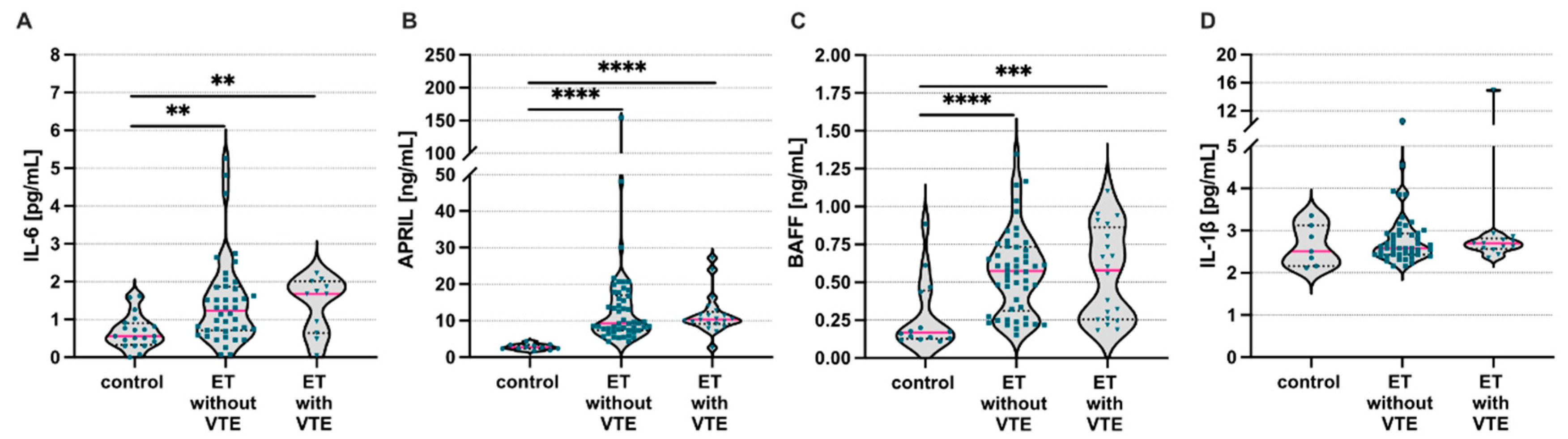
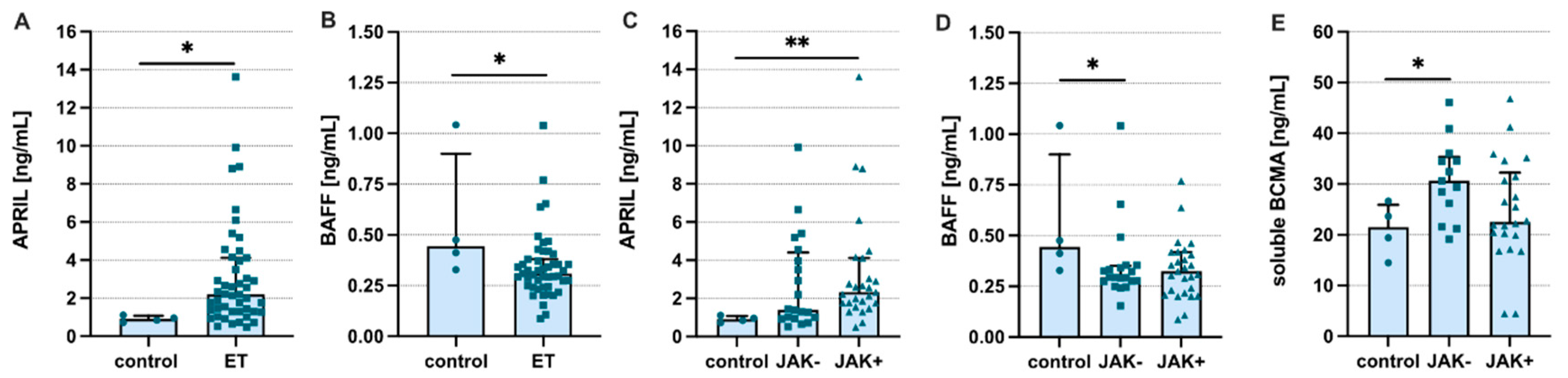
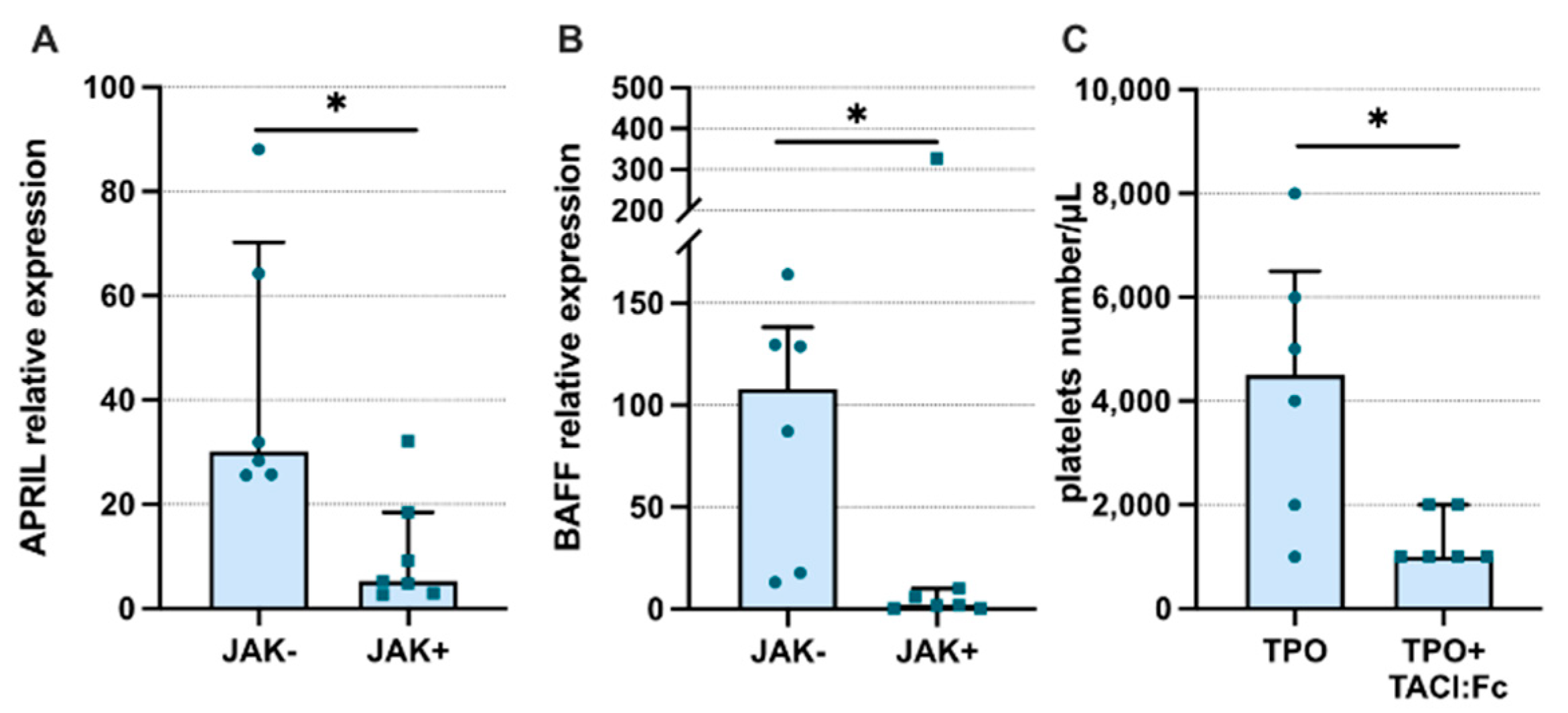
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, M.H.; Avraham, H.; Groopman, J.E. The regulation of megakaryocytopoiesis. Blood Rev. 1995, 9, 1–6. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, R.; Han, Z.; Zhou, B.; Liang, L.; Lu, M. TPO-independent megakaryocytopoiesis. Crit Rev. Oncol. Hematol. 2008, 65, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A. Molecular and transcriptional regulation of megakaryocyte differentiation. Stem Cells 2001, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Levine, R.; Tong, W.; Wernig, G.; Pikman, Y.; Zarnegar, S.; Gilliland, D.G.; Lodish, H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc. Natl. Acad. Sci. USA 2005, 102, 18962–18967. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Pistoia, V. Production of cytokines by human B cells in health and disease. Immunol. Today 1997, 18, 343–350. [Google Scholar] [CrossRef]
- De Maria, R.; Zeuner, A.; Eramo, A.; Domenichelli, C.; Bonci, D.; Grignani, F.; Srinivasula, S.M.; Alnemri, E.S.; Testa, U.; Peschle, C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 1999, 401, 489–493. [Google Scholar] [CrossRef]
- Bossen, C.; Ingold, K.; Tardivel, A.; Bodmer, J.L.; Gaide, O.; Hertig, S.; Ambrose, C.; Tschopp, J.; Schneider, P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 2006, 281, 13964–13971. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Veyrune, J.L.; De Vos, J.; Klein, B. APRIL is overexpressed in cancer: Link with tumor progression. BMC Cancer 2009, 9, 83. [Google Scholar] [CrossRef]
- Bolkun, L.; Lemancewicz, D.; Jablonska, E.; Szumowska, A.; Bolkun-Skornicka, U.; Ratajczak-Wrona, W.; Dzieciol, J.; Kloczko, J. The impact of TNF superfamily molecules on overall survival in acute myeloid leukaemia: Correlation with biological and clinical features. Ann. Hematol. 2015, 94, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Bolkun, L.; Grubczak, K.; Schneider, G.; Zembko, P.; Radzikowska, U.; Singh, P.; Kloczko, J.; Ratajczak, M.Z.; Moniuszko, M.; Eljaszewicz, A. Involvement of BAFF and APRIL in Resistance to Apoptosis of Acute Myeloid Leukemia. J. Cancer 2016, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Warakomska, M.; Tynecka, M.; Lemancewicz, D.; Grubczak, K.; Dzieciol, J.; Moniuszko, M.; Eljaszewicz, A.; Bolkun, L. The effects of BAFF and APRIL signaling on non-small cell lung cancer cell proliferation and invasiveness. Oncol. Lett. 2021, 22, 728. [Google Scholar] [CrossRef]
- Bonci, D.; Hahne, M.; Felli, N.; Peschle, C.; De Maria, R. Potential role of APRIL as autocrine growth factor for megakaryocytopoiesis. Blood 2004, 104, 3169–3172. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, D.; Croci, G.A.; Bucelli, C.; Tabano, S.; Cannone, M.G.; Gaudioso, G.; Barbanti, M.C.; Barbullushi, K.; Bianchi, P.; Fermo, E.; et al. Triple-Negative Essential Thrombocythemia: Clinical-Pathological and Molecular Features. A Single-Center Cohort Study. Front. Oncol. 2021, 11, 637116. [Google Scholar] [CrossRef]
- Guerriero, R.; Testa, U.; Gabbianelli, M.; Mattia, G.; Montesoro, E.; Macioce, G.; Pace, A.; Ziegler, B.; Hassan, H.J.; Peschle, C. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood 1995, 86, 3725–3736. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, C.J.; Powell, D.S.; Evans, M.J.; Thomas-Patterson, D.; Johnke, R.M. Enhanced platelet recovery in myelosuppressed mice treated with interleukin-1 and macrophage colony-stimulating factor: Potential interactions with cytokines having megakaryocyte colony-stimulating activity. J. Interferon Cytokine Res. 1996, 16, 187–194. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Chandrasekhar, C. Hematopoietic factor-induced synthesis of von Willebrand factor by the Dami human megakaryoblastic cell line and by normal human megakaryocytes. Exp. Hematol. 1991, 19, 53–58. [Google Scholar]
- Dechkajorn, W.; Benjathummarak, S.; Kumsiri, R.; Maneerat, Y. The role of the BAFF/APRIL system in the T cell-independent specific response to blood stage Plasmodium falciparum hemozoin. Cytokine 2018, 111, 445–453. [Google Scholar] [CrossRef]
- MacLennan, I.; Vinuesa, C. Dendritic cells, BAFF, and APRIL: Innate players in adaptive antibody responses. Immunity 2002, 17, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Shurin, M.R.; Ma, Y.; Keskinov, A.A.; Zhao, R.; Lokshin, A.; Agassandian, M.; Shurin, G.V. BAFF and APRIL from Activin A-Treated Dendritic Cells Upregulate the Antitumor Efficacy of Dendritic Cells In Vivo. Cancer Res. 2016, 76, 4959–4969. [Google Scholar] [CrossRef] [Green Version]
- Parsa, R.; Lund, H.; Georgoudaki, A.M.; Zhang, X.M.; Ortlieb Guerreiro-Cacais, A.; Grommisch, D.; Warnecke, A.; Croxford, A.L.; Jagodic, M.; Becher, B.; et al. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J. Exp. Med. 2016, 213, 1537–1553. [Google Scholar] [CrossRef] [Green Version]
- Schwaller, J.; Schneider, P.; Mhawech-Fauceglia, P.; McKee, T.; Myit, S.; Matthes, T.; Tschopp, J.; Donze, O.; Le Gal, F.A.; Huard, B. Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B-cell lymphoma aggressiveness. Blood 2007, 109, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Barbui, T.; Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012, 120, 5128–5133; quiz 5252. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.G.; Ng, C.H.; Woehl, B.; Sutherland, A.P.; Huo, J.; Xu, S.; Mackay, F.; Lam, K.P. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur. J. Immunol. 2006, 36, 1837–1846. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, S.C.; Kao, C.W.; Hsieh, R.K.; Chang, M.C.; Chang, Y.F.; Lim, K.H.; Chen, C.G. B cells facilitate platelet production mediated by cytokines in patients with essential thrombocythaemia. Thromb. Haemost. 2014, 112, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Chuen, C.K.; Li, K.; Yang, M.; Fok, T.F.; Li, C.K.; Chui, C.M.; Yuen, P.M. Interleukin-1beta up-regulates the expression of thrombopoietin and transcription factors c-Jun, c-Fos, GATA-1, and NF-E2 in megakaryocytic cells. J. Lab. Clin. Med. 2004, 143, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Gründer, A.; Hadlich, T.; Wehrle, J.; Gothwal, M.; Bogeska, R.; Seeger, T.S.; Kayser, S.; Pham, K.B.; Jutzi, J.S.; et al. A novel murine model of myeloproliferative disorders generated by overexpression of the transcription factor NF-E2. J. Exp. Med. 2012, 209, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.; Marchetti, M.; Vignoli, A.; Balducci, D.; Barbui, T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp. Hematol. 2005, 33, 523–530. [Google Scholar] [CrossRef]
- Landolfi, R.; Di Gennaro, L. Pathophysiology of thrombosis in myeloproliferative neoplasms. Haematologica 2011, 96, 183–186. [Google Scholar] [CrossRef]
- Hahne, M.; Kataoka, T.; Schröter, M.; Hofmann, K.; Irmler, M.; Bodmer, J.L.; Schneider, P.; Bornand, T.; Holler, N.; French, L.E.; et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 1998, 188, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Ware, C.F. APRIL and BAFF connect autoimmunity and cancer. J. Exp. Med. 2000, 192, F35–F38. [Google Scholar] [CrossRef]
- Rennert, P.; Schneider, P.; Cachero, T.G.; Thompson, J.; Trabach, L.; Hertig, S.; Holler, N.; Qian, F.; Mullen, C.; Strauch, K.; et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 2000, 192, 1677–1684. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, J.; Planelles, L.; de Jong-Odding, J.; Hardenberg, G.; Pals, S.T.; Hahne, M.; Spaargaren, M.; Medema, J.P. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005, 12, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Ingold, K.; Zumsteg, A.; Tardivel, A.; Huard, B.; Steiner, Q.G.; Cachero, T.G.; Qiang, F.; Gorelik, L.; Kalled, S.L.; Acha-Orbea, H.; et al. Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 2005, 201, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Couchman, J.R. Syndecans: Proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell. Biol. 2003, 4, 926–937. [Google Scholar] [CrossRef]
- Filmus, J. Glypicans in growth control and cancer. Glycobiology 2001, 11, 19R–23R. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.F.; Lin, L.; Xing, L.; Li, Y.; Yu, T.; Anderson, K.C.; Tai, Y.T. BCMA-Targeting Therapy: Driving a New Era of Immunotherapy in Multiple Myeloma. Cancers 2020, 12, 1473. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
| Parameter | All Donors | ET Patients | ||||
|---|---|---|---|---|---|---|
| ET (n = 109) | Control (n = 40) | p | JAK2+ (n = 75) | JAK2- (n = 34) | p | |
| age (min–max) | 59 (23–87) | 58 (23–85) | p = 0.9 | 58 (23–84) | 59 (25–87) | p = 0.88 |
| female/male | 75/39 | 25/15 | p = 0.9 | 52/27 | 27/12 | p = 0.79 |
| RBC (×106/µL) | 4.85 (4.4–5.17) | 4.12 (3.9–4.45) | p = 0.09 | 4.97 (4.6–5.29) | 4.725 (4.14–5.07) | p = 0.22 |
| HGB (g/dL) | 14.4 (13.1–15.3) | 14.1 (12.3–15.4) | p = 0.2 | 14.7 (13.2–15.5) | 13.8 (12.8–14.9) | p = 0.1 |
| HCT (%) | 43.5 (39.45–47) | 42 (39.1–45.1) | p = 0.09 | 44.1 (39.9–47.9) | 40.8 (37.7–45.0) | p = 0.02 |
| WBC (×103/µL) | 9.71 (7.9–11.83) | 7.12 (5.1–9.2) | p = 0.01 | 10.32 (8.3–12.53) | 8.9 (6.46–10.29) | p = 0.002 |
| PLT (×103/µL)) | 828 (678.5–994) | 255 (166–340) | p < 0.01 | 876 (712–1072) | 764.5 (610–941.3) | p = 0.06 |
| history of thrombosis (patients) | n = 20 | - | - | n = 13 | n = 7 | - |
| risk stratification for thrombosis (IPSET-t) | ||||||
| low | n = 44 | - | - | n = 22 | n = 20 | - |
| intermediate | n = 53 | - | - | n = 30 | n = 23 | - |
| high | n = 17 | - | - | n = 10 | n = 7 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolkun, L.; Tynecka, M.; Wasiluk, T.; Piszcz, J.; Starosz, A.; Grubczak, K.; Moniuszko, M.; Eljaszewicz, A. A Proliferation-Inducing Ligand and B-Cell Activating Factor Are Upregulated in Patients with Essential Thrombocythemia. J. Clin. Med. 2022, 11, 4663. https://doi.org/10.3390/jcm11164663
Bolkun L, Tynecka M, Wasiluk T, Piszcz J, Starosz A, Grubczak K, Moniuszko M, Eljaszewicz A. A Proliferation-Inducing Ligand and B-Cell Activating Factor Are Upregulated in Patients with Essential Thrombocythemia. Journal of Clinical Medicine. 2022; 11(16):4663. https://doi.org/10.3390/jcm11164663
Chicago/Turabian StyleBolkun, Lukasz, Marlena Tynecka, Tomasz Wasiluk, Jaroslaw Piszcz, Aleksandra Starosz, Kamil Grubczak, Marcin Moniuszko, and Andrzej Eljaszewicz. 2022. "A Proliferation-Inducing Ligand and B-Cell Activating Factor Are Upregulated in Patients with Essential Thrombocythemia" Journal of Clinical Medicine 11, no. 16: 4663. https://doi.org/10.3390/jcm11164663
APA StyleBolkun, L., Tynecka, M., Wasiluk, T., Piszcz, J., Starosz, A., Grubczak, K., Moniuszko, M., & Eljaszewicz, A. (2022). A Proliferation-Inducing Ligand and B-Cell Activating Factor Are Upregulated in Patients with Essential Thrombocythemia. Journal of Clinical Medicine, 11(16), 4663. https://doi.org/10.3390/jcm11164663






