Transcriptomic Analysis of Human Keratinocytes Treated with Galactomyces Ferment Filtrate, a Beneficial Cosmetic Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Treatment and Sample Preparation for Microarray Analysis
2.2. Statistical Analysis of Microarray Data
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukahara, K.; Sugata, K.; Osanai, O.; Ohuchi, A.; Miyauchi, Y.; Takizawa, M.; Hotta, M.; Kitahara, T. Comparison of age-related changes in facial wrinkles and sagging in the skin of Japanese, Chinese and Thai women. J. Dermatol. Sci. 2007, 47, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily fluctuation of facial pore area, roughness and redness among young Japanese women; Beneficial effects of Galactomyces ferment filtrate containing antioxidative skin care formula. J. Clin. Med. 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Inoue, Y.; Hsueh, K.; Liang, Z.; Yan, X.; Yoshii, T.; Furue, M. Characterization of comprehensive appearances of skin ageing: An 11-year longitudinal study on facial skin ageing in Japanese females at Akita. J. Dermatol. Sci. 2011, 64, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Munakata, Y.; Yan, X.; Tsuji, G.; Furue, M. Enhanced fluctuations in facial pore size, redness, and TEWL caused by mask usage are normalized by the application of a moisturizer. J. Clin. Med. 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015, 40, 786–793. [Google Scholar] [CrossRef]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef]
- Tsai, H.H.; Chen, Y.C.; Lee, W.R.; Hu, C.H.; Hakozaki, T.; Yoshii, T.; Shen, S.C. Inhibition of inflammatory nitric oxide production and epidermis damages by Saccharomycopsis ferment filtrate. J. Dermatol. Sci. 2006, 42, 249–257. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidants cinnamaldehyde and Galactomyces fermentation filtrate downregulate senescence marker CDKN2A/p16INK4A via NRF2 activation in keratinocytes. J. Dermatol. Sci. 2019, 96, 53–56. [Google Scholar] [CrossRef]
- Cooper, J.K.W.; Koshoffer, A.; Kadekaro, A.L.; Hakozaki, T.; Boissy, R. Galactomyces ferment filtrate suppresses reactive oxygen species generation and promotes cellular redox balance in human melanocytes via Nrf2-ARE pathway. J. Clin. Cosmet. Dermatol. 2019, 3. [Google Scholar] [CrossRef]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Aono, S.; Hirai, Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp. Cell Res. 2008, 314, 3326–3339. [Google Scholar] [CrossRef]
- Olsen, E.; Rasmussen, H.H.; Celis, J.E. Identification of proteins that are abnormally regulated in differentiated cultured human keratinocytes. Electrophoresis 1995, 16, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Farwanah, H.; Willrodt, A.H.; Huebner, A.J.; Sandhoff, K.; Roop, D.; Hohl, D.; Bloch, W.; Werner, S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012, 4, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. 2016, 13, 1. [Google Scholar] [CrossRef]
- Hasegawa, T.; Feng, Z.; Yan, Z.; Ngo, K.H.; Hosoi, J.; Demehri, S. Reduction in human epidermal Langerhans cells with age is associated with decline in CXCL14-mediated recruitment of CD14+ monocytes. J. Cosmet. Dermatol. Sci. Appl. 2020, 140, 1327–1334. [Google Scholar] [CrossRef]
- Wood, L.C.; Stalder, A.K.; Liou, A.; Campbell, I.L.; Grunfeld, C.; Elias, P.M.; Feingold, K.R. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp. Dermatol. 1997, 6, 98–104. [Google Scholar] [CrossRef]
- Terunuma, A.; Aiba, S.; Tagami, H. Cytokine mRNA profiles in cultured human skin component cells exposed to various chemicals: A simulation model of epicutaneous stimuli induced by skin barrier perturbation in comparison with that due to exposure to haptens or irritant. J. Dermatol. Sci. 2001, 26, 85–93. [Google Scholar] [CrossRef]
- Engelhart, K.; El Hindi, T.; Biesalski, H.K.; Pfitzner, I. In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch. Dermatol. Res. 2005, 297, 1–9. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Use of telomerase to create bioengineered tissues. Ann. NY Acad. Sci. 2005, 1057, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Card, P.D.; Chen, J.; Li, L.; Laughlin, T.; Jarrold, B.; Zhao, W.; Benham, A.M.; Määttä, A.T.; Hawkins, T.J.; et al. A potential role of keratinocyte-derived bilirubin in human skin yellowness and its amelioration by sucrose laurate/dilaurate. Int. J. Mol. Sci. 2022, 23, 5884. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.R.; Hakozaki, T.; Yoshii, T.; Chen, T.Y.; Pang, J.H.S. Up-regulation of tight junction-related proteins and increase of human epidermal keratinocytes barrier function by Saccharomycosis ferment filtrate. J. Cosm. Dermatol. Sci. Appl. 2011, 1, 15–24. [Google Scholar]
- Purba, T.S.; Ng’andu, K.; Brunken, L.; Smart, E.; Mitchell, E.; Hassan, N.; O’Brien, A.; Mellor, C.; Jackson, J.; Shahmalak, A.; et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol. Med. 2019, 11, e11031. [Google Scholar] [CrossRef]
- Bygum, A.; Fagerberg, C.R.; Clemmensen, O.J.; Fiebig, B.; Hafner, C. Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med. Genet. 2011, 12, 79. [Google Scholar] [CrossRef]
- Duperret, E.K.; Oh, S.J.; McNeal, A.; Prouty, S.M.; Ridky, T.W. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle 2014, 13, 1551–1559. [Google Scholar] [CrossRef]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef]
- Hashimoto-Hachiya, A.; Tsuji, G.; Murai, M.; Yan, X.; Furue, M. Upregulation of FLG, LOR, and IVL Expression by Rhodiola crenulata Root Extract via Aryl Hydrocarbon Receptor: Differential Involvement of OVOL1. Int. J. Mol. Sci. 2018, 19, 1654. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef]
- Rusni, S.; Sassa, M.; Takagi, T.; Kinoshita, M.; Takehana, Y.; Inoue, K. Establishment of cytochrome P450 1a gene-knockout Javanese medaka, Oryzias javanicus, which distinguishes toxicity modes of the polycyclic aromatic hydrocarbons, pyrene and phenanthrene. Mar. Pollut. Bull. 2022, 178, 113578. [Google Scholar] [CrossRef] [PubMed]
- Christmann, C.; Zenker, S.; Martens, L.; Hübner, J.; Loser, K.; Vogl, T.; Roth, J. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front. Immunol. 2021, 11, 599947. [Google Scholar] [CrossRef] [PubMed]
- Skeate, J.G.; Porras, T.B.; Woodham, A.W.; Jang, J.K.; Taylor, J.R.; Brand, H.E.; Kelly, T.J.; Jung, J.U.; Da Silva, D.M.; Yuan, W.; et al. Herpes simplex virus downregulation of secretory leukocyte protease inhibitor enhances human papillomavirus type 16 infection. J. Gen. Virol. 2016, 97, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.C.; Piña-Paz, S.; Rotty, J.D.; Hickerson, R.P.; Kaspar, R.L.; Balmain, A.; Coulombe, P.A. Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc. Natl. Acad. Sci. USA 2013, 110, 19537–19542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, S.T.; Chang, S.H.; Chuang, K.C.; Wang, H.Y.; Kao, J.K.; Liang, S.M.; Wu, C.Y.; Kao, S.H.; Chen, Y.J.; et al. Imiquimod exerts antitumor effects by inducing immunogenic cell death and is enhanced by the glycolytic inhibitor 2-deoxyglucose. J. Investig. Dermatol. 2020, 140, 1771–1783. [Google Scholar] [CrossRef]
- Feng, M.; Marjon, K.D.; Zhu, F.; Weissman-Tsukamoto, R.; Levett, A.; Sullivan, K.; Kao, K.S.; Markovic, M.; Bump, P.A.; Jackson, H.M.; et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat. Commun. 2018, 9, 3194. [Google Scholar] [CrossRef]
- Krysko, D.V.; Ravichandran, K.S.; Vandenabeele, P. Macrophages regulate the clearance of living cells by calreticulin. Nat. Commun. 2018, 9, 4644. [Google Scholar] [CrossRef]
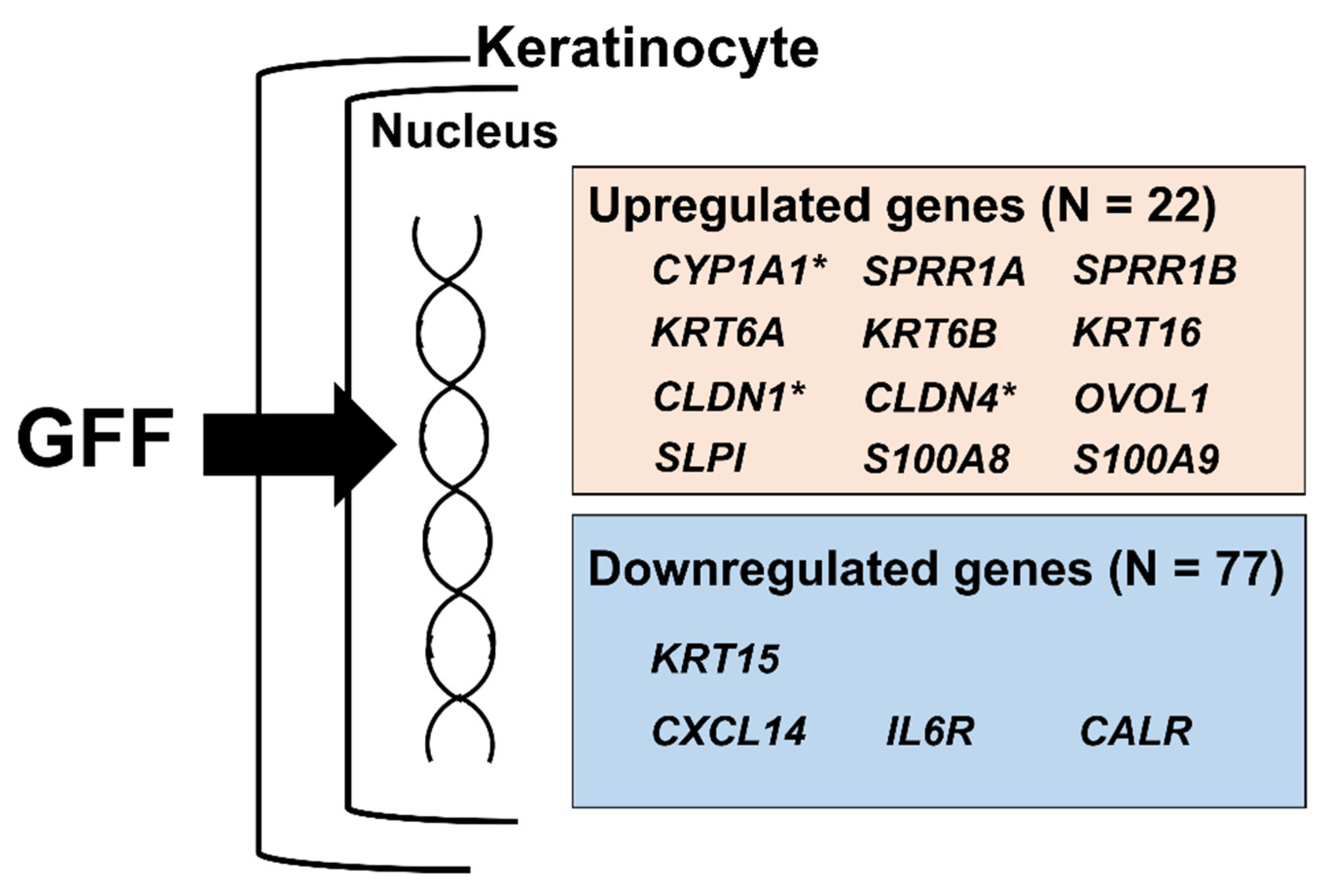
| Gene | GFF Lot | Gene | GFF Lot | ||||
|---|---|---|---|---|---|---|---|
| Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | ||
| KRT13 | 2.357 * | 2.488 | 2.351 | KRT6A | 1.684 | 1.842 | 1.693 |
| PI3 | 2.310 | 2.439 | 2.224 | CLDN1 | 1.717 | 1.659 | 1.619 |
| SPRR1B | 2.247 | 2.382 | 2.063 | GAL | 1.600 | 1.702 | 1.669 |
| KRT6B | 2.123 | 2.152 | 2.120 | SERPINB7 | 1.645 | 1.656 | 1.667 |
| UPK1B | 1.957 | 2.082 | 1.955 | KRTAP2 | 1.664 | 1.555 | 1.736 |
| SERPINB2 | 1.920 | 1.962 | 1.962 | SPRR1A | 1.525 | 1.814 | 1.601 |
| PLK2 | 1.981 | 1.915 | 1.847 | GPRC5A | 1.763 | 1.590 | 1.582 |
| CTSC | 1.920 | 1.936 | 1.729 | MAL2 | 1.646 | 1.709 | 1.552 |
| KRT16 | 1.736 | 1.904 | 1.709 | ZNF750 | 1.542 | 1.740 | 1.602 |
| CYP1A1 | 1.525 | 2.208 | 1.597 | CLDN4 | 1.639 | 1.627 | 1.517 |
| SERPINB1 | 1.791 | 1.753 | 1.697 | SLPI | 1.545 | 1.572 | 1.520 |
| Gene | GFF Lot | Gene | GFF Lot | ||||
|---|---|---|---|---|---|---|---|
| Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | ||
| CXCL14 | 0.244 * | 0.257 | 0.302 | TSC22D3 | 0.628 | 0.703 | 0.667 |
| HERPUD1 | 0.444 | 0.416 | 0.447 | ISG15 | 0.644 | 0.664 | 0.696 |
| LTB | 0.460 | 0.402 | 0.460 | MIR4680 | 0.659 | 0.675 | 0.671 |
| HSPA5 | 0.481 | 0.456 | 0.504 | FILIP1L | 0.669 | 0.658 | 0.685 |
| KRT15 | 0.507 | 0.471 | 0.545 | PRKCDBP | 0.685 | 0.642 | 0.694 |
| CALR | 0.556 | 0.497 | 0.528 | RAB7B | 0.656 | 0.686 | 0.682 |
| GLUL | 0.501 | 0.544 | 0.559 | PDIA3 | 0.669 | 0.684 | 0.684 |
| LGALS7 | 0.494 | 0.559 | 0.562 | WNT10A | 0.657 | 0.679 | 0.703 |
| MANF | 0.548 | 0.552 | 0.561 | ZBTB16 | 0.645 | 0.674 | 0.724 |
| VAV3 | 0.539 | 0.555 | 0.570 | DST | 0.698 | 0.653 | 0.696 |
| CRELD2 | 0.559 | 0.546 | 0.559 | IMPA2 | 0.653 | 0.698 | 0.699 |
| PDIA4 | 0.549 | 0.555 | 0.563 | TNS3 | 0.663 | 0.667 | 0.720 |
| IFIT1 | 0.556 | 0.579 | 0.577 | P4HB | 0.667 | 0.686 | 0.698 |
| AHNAK2 | 0.568 | 0.562 | 0.612 | DDX60 | 0.696 | 0.670 | 0.686 |
| IFITM1 | 0.565 | 0.581 | 0.600 | ST6GALNAC2 | 0.684 | 0.698 | 0.681 |
| HSP90B1 | 0.593 | 0.571 | 0.590 | HEG1 | 0.691 | 0.683 | 0.708 |
| NUCB2 | 0.581 | 0.564 | 0.610 | MICALL2 | 0.685 | 0.716 | 0.682 |
| PNRC1 | 0.578 | 0.574 | 0.612 | IFIT2 | 0.708 | 0.699 | 0.685 |
| SDF2L1 | 0.593 | 0.578 | 0.595 | IFI44 | 0.682 | 0.714 | 0.698 |
| SULF2 | 0.553 | 0.589 | 0.631 | ITGB8 | 0.675 | 0.705 | 0.723 |
| FLRT2 | 0.599 | 0.578 | 0.605 | SOX6 | 0.683 | 0.690 | 0.741 |
| FGFR3 | 0.563 | 0.628 | 0.636 | PIK3R1 | 0.683 | 0.702 | 0.742 |
| COL7A1 | 0.596 | 0.615 | 0.633 | CLCA2 | 0.710 | 0.703 | 0.731 |
| METTL7A | 0.582 | 0.625 | 0.644 | SMIM14 | 0.692 | 0.746 | 0.714 |
| PRSS23 | 0.602 | 0.594 | 0.665 | C1R | 0.718 | 0.723 | 0.714 |
| ASS1 | 0.620 | 0.605 | 0.638 | DNAJB9 | 0.737 | 0.706 | 0.713 |
| HYOU1 | 0.612 | 0.616 | 0.646 | LGALS1 | 0.700 | 0.716 | 0.741 |
| DLL1 | 0.648 | 0.622 | 0.648 | SEL1L | 0.733 | 0.705 | 0.722 |
| GJB2 | 0.616 | 0.625 | 0.683 | PBX1 | 0.701 | 0.727 | 0.742 |
| TNNI2 | 0.556 | 0.660 | 0.717 | PSMB9 | 0.693 | 0.744 | 0.745 |
| PDIA6 | 0.642 | 0.645 | 0.651 | PPIB | 0.729 | 0.713 | 0.742 |
| HTRA1 | 0.622 | 0.663 | 0.655 | IL6R | 0.725 | 0.725 | 0.741 |
| SYT8 | 0.605 | 0.665 | 0.684 | ETS2 | 0.719 | 0.742 | 0.731 |
| DLK2 | 0.655 | 0.629 | 0.672 | LFNG | 0.726 | 0.724 | 0.745 |
| CDK2AP2 | 0.651 | 0.656 | 0.652 | DHX58 | 0.723 | 0.734 | 0.744 |
| ACKR3 | 0.635 | 0.622 | 0.705 | OLFML2A | 0.743 | 0.723 | 0.736 |
| TGFBI | 0.578 | 0.700 | 0.689 | TMEM50B | 0.749 | 0.722 | 0.738 |
| IRF9 | 0.652 | 0.652 | 0.673 | AGR2 | 0.748 | 0.744 | 0.734 |
| TNFRSF21 | 0.613 | 0.693 | 0.690 | ||||
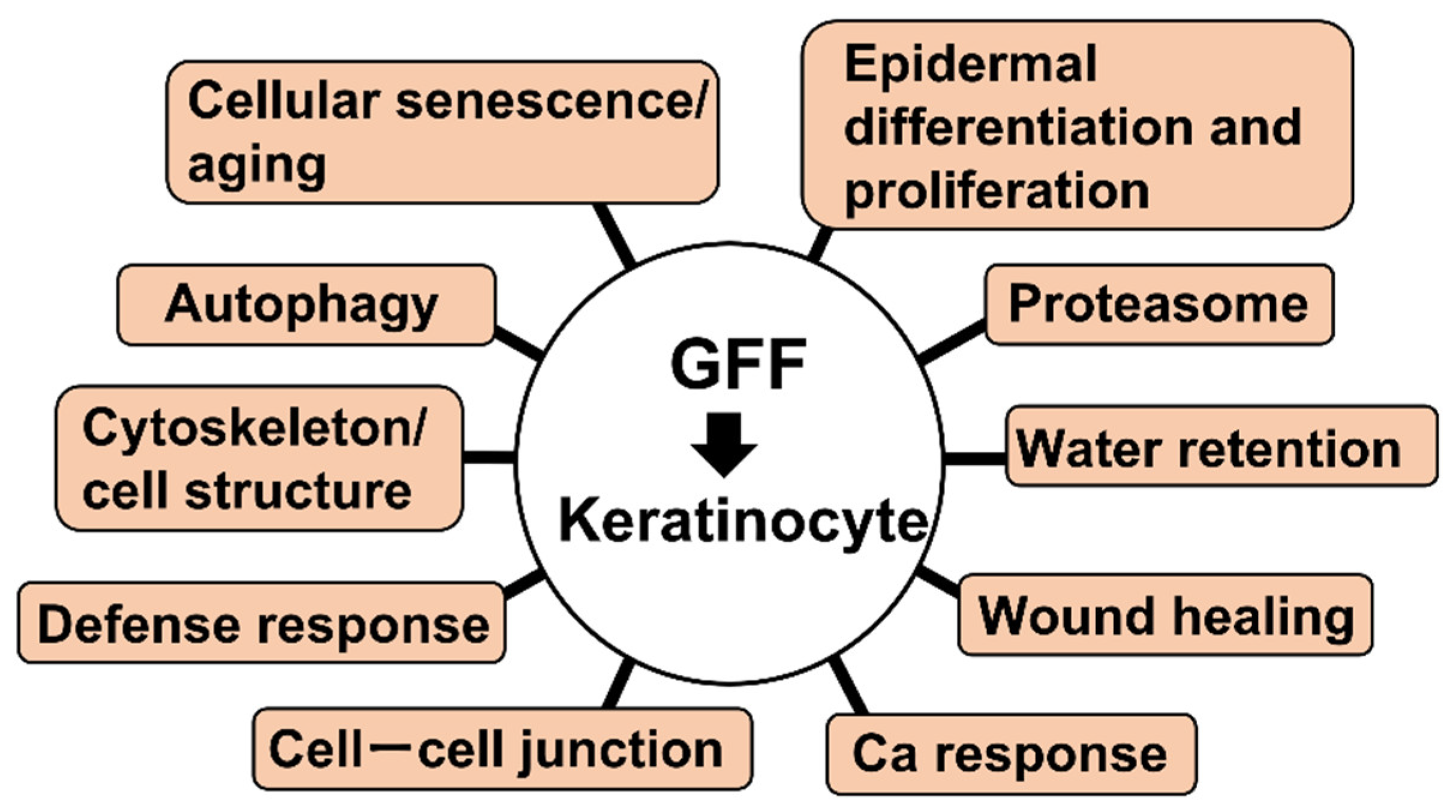
| Group | Term | Gene | tKC RNA Expression |
|---|---|---|---|
| Cellular senescence/aging | GO:0090342 regulation of cell aging | PLK2 | Up |
| GO:0090398 cellular senescence GO:0007569 cell aging | PLK2 | Up | |
| CALR | Down | ||
| Autophagy | GO:0006914 autophagy GO:0010508 positive regulation of autophagy | PLK2 | Up |
| Cytoskeleton/cell structure | GO:0000226 microtubule cytoskeleton organization | PLK2 | Up |
| CDK2AP2, DST | Down | ||
| GO:0007015 actin filament organization | HSP90B1, MICALL2, PIK3R1 | Down | |
| GO:0007018 microtubule-based movement GO:0045104 intermediate filament cytoskeleton organization | DST | Down | |
| Defense response | GO:0031349 positive regulation of defense response | CTSC | Up |
| DDX60, DHX58, PSMB9 | Down | ||
| Cell–cell junction | GO:0007043 cell–cell junction assembly | CLDN1 | Up |
| GJB2, HEG1, MICALL2 | Down | ||
| GO:0007044 cell–substrate junction assembly | DST | Down | |
| GO:0120192 tight junction assembly | CLDN1 | Up | |
| MICALL2 | Down | ||
| Proteasome | GO:0010498 proteasomal protein catabolic process | PLK2 | Up |
| DNAJB9, HERPUD1, HSP90B1, HSPA5, PSMB9, SDF2L1, SEL1L | Down | ||
| GO:0043161 proteasome-mediated ubiquitin-dependent protein catabolic process | PLK2 | Up | |
| DNAJB9, HERPUD1, HSP90B1, HSPA5, PSMB9, SEL1L | Down | ||
| Epidermal differentiation and proliferation | GO:0008544 epidermis development | GAL, KRT6A, KRT6B, KRT13, PI3, SPRR1A, SPRR1B, ZNF750 | Up |
| COL7A1, DLL1, KRT15, WNT10A | Down | ||
| GO:0009913 epidermal cell differentiation | KRT6A, KRT6B, KRT13, PI3, SPRR1A, SPRR1B | Up | |
| DLL1, KRT15 | Down | ||
| GO:0030216 keratinocyte differentiation GO:0031424 keratinization | KRT6A, KRT6B, KRT13, PI3, SPRR1A, SPRR1B | Up | |
| KRT15 | Down | ||
| GO:0033561 regulation of water loss via skin GO:0061436 establishment of skin barrier | CLDN1, CLDN4 | Up | |
| GO:0070268 cornification | KRT6A, KRT6B, KRT13, PI3, SPRR1A, SPRR1B | Up | |
| KRT15 | Down | ||
| Water retention | GO:0030104 water homeostasis | CLDN1, CLDN4 | Up |
| Wound healing | GO:0061041 regulation of wound healing | SERPINB2 | Up |
| Ca response | GO:0071277 cellular response to calcium ion | HSPA5, SYT8 | Down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, A.; Sakae, N.; Yan, X.; Hakozaki, T.; Zhao, W.; Laughlin, T.; Furue, M. Transcriptomic Analysis of Human Keratinocytes Treated with Galactomyces Ferment Filtrate, a Beneficial Cosmetic Ingredient. J. Clin. Med. 2022, 11, 4645. https://doi.org/10.3390/jcm11164645
Nakajima A, Sakae N, Yan X, Hakozaki T, Zhao W, Laughlin T, Furue M. Transcriptomic Analysis of Human Keratinocytes Treated with Galactomyces Ferment Filtrate, a Beneficial Cosmetic Ingredient. Journal of Clinical Medicine. 2022; 11(16):4645. https://doi.org/10.3390/jcm11164645
Chicago/Turabian StyleNakajima, Akiko, Nahoko Sakae, Xianghong Yan, Tomohiro Hakozaki, Wenzhu Zhao, Timothy Laughlin, and Masutaka Furue. 2022. "Transcriptomic Analysis of Human Keratinocytes Treated with Galactomyces Ferment Filtrate, a Beneficial Cosmetic Ingredient" Journal of Clinical Medicine 11, no. 16: 4645. https://doi.org/10.3390/jcm11164645
APA StyleNakajima, A., Sakae, N., Yan, X., Hakozaki, T., Zhao, W., Laughlin, T., & Furue, M. (2022). Transcriptomic Analysis of Human Keratinocytes Treated with Galactomyces Ferment Filtrate, a Beneficial Cosmetic Ingredient. Journal of Clinical Medicine, 11(16), 4645. https://doi.org/10.3390/jcm11164645






