A Case of 123I-Metaiodobenzylguanidine Scintigraphy-Negative Pheochromocytoma with a Tumor-Developing Mutation in the RET Gene
Abstract
1. Introduction
2. Case Presentation
3. Materials and Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yonamine, M.; Wasano, K.; Aita, Y.; Sugasawa, T.; Takahashi, K.; Kawakami, Y.; Shimano, H.; Nishiyama, H.; Hara, H.; Naruse, M.; et al. Prevalence of germline variants in a large cohort of Japanese patients with pheochromocytoma and/or paraganglioma. Cancers 2021, 13, 4014. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Takekoshi, K.; Horii, A.; Morimoto, R.; Imai, T.; Oki, Y.; Saito, T.; Midorikawa, S.; Arao, T.; Sugisawa, C.; et al. Clinicopathological study of SDHB mutation-related pheochromocytoma and sympathetic paraganglioma. Endocr. Relat. Cancer 2014, 21, L13–L16. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Gao, X.; Pecori, A.; Nakamura, Y.; Tezuka, Y.; Omata, K.; Ono, Y.; Morimoto, R.; Satoh, F.; Sasano, H. Recent advances in histopathological and molecular diagnosis in pheochromocytoma and paraganglioma: Challenges for predicting metastasis in individual patients. Front. Endocrinol. 2020, 11, 587769. [Google Scholar] [CrossRef]
- Brandi, M.L.; Gagel, R.F.; Angeli, A.; Bilezikian, J.P.; Beck-Peccoz, P.; Bordi, C.; Conte-Devolx, B.; Falchetti, A.; Gheri, R.G.; Libroia, A.; et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 2001, 86, 5658–5671. [Google Scholar] [CrossRef]
- Bomanji, J.; Levison, D.A.; Flatman, W.D.; Horne, T.; Bouloux, P.M.; Ross, G.; Britton, K.E.; Besser, G.M. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: A histopathological comparison. J. Nucl. Med. 1987, 28, 973–978. [Google Scholar]
- Havekes, B.; Lai, E.W.; Corssmit, E.P.; Romijn, J.A.; Timmers, H.J.; Pacak, K. Detection and treatment of pheochromocytomas and paragangliomas: Current standing of MIBG scintigraphy and future role of PET imaging. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 419–429. [Google Scholar]
- Ilias, I.; Divgi, C.; Pacak, K. Current role of metaiodobenzylguanidine in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin. Nucl. Med. 2011, 41, 364–368. [Google Scholar] [CrossRef]
- Wachtel, H.; Hutchens, T.; Baraban, E.; Schwartz, L.E.; Montone, K.; Baloch, Z.; LiVolsi, V.; Krumeich, L.; Fraker, D.L.; Nathanson, K.L.; et al. Predicting metastatic potential in pheochromocytoma and paraganglioma: A comparison of PASS and GAPP scoring systems. J. Clin. Endocrinol. Metab. 2020, 105, e4661–e4670. [Google Scholar] [CrossRef]
- Berends, A.M.A.; Eisenhofer, G.; Fishbein, L.; Horst-Schrivers, A.N.A.V.; Kema, I.P.; Links, T.P.; Lenders, J.W.M.; Kerstens, M.N. Intricacies of the molecular machinery of catecholamine biosynthesis and secretion by chromaffin cells of the normal adrenal medulla and in pheochromocytoma and paraganglioma. Cancers 2019, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, D.R.; Ceolin, L.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Maciel, L.M.; Maia, A.L. Role of RET genetic variants in MEN2-associated pheochromocytoma. Eur. J. Endocrinol. 2014, 170, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Kölby, L.; Bernhardt, P.; Levin-Jakobsen, A.M.; Johanson, V.; Wängberg, B.; Ahlman, H.; Forssell-Aronsson, E.; Nilsson, O. Uptake of meta-iodobenzylguanidine in neuroendocrine tumours is mediated by vesicular monoamine transporters. Br. J. Cancer 2003, 89, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Bacca, A.; Pucci, A.; Lorenzini, D.; Chiacchio, S.; Volterrani, D.; Ferrari, M.; Sellari Franceschini, S.; Materazzi, G.; Basolo, F.; Bernini, G. Vesicular monoamine transporters expression in pheochromocytomas and paragangliomas according to scintigraphy and positron emission tomography behavior. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 396–401. [Google Scholar] [CrossRef]
- Fishbein, L.; Wilkerson, M.D. Chromaffin cell biology: Inferences from the Cancer Genome Atlas. Cell Tissue Res. 2018, 372, 339–346. [Google Scholar] [CrossRef]
- Huynh, T.T.; Pacak, K.; Brouwers, F.M.; Abu-Asab, M.S.; Worrell, R.A.; Walther, M.M.; Elkahloun, A.G.; Goldstein, D.S.; Cleary, S.; Eisenhofer, G. Different expression of catecholamine transporters in phaeochromocytomas from patients with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Eur. J. Endocrinol. 2005, 153, 551–563. [Google Scholar] [CrossRef][Green Version]
- Mackenzie, I.S.; Gurnell, M.; Balan, K.K.; Simpson, H.; Chatterjee, K.; Brown, M.J. The use of 18-fluoro-dihydroxyphenylalanine and 18-fluorodeoxyglucose positron emission tomography scanning in the assessment of metaiodobenzylguanidine-negative phaeochromocytoma. Eur. J. Endocrinol. 2007, 157, 533–537. [Google Scholar] [CrossRef]
- Mehta, N.; Paniker, V.; Shah, A. MIBG negative pheochromocytoma. J. Assoc. Physicians India 2010, 58, 198–199. [Google Scholar]
- Kurisaki-Arakawa, A.; Saito, T.; Takahashi, M.; Mitani, K.; Yao, T. A case of (123)I-MIBG scintigram-negative functioning pheochromocytoma: Immunohistochemical and molecular analysis with review of literature. Int. J. Clin. Exp. Pathol. 2014, 7, 4438–4447. [Google Scholar]
- Fonte, J.S.; Robles, J.F.; Chen, C.C.; Reynolds, J.; Whatley, M.; Ling, A.; Mercado-Asis, L.B.; Adams, K.T.; Martucci, V.; Fojo, T.; et al. False-negative ¹²³I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr. Relat. Cancer 2012, 19, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rastogi, A.; Kumar, S.; Bhansali, A. Metastatic pheochromocytoma in MEN 2A: A rare association. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Casanova, S.; Rosenberg-Bourgin, M.; Farkas, D.; Calmettes, C.; Feingold, N.; Heshmati, H.M.; Cohen, R.; Conte-Devolx, B.; Guillausseau, P.J.; Houdent, C. Phaeochromocytoma in multiple endocrine neoplasia type 2 A: Survey of 100 cases. Clin. Endocrinol. 1993, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Hinze, R.; Machens, A.; Schneider, U.; Holzhausen, H.J.; Dralle, H.; Rath, F.W. Simultaneously occurring liver metastases of pheochromocytoma and medullary thyroid carcinoma--a diagnostic pitfall with clinical implications for patients with multiple endocrine neoplasia type 2a. Pathol. Res. Pract. 2000, 196, 477–481. [Google Scholar] [CrossRef]
- Tural, S.; Yuce, M.; Polat, A.K.; Tekcan, E.; Celik, B.Z.; Karabacak, U.; Kara, N. Novel RET Proto-oncogene variants identified in Turkish patients with thyroid carcinoma. Gene 2020, 746, 144611. [Google Scholar] [CrossRef]
- Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Leiria, L.; Crispim, D.; Ashton-Prolla, P.; Prolla, P.A.; Maia, A.L. Additive effect of RET polymorphisms on sporadic medullary thyroid carcinoma susceptibility and tumor aggressiveness. Eur. J. Endocrinol. 2012, 166, 847–854. [Google Scholar] [CrossRef]
- Lebeault, M.; Pinson, S.; Guillaud-Bataille, M.; Gimenez-Roqueplo, A.P.; Carrie, A.; Barbu, V.; Pigny, P.; Bezieau, S.; Rey, J.M.; Delvincourt, C.; et al. Nationwide french study of RET variants detected from 2003 to 2013 suggests a possible influence of polymorphisms as modifiers. Thyroid 2017, 27, 1511–1522. [Google Scholar] [CrossRef]
- de Groot, J.W.; Links, T.P.; Plukker, J.T.; Lips, C.J.; Hofstra, R.M. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr. Rev. 2006, 27, 535–560. [Google Scholar] [CrossRef]
- Lantieri, F.; Caroli, F.; Ceccherini, I.; Griseri, P. The involvement of the RET variant G691S in medullary thyroid carcinoma enlightened by a meta-analysis study. Int. J. Cancer 2013, 132, 2808–2819. [Google Scholar] [CrossRef]
- Cebrian, A.; Lesueur, F.; Martin, S.; Leyland, J.; Ahmed, S.; Luccarini, C.; Smith, P.L.; Luben, R.; Whittaker, J.; Pharoah, P.D.; et al. Polymorphisms in the initiators of RET (rearranged during transfection) signaling pathway and susceptibility to sporadic medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2005, 90, 6268–6274. [Google Scholar] [CrossRef]
- Lam, A.K. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef]
- Kimura, N.; Ishikawa, M.; Shigematsu, K. Colorectal paragangliomas with immunohistochemical deficiency of succinate dehydrogenase subunit B. Endocr. J. 2022, 69, 523–528. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yoshida, Y.; Ozeki, Y.; Okamoto, M.; Gotoh, K.; Masaki, T.; Nishida, H.; Shibuya, T.; Shin, T.; Daa, T.; et al. Dopamine-secreting pheochromocytoma and paraganglioma. J. Endocr. Soc. 2021, 5, bvab163. [Google Scholar] [CrossRef]
- Darlow, J.M.; Molloy, N.H.; Green, A.J.; Puri, P.; Barton, D.E. The increased incidence of the RET p.Gly691Ser variant in French-Canadian vesicoureteric reflux patients is not replicated by a larger study in Ireland. Hum. Mutat. 2009, 30, E612–E617. [Google Scholar] [CrossRef]
- Anson, B.J.; Daseler, E.H. Common variations in renal anatomy, affecting blood supply, form, and topography. Surg. Gynecol. Obstet. 1961, 112, 439–449. [Google Scholar]
- Stojadinovic, D.; Zivanovic-Macuzic, I.; Sazdanovic, P.; Jeremic, D.; Jakovcevski, M.; Minic, M.; Kovacevic, M. Concomitant multiple anomalies of renal vessels and collecting system. Folia Morphol. 2020, 79, 627–633. [Google Scholar] [CrossRef]
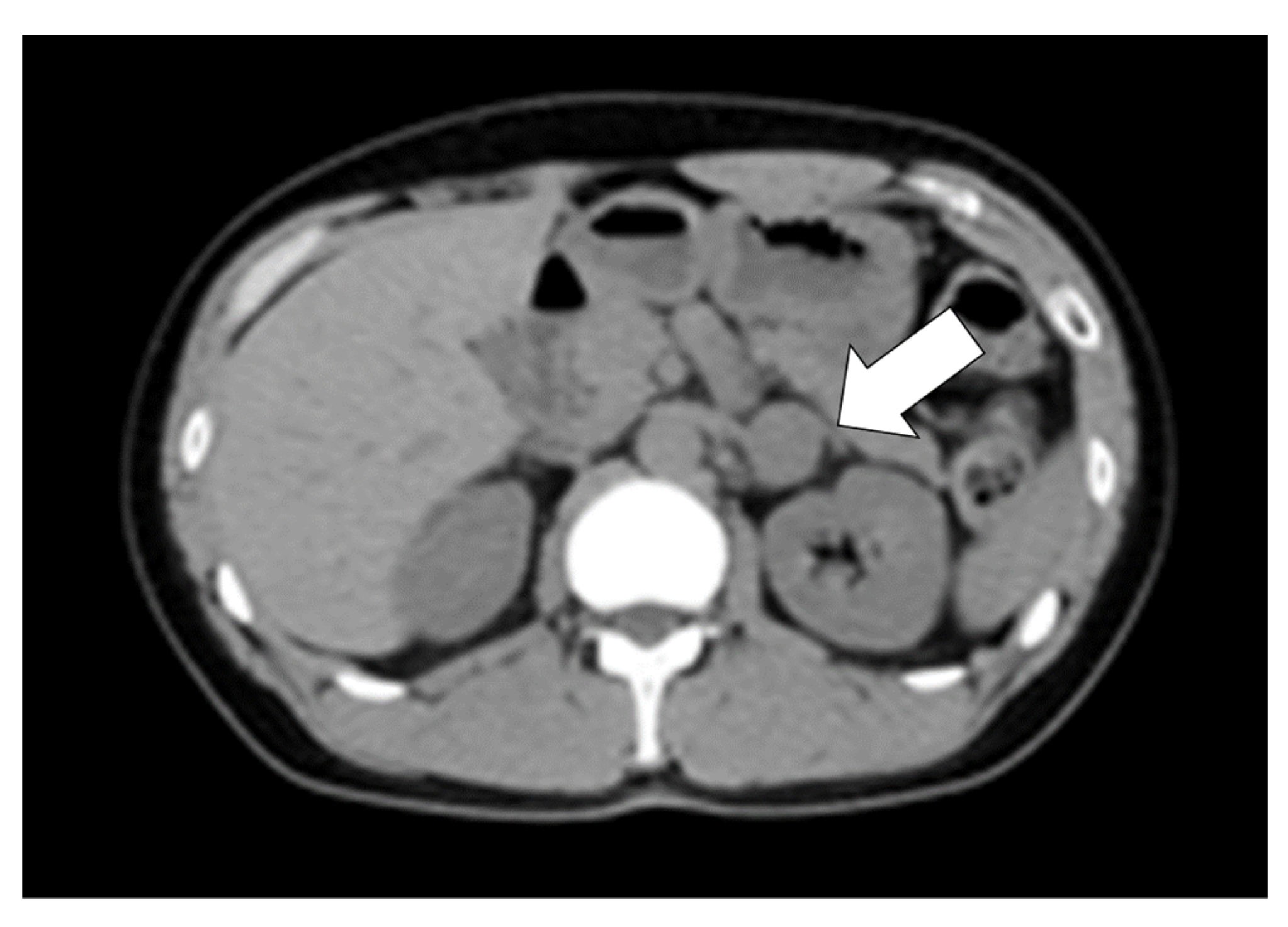
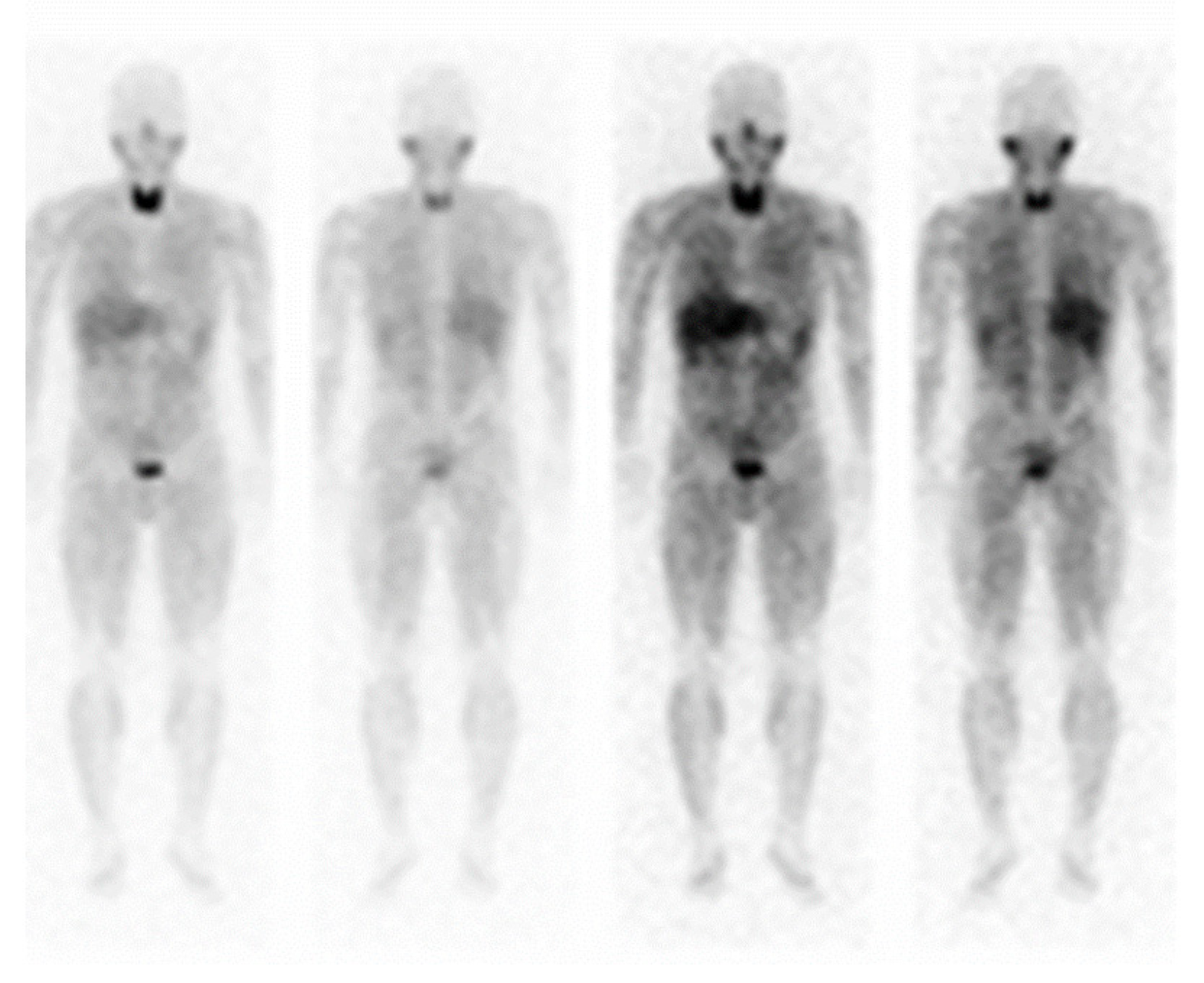

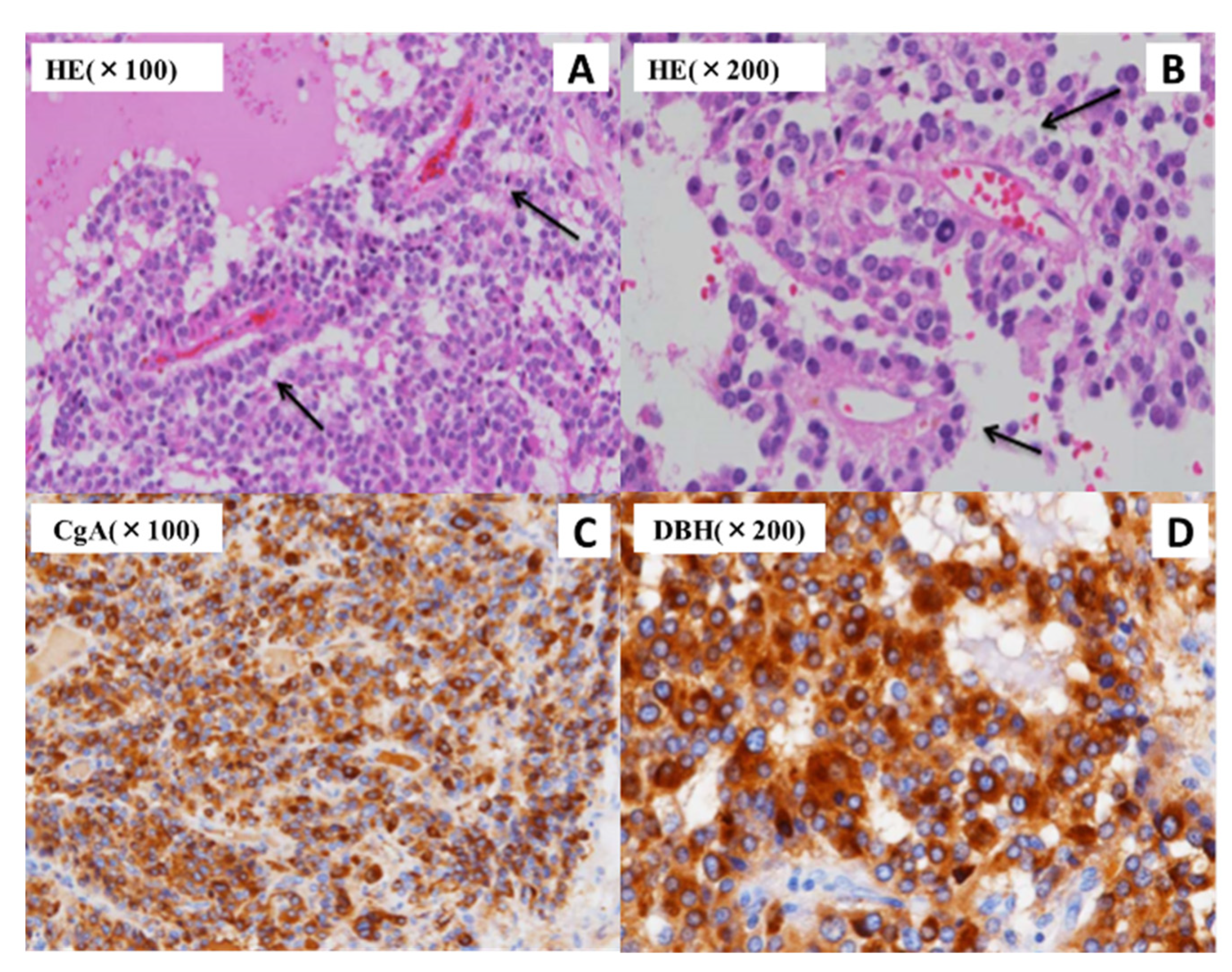
| Parameter | Value | Unit | Reference Range | Parameter | Value | Unit | Reference Range |
|---|---|---|---|---|---|---|---|
| <Blood chemistry data> | <Endocrinological data> | ||||||
| Total protein | 6.6 | g/dL | 6.7–8.3 | ACTH | 30.1 | pg/mL | 7.2–63.3 |
| Albumin | 4.4 | g/dL | 4.1–5.1 | Cortisol | 10.7 | mg/dL | 1.0–19.3 |
| AST | 18 | U/L | 13–30 | Growth hormone | 0.06 | ng/mL | 0.13–9.88 |
| ALT | 20 | U/L | 10–42 | IGF-1 | 132 | ng/mL | 103–287 |
| γ-GTP | 14 | U/L | 13–64 | PRA | 2.2 | ng/nL/hr | 0.3–2.9 |
| BUN | 8.9 | mg/dL | 8.0–20.0 | PAC | 18.6 | ng/dL | 3.0–15.9 |
| Creatinine | 0.77 | mg/dL | 0.65–1.07 | LH | 3.44 | mIU/mL | 2.20–8.40 |
| Na | 140 | mEq/L | 138–145 | FSH | 10.2 | mIU/mL | 1.8–12.0 |
| K | 4.2 | mEq/L | 3.6–4.8 | Prolactin | 7.24 | ng/mL | 1.50–9.70 |
| Cl | 106 | mEq/L | 101–108 | TSH | 1.32 | μIU/mL | 0.500–5.000 |
| Ca | 9 | mEq/L | 8.8–10.1 | Free T3 | 2.6 | pg/mL | 2.3–4.3 |
| IP | 3.1 | mEq/L | 2.7–4.6 | Free T4 | 1 | ng/dL | 0.9–1.7 |
| Mg | 2.1 | mg/dL | 1.8–2.6 | Calcitonin | 1.34 | pg/mL | <5.15 |
| CRP | <0.01 | mg/dL | 0.00–0.14 | PTH-intact | 6.12 | pg/mL | 10–65 |
| BNP | 10.2 | pg/mL | 0.0–18.4 | Adrenaline | 0.05 | ng/mL | <0.10 |
| Total cholesterol | 185 | mg/dL | 130–219 | Dopamine | 0.14 | ng/mL | <0.03 |
| Triglyceride | 49 | mg/dL | 36–150 | <Urinary data> | |||
| HDL-cholesterol | 80 | mg/dL | 41–67 | Adrenaline | 54.8 | μg/day | 3.0–41.0 |
| LDL-cholesterol | 94 | mg/dL | 70–139 | Noradrenaline | 3650 | μg/day | 31.0–160.0 |
| HbA1c | 6.0 | % | 4.6–6.2 | Metanephrine | 0.2 | mg/day | 0.05–0.20 |
| Blood glucose | 127 | mg/dL | 10–110 | Normetanephrine | 4 | mg/day | 0.10–0.28 |
| CPR | 15.5 | ng/mL | 0.80–2.50 | Cortisol | 135.5 | μg/day | 4.3–176.0 |
| Anti-GAD antibody | <0.5 | U/mL | <5.0 | Aldosterone | 11.2 | μg/day | 1.0–19.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, H.; Tsurutani, Y.; Sunouchi, T.; Hoshino, Y.; Hirose, R.; Katsuragawa, S.; Kimura, N.; Saito, J.; Nishikawa, T. A Case of 123I-Metaiodobenzylguanidine Scintigraphy-Negative Pheochromocytoma with a Tumor-Developing Mutation in the RET Gene. J. Clin. Med. 2022, 11, 4624. https://doi.org/10.3390/jcm11154624
Kubo H, Tsurutani Y, Sunouchi T, Hoshino Y, Hirose R, Katsuragawa S, Kimura N, Saito J, Nishikawa T. A Case of 123I-Metaiodobenzylguanidine Scintigraphy-Negative Pheochromocytoma with a Tumor-Developing Mutation in the RET Gene. Journal of Clinical Medicine. 2022; 11(15):4624. https://doi.org/10.3390/jcm11154624
Chicago/Turabian StyleKubo, Haremaru, Yuya Tsurutani, Takashi Sunouchi, Yoshitomo Hoshino, Rei Hirose, Sho Katsuragawa, Noriko Kimura, Jun Saito, and Tetsuo Nishikawa. 2022. "A Case of 123I-Metaiodobenzylguanidine Scintigraphy-Negative Pheochromocytoma with a Tumor-Developing Mutation in the RET Gene" Journal of Clinical Medicine 11, no. 15: 4624. https://doi.org/10.3390/jcm11154624
APA StyleKubo, H., Tsurutani, Y., Sunouchi, T., Hoshino, Y., Hirose, R., Katsuragawa, S., Kimura, N., Saito, J., & Nishikawa, T. (2022). A Case of 123I-Metaiodobenzylguanidine Scintigraphy-Negative Pheochromocytoma with a Tumor-Developing Mutation in the RET Gene. Journal of Clinical Medicine, 11(15), 4624. https://doi.org/10.3390/jcm11154624






