“Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Selection and Study Design
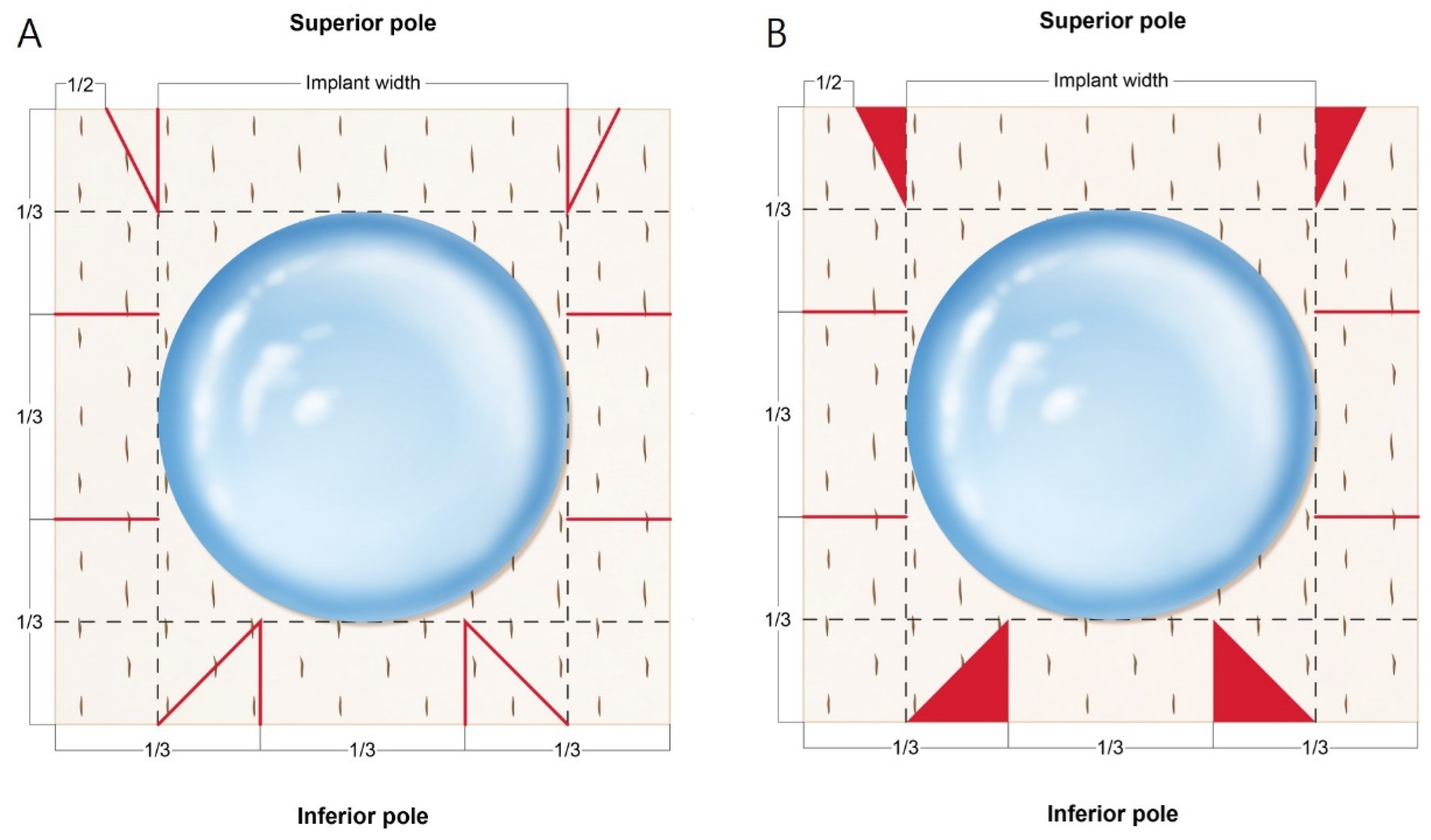
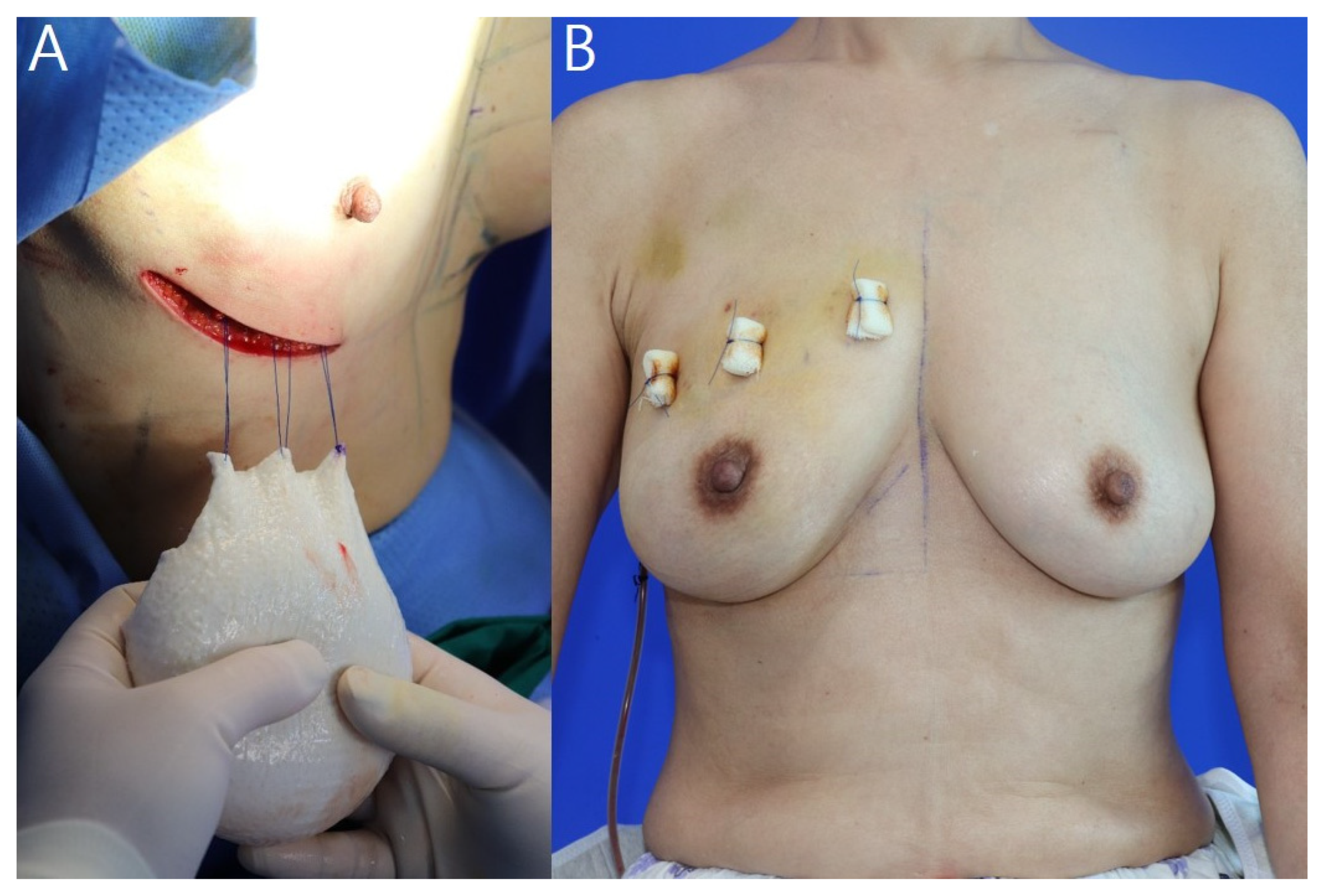
2.2. Surgical Procedures and ADM Warpping Technique
2.3. Satisfaction Survey
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.J.; Kang, S.G.; Kim, E.K.; Song, S.Y.; Lee, L.S.; Lee, J.H.; Jin, U.S. Current status of and trends in post-mastectomy breast reconstruction in Korea. Arch. Plast. Surg. 2020, 47, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Society of Plastic Surgeons. Breast Reconstruction Statistics. 2020. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 22 July 2016).
- Young, W.A.; Degnim, A.C.; Hoskin, T.L.; Jakub, J.W.; Nguyen, M.D.; Tran, N.V.; Harless, C.A.; Manrique, O.J.; Boughey, J.C.; Hieken, T.J. Outcomes of >1300 nipple-sparing mastectomies with immediate reconstruction: The impact of expanding indications on complications. Ann. Surg. Oncol. 2019, 26, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, R.K.; Guthrie, R.H. Reconstruction of the female breast following radical mastectomy. Plast. Reconstr. Surg. 1971, 47, 565–567. [Google Scholar] [CrossRef]
- Rebowe, R.E.; Allred, L.J.; Nahabedian, M.Y. The evolution from subcutaneous to prepectoral prosthetic breast reconstruction. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1797. [Google Scholar] [CrossRef] [PubMed]
- Ter Louw, R.P.; Nahabedian, M.Y. Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. 2017, 140, 51S–59S. [Google Scholar] [CrossRef]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Strom-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Cocilovo, C. Two-stage prosthetic breast reconstruction: A comparison between prepectoral and partial subpectoral techniques. Plast. Reconstr. Surg. 2017, 140, 22S–30S. [Google Scholar] [CrossRef]
- Hammond, D.C.; Schmitt, W.P.; O’Connor, E.A. Treatment of Breast Animation Deformity in Implant-Based Reconstruction with Pocket Change to the Subcutaneous Position. Plast. Reconstr. Surg. 2015, 135, 1540–1544. [Google Scholar] [CrossRef]
- Salzberg, C.A. Nonexpansive Immediate Breast Reconstruction Using Human Acellular Tissue Matrix Graft (AlloDerm). Ann. Plast. Surg. 2006, 57, 1–5. [Google Scholar] [CrossRef]
- Ibrahim, A.M.S.; Koolen, P.G.; Ashraf, A.A.; Kim, K.; Mureau, M.A.M.; Lee, B.T.; Lin, S.J. Acellular Dermal Matrix in Reconstructive Breast Surgery: Survey of Current Practice among Plastic Surgeons. Plast. Reconstr. Surg. Glob. Open. 2015, 3, e381. [Google Scholar] [CrossRef]
- Neamonitou, F.; Mylvaganma, S.; Salem, F.; Vidya, R. Outcome of complete acellular dermal matrix wrap with polyurethane implant in immediate prepectoral breast reconstruction. Arch. Plast. Surg. 2020, 47, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.S.; Lee, J.H.; Lee, J.W.; Lee, J.; Park, H.Y.; Yang, J.D. Prepectoral breast reconstruction with complete implant coverage using double-crossed acellular dermal matrixs. Gland Surg. 2019, 8, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Vidya, R.; Iqbal, F.M. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clin. Breast Cancer 2017, 17, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Cano, S.J.; Klassen, A.F.; Scott, A.M.; Cordeiro, P.G.; Pusic, A.L. The BREAST-Q: Further Validation in Independent Clinical Samples. Plast. Reconstr. Surg. 2012, 129, 293–302. [Google Scholar] [CrossRef]
- Dikmans, R.E.G.; Nene, L.E.H.; Bouman, M.B.; de Vet, H.C.W.; Mureau, M.A.M.; Buncamper, M.E.; Winters, H.A.H.; Ritt, M.J.P.F.; Mullender, M.G. The Aesthetic Items Scale: A Tool for the Evaluation of Aesthetic Outcome after Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1254. [Google Scholar] [CrossRef]
- Larrimore, C.; Jaghab, A. A Rare Case of Breast Implant-Associated Diffuse Large B-Cell Lymphoma. Case Rep. Oncol. Med. 2019, 2019, 1801942. [Google Scholar] [CrossRef] [Green Version]
- Reitsamer, R.; Peintinger, F.; Klaassen-Federspiel, F.; Sir, A. Prepectoral direct-to-implant breast reconstruction with complete ADM or synthetic mesh coverage e 36-Months follow-up in 200 reconstructed breasts. Breast 2019, 48, 32–37. [Google Scholar] [CrossRef]
- Cuomo, R.; Giardino, F.R.; Neri, A.; Nisi, G.; Brandi, C.; Zerini, I.; Jingjian, H.; Grimaldi, L. Optimization of Prepectoral Breast Reconstruction. Breast Care 2021, 16, 36–42. [Google Scholar]
- Yi, A.; Kim, H.H.; Shin, H.J.; Huh, M.O.; Ahn, S.D.; Seo, B.K. Radiation-Induced Complications after Breast Cancer Radiation Therapy: A Pictorial Review of Multimodality Imaging Findings. Korean J. Radiol. 2009, 10, 49–507. [Google Scholar] [CrossRef] [Green Version]
- Apte, A.; Walsh, M.; Balaji, P.; Khor, B.; Chandrasekharan, S.; Chakravorty, A. Single stage immediate breast reconstruction with acellular dermal matrix and implant: Defining the risks and outcomes of post-mastectomy radiotherapy. Surgeon 2020, 18, 202–207. [Google Scholar]
- Mokhtari-Hessari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Downs, R.K.; Hedges, K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction—A Case Series. Plast. Reconstr. Surg. Glob. Open 2016, 4, e821. [Google Scholar] [CrossRef] [PubMed]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population with BRCA Mutations: A Multi-institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pusic, A.L.; Matros, E.; Fine, N.; Buchel, E.; Gordillo, G.M.; Hamill, J.B.; Kim, H.M.; Qi, J.; Albornoz, C.; Klassen, A.F.; et al. Patient-reported outcomes 1 year after immediate breast reconstruction: Results of the mastectomy reconstruction outcomes consortium study. J. Clin. Oncol. 2017, 35, 2499–2506. [Google Scholar] [CrossRef]
- Hu, E.S.; Pusic, A.L.; Waljee, J.F.; Khun, L.; Hawley, S.T.; Wilkins, E.; Alderman, A.K. Patient-Reported Aesthetic Satisfaction with Breast Reconstruction during the Long-Term Survivorship Period. Plast. Reconstr. Surg. 2009, 124, 1–8. [Google Scholar] [CrossRef]
- Paydar, K.Z.; Wirth, G.A.; Mowlds, D.S. Prepectoral Breast Reconstruction with Fenestrated Acellular Dermal Matrix: A Novel Design. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1712. [Google Scholar] [CrossRef]
- Palaia, D.A.; Arthur, K.S.; Cahan, A.C.; Rosenberg, M.H. Incidence of Seromas and Infections Using Fenestrated versus Nonfenestrated Acellular Dermal Matrix in Breast Reconstructions. Plast. Reconstr. Surg. Glob. Open 2015, 3, e569. [Google Scholar] [CrossRef]
- Fasnot, J.; Kovach, S.J., 3rd; Serletti, J.M. Acellular Dermal Matrix: General Principles for the Plastic Surgeon. Aesthetic Surg. J. 2011, 31, 5S–12S. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, H.G.; Lee, W.J. Characterization and tissue incorporation of cross-linked human acellular dermal matrix. Bio-materials 2015, 44, 195–205. [Google Scholar] [CrossRef]
- Gwak, H.K.; Jeon, Y.W.; Lim, S.T.; Park, S.Y.; Suh, Y.J. Volume replacement with diced acellular dermal matrix in oncoplastic breast-conserving surgery: A prospective single-center experience. World J. Surg. Oncol. 2020, 18, 60. [Google Scholar] [CrossRef]
- Pittman, T.A.; Abbate, O.A.; Economides, J.M. The P1 Method: Prepectoral Breast Reconstruction to Minimize the Palpable Implant Edge and Upper Pole Rippling. Ann. Plast. Surg. 2018, 80, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Oleck, N.C.; Gu, C.; Pyfer, B.J.; Phillips, B.T. Defining Mastectomy Skin Flap Necrosis: A Systematic Review of the Literature and a Call for Standardization. Plast. Reconstr. Surg. 2018, 149, 858e–866e. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.N.; Gerety, P.A.; Serletti, J.M.; Kovach, S.J.; Fischer, J.P. A Systematic Review and Head-to-Head Meta-Analysis of Outcomes following Direct-to-Implant versus Conventional Two-Stage Implant Reconstruction. Plast. Reconstr. Surg. 2015, 136, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Algaithy, Z.K.; Petit, J.Y.; Lohsiriwat, V.; Maisonneuve, P.; Rey, P.C.; Baros, N.; Lai, H.; Mulas, P.; Barbalho, D.M.; Veronesi, P.; et al. Nipple sparing mastectomy: Can we predict the factors predisposing to necrosis? Eur. J. Surg. Oncol. 2012, 38, 125–129. [Google Scholar] [CrossRef]



| Variables | Value * |
|---|---|
| Total number of patients | 56 |
| Reconstruction side | |
| Right | 30 (53.6%) |
| Left | 26 (46.4%) |
| Age (years) | 50.2 ± 8.9 (32–73) |
| Weight (kg) | 61.3 ± 9.6 (47–102) |
| Height (cm) | 160.1 ± 5.5 (149–170) |
| BMI (kg/m2) | 24.0 ± 3.8 (18.1–37.0) |
| Follow-up period | 13.8 ± 1.5 (12–16) |
| Preoperative breast shape | |
| Tubular | 5 (8.9%) |
| Round | 11 (19.6%) |
| Conical | 19 (33.9%) |
| Wide | 15 (26.9%) |
| Narrow | 6 (10.7%) |
| Ptotic breasts | 27 (48.2%) |
| Sternal notch to nipple distance (cm) | |
| Right | 22.2 ± 3.1 (14.5–32.5) |
| Left | 22.0 ± 3.0 (15.5–31.0) |
| Chemotherapy | 34 (60.7%) |
| Neoadjuvant chemotherapy | 13 (23.2%) |
| Adjuvant chemotherapy | 21 (37.5%) |
| Complications | |
| Seroma | 1 (1.8) |
| Hematoma | 1 (1.8) |
| Infection | 0 |
| Mastectomy flap or nipple necrosis | 0 |
| Implant malposition, rotation | 0 |
| ADM non-incorporation | 0 |
| Variables | Value * |
|---|---|
| Breast base width (cm) | 13.4 ± 1.8 (10.5–17.5) |
| Days until JP drain removed | 10.4 ± 1.5 (7.0–15.0) |
| Resected breast tissue weight (g) | 274.3 ± 111.4 (77–742) |
| Mentor silicone implant volume (cc) | 230.0 ± 78.4 (130–425) |
| 100–199 | 23 (41.1%) |
| 200–299 | 24 (42.9%) |
| ≥300 | 9 (16.0%) |
| ADM size (cm2) | |
| 16 × 16 | 26 (46.4%) |
| 18 × 18 | 23 (41.1%) |
| 20 × 20 | 7 (12.5%) |
| Value (Range) | |
|---|---|
| BREAST-Q score | 82.6 ± 12.4 (58–100) |
| Median AIS scores | |
| Volume | 4.4 ± 0.6 (3.4–4.8) |
| Shape | 4.5 ± 0.6 (3.3–4.8) |
| Symmetry | 4.1 ± 0.8 (2.9–4.3) |
| Scar | 4.1 ± 0.8 (3.1–4.6) |
| Nipple-areolar complex | 4.2 ± 0.6 (3.2–4.6) |
| Total AIS | 21.0 ± 0.3 (19.8–22.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-i.; Kim, B.-s.; Park, J.-h.; Yi, H.-s.; Kim, H.-y.; Choi, J.-h.; Jung, S.-u.; Kim, Y.-s. “Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction. J. Clin. Med. 2022, 11, 4592. https://doi.org/10.3390/jcm11154592
Kim H-i, Kim B-s, Park J-h, Yi H-s, Kim H-y, Choi J-h, Jung S-u, Kim Y-s. “Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction. Journal of Clinical Medicine. 2022; 11(15):4592. https://doi.org/10.3390/jcm11154592
Chicago/Turabian StyleKim, Hong-il, Byeong-seok Kim, Jin-hyung Park, Hyung-suk Yi, Hyo-young Kim, Jin-hyuk Choi, Sung-ui Jung, and Yoon-soo Kim. 2022. "“Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction" Journal of Clinical Medicine 11, no. 15: 4592. https://doi.org/10.3390/jcm11154592
APA StyleKim, H.-i., Kim, B.-s., Park, J.-h., Yi, H.-s., Kim, H.-y., Choi, J.-h., Jung, S.-u., & Kim, Y.-s. (2022). “Tear-Drop Appearance” Wrap: A Novel Implant Coverage Method for Creating Natural Contour in Prepectoral Prosthetic-Based Breast Reconstruction. Journal of Clinical Medicine, 11(15), 4592. https://doi.org/10.3390/jcm11154592






