Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics
Abstract
1. Background and Introduction
2. Materials and Methods
2.1. Surgical Indication
2.2. Clinical Setting and Patient Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Associated Conditions
2.3. Assessments and Outcomes
2.3.1. Neurological and Neuroradiological Examination, and Follow up
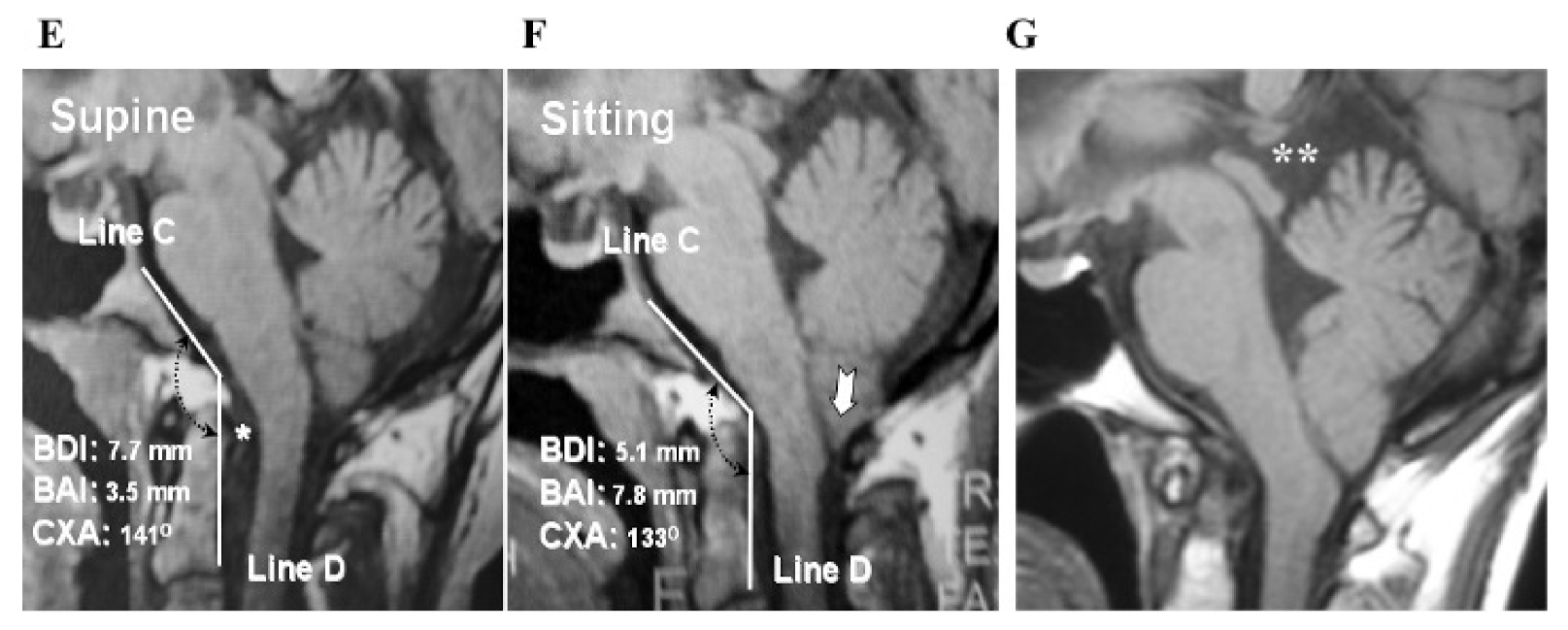
2.3.2. Volumetric Calculations, Morphometric Measurements, and Data Collection
2.3.3. Statistical Analyses
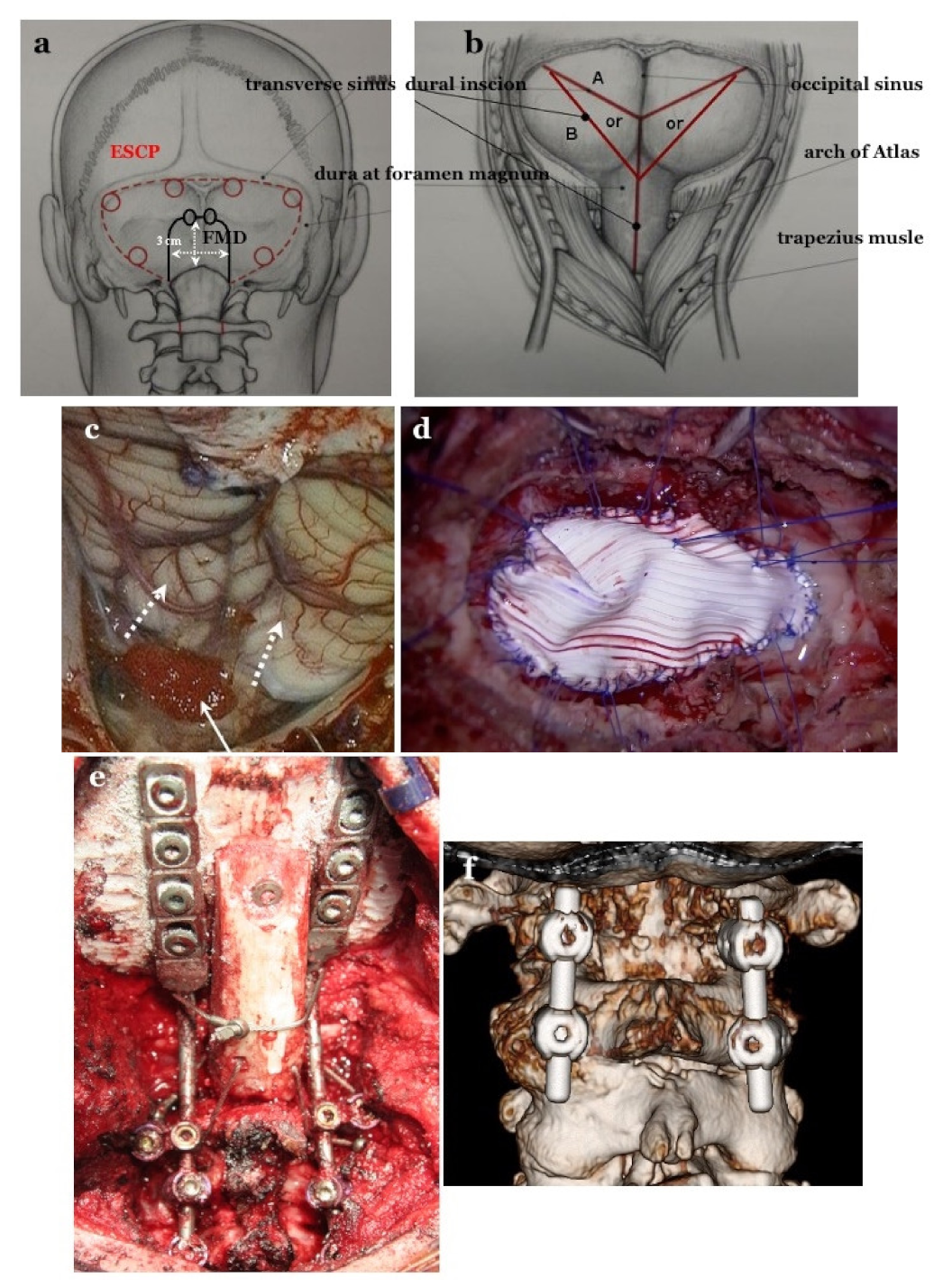
2.4. Selection of Operative Procedures of Posterior Fossa Decompression and CCF
2.4.1. Foramen Magnum Decompression (FMD) for CM-I Type B and CM-Borderline
2.4.2. Expansive Suboccipital Cranioplasty (ECSP) for CM-I Type C
2.4.3. CCF for CM-I with CCJ Instability
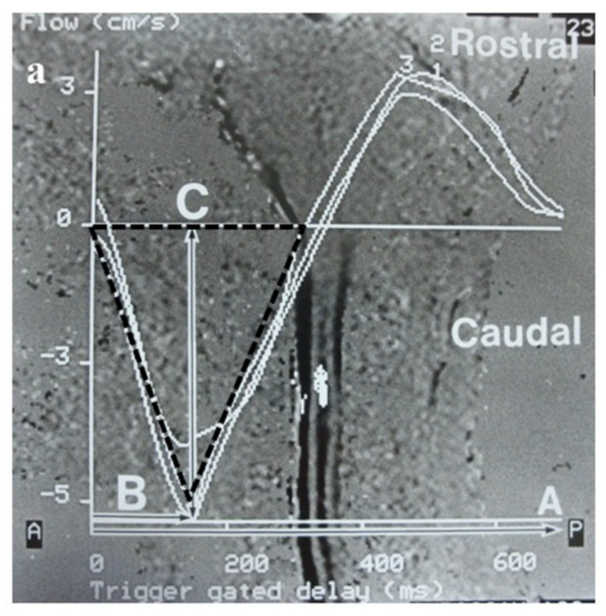
2.5. Evaluation of CSF Flow Dynamics
2.5.1. Evaluation of CSF Flow Dynamics Using Intraoperative CDU
2.5.2. Measurements of CSF Space of the Major Cistern and Evaluation of CSF Flow Dynamics Using MRI Pre- and Post-Operatively
3. Results
3.1. Neurological Symptoms and Signs in Each Type and Its Outcome
3.2. Outcome of Posterior Fossa Decompression (FMD and ESCP)
3.3. CCF Outcome
3.4. Complications, Side Effects, and Re-Operation Cases
3.4.1. Complication and Side Effects of FMD and ESCP
3.4.2. Complications and Side Effects of CCF
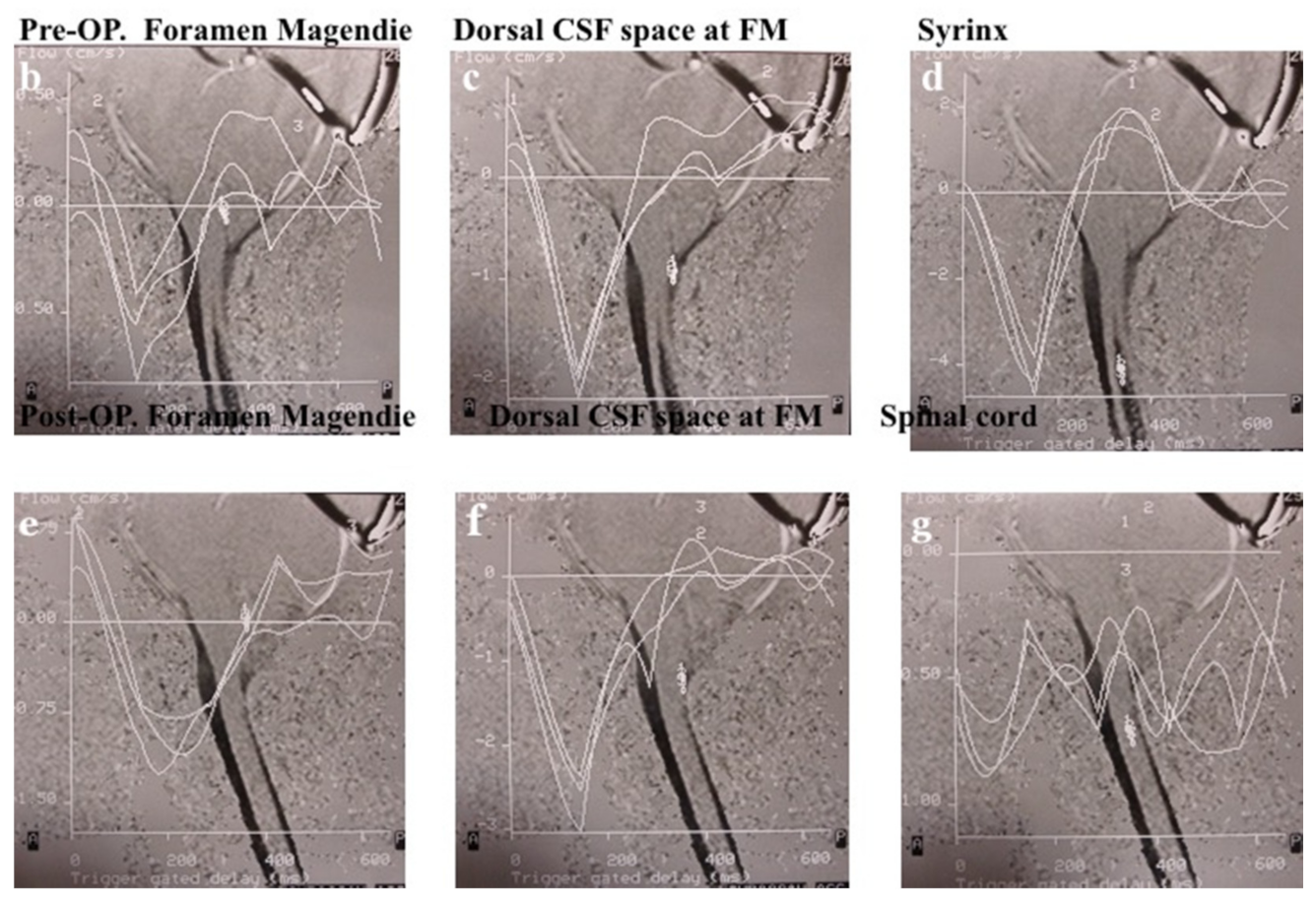
3.5. Evaluation of CSF Space and CSF Flow Dynamics
3.5.1. Evaluation by Intraoperative CDU
3.5.2. Pre- and Post-Operative CSF Space in the Major Cistern and CSF Flow Dynamics by Cine PC MRI
4. Discussion
4.1. Selection of Surgical Intervention Based on the Mechanism of Hindbrain Ptosis
4.2. Outcome of Surgical Intervention
4.2.1. Outcomes of Posterior Fossa Decompression (FMD and ESCP)
4.2.2. Outcome of CCF for CCJ Instability
4.3. CSF Dynamics and Pathogenesis of Syringomyelia
4.3.1. Study Outcome by MRI and Cine PC MRI
4.3.2. Pathophysiology of Syringomyelia Based on our Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM-I | Chiari malformation type I |
| CCF | craniocervical fixation |
| CCJ | craniocervical junction |
| CDU | color Doppler ultrasonography |
| CSF | cerebrospinal fluid |
| C1/2 FIX | atlanto–axial posterior lateral fixation |
| ESCP | expansive suboccipital cranioplasty |
| FMD | foramen magnum decompression |
| OCF | occipito–cervical posterior lateral fixation |
References
- Nishikawa, M.; Sakamoto, H.; Hakuba, A.; Nakanishi, N.; Inoue, Y. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J. Neurosurg. 1997, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Chou, M.W.; Trinidal, E.M.; Mandell, M.; Wolpert, C.; Speer, C.A. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Nishikawa, M.; Kula, W.R.; Dlugacz, D.Y. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir. Wien 2010, 152, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentations. Neuroradiology 1993, 55, 113–118. [Google Scholar] [CrossRef]
- Badie, B.; Mendoza, D.; Batzdorf, U. Posterior cranial fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery 1995, 37, 214–218. [Google Scholar] [CrossRef]
- Noudel, R.; Gomis, P.; Sotorares, G.; Bazin, A.; Pierot, L.; Pruvo, J.-P.; Bordet, R.; Roche, P.H. Posterior fossa volumetric increase after surgery for Chiari malformation type I: A quantitative study of the posterior cranial fossa. J. Neurosurg. 2011, 115, 647–658. [Google Scholar] [CrossRef]
- Karagoz, F.; Izgi, N.; Sencer, S.K. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir. Wien 2012, 144, 165–171. [Google Scholar] [CrossRef]
- Alperin, N.; Loftus, J.R.; Oliu, C.J.; Bagci, A.M.; Lee, S.H.; Ertl-Wagner, B.; Green, B.; Sekula, R. Magnetic resonance imaging measures of posterior cranial fossa morphology and cerebrospinal fluid physiology in Chiari malformation type I. Neurosurgery 2014, 75, 515–522. [Google Scholar] [CrossRef]
- Speer, M.C.; George, T.M.; Enterline, D.S.; Franklin, A.; Wolpert, C.M.; Milhorat, T.H. A genetic hypothesis for Chiari I malformation with or without syringomyelia. Neurosurg. Focus 2000, 8, E121. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E. Whole brain segmentation labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Bolognese, P.A.; Nishikawa, M.; McDonnell, N.B.; Francomano, C.A. Syndrome of occipitoatlantoaxial hypermobility, functional cranial settling and Chiari malformation I patients with hereditary disorders of connective tissue. J. Neurosurg. Spine 2007, 7, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Bolognese, P.A.; Nishikawa, M.; Francomano, C.A.; McDonnell, N.B.; Roonprapunt, C.; Kula, R.W. Association of Chiari malformation type I and tethered cord syndrome: Preliminary results of section filumn terminale. Surg. Neurol. 2009, 71, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Jain, S.; Shah, A. Radiological evaluation of 510 cases of basilar invagination with evidence od atlantoaxial instability (Group a basilar invagination). World Neurosurg. 2018, 110, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Gore, S.; Shah, A.; Dharukar, P.; Vutha, R.; Patil, A. Atlantoaxial fixation for Chiari 1 formation in pediatric age group patients: Report of treatment in 33 patients. World Neurosurg. 2018, 111, e668–e677. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kaswa, A.; Shah, A. Atlantoaxial fixation for treatment of Chiari formation and syringomyelia with no craniovertebral bone anomaly: Report an experience with 57 cases. Acta Neurochir. Suppl. 2018, 125, 101–110. [Google Scholar]
- Nishikawa, M.; Bolognese, P.A.; Kula, R.W.; Ikuno Ohata, K. Pathogenesis and classification of Chiari malformation type I based on the mechanism of ptosis of the brain stem and cerebellum: A morphometric study of the posterior cranial fossa and craniovertebral junction. J. Neurol. Surg. B 2021, 82, 277–284. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Bolognese, P.A. Tailored operative technique for Chiari type I malformation using intraoperative color Doppler ultrasonography. Neurosurgery 2003, 53, 890–905. [Google Scholar] [CrossRef]
- Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.-I.; Konno, S.-I.; Miyamoto, M.; Seichi, A.; Shimamura, T.; Shirado, O.; Taguchi, T.; et al. An outcome measure for patients with cervical myelopathy: Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 1. J. Ortho. Sci. 2007, 12, 227–240. [Google Scholar] [CrossRef]
- Nishikawa, M.; Bolognese, P.A.; Kula, R.W.; Ikuno, H.; Takami, T.; Ohata, K. Surgical management of Chiari malformation: Preliminary results of surgeries for mechanisms of ptosis of the brain stem and cerebellum. J. Neurol. Surg. B 2021, 82, 264–272. [Google Scholar] [CrossRef]
- Oaks, J. Chiari malformation and syringomyelia. In Principles of Neurosurgery; Rengachary, S.S., Williams, R.H., Eds.; Mosby-Wolf: London, UK, 1994; p. 9.1.189. [Google Scholar]
- Sakamoto, H.; Nishikawa, M.; Hakuba, A.; Tokunou, N.; Nakanishi, N.; Nishimura, S. Expansive suboccipital cranioplasty for the treatment of syringomyelia associated with Chiari malformation. Acta Neurochir. Wien 1999, 123, 949–961. [Google Scholar] [CrossRef]
- Winegar, C.D.; Lawrence, J.P.; Friel, B.C.; Fernandez, C. A systemic review of occipital cervical fusion: Technique and outcome. J. Neurosurg. Spine 2010, 13, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Desai, K. Surgery of syringomyela: An analysis based on 163 surgical cases. Acta Neurochir. Wien 2000, 142, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Ohata, K.; Baba, M.; Terakawa, Y.; Hara, M. Chiari I malformation associated with ventral compression and instability: One staged posterior decompression and fusion with a new instrumentation technique. Neurosurgery 2004, 54, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-G.; Jiang, L.; Zhang, H.-B.; Liu, B.; Wang, J.-R.; Jia, J.-W.; Chen, W. Monitoring of cerebrospinal fluid flow by intraoperative ultrasound in patients with Chiari I malformation. Clin. Neurol. Neurosurg. 2011, 113, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Haughton, V.M.; Korosec, F.R.; Medow, J.E.; Dolar, M.T.; Iskandar, B.J. Peak systolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control paticipants. AJNR 2003, 24, 169–176. [Google Scholar]
- Clarke, E.C.; Stoodley, M.A.; Bilston, L. Change in temporal flow characteristics of CSF in Chiari malformation type I with and without syringomyelia: Implications for theory of syrinx development. J. Neurosurg. 2013, 118, 1135–1140. [Google Scholar] [CrossRef]
- Bond, A.E.; Jane, J.A., Sr.; Liu, K.C.; Oldfield, E.H. Changes in cerebrospinal fluid flow assessed using intraoperative MRI during posterior fossa decompression for Chiari malformation. J. Neurosurg. 2015, 122, 1068–1107. [Google Scholar] [CrossRef]
- Vinje, V.; Brucker, J.; Rongnes, M.; Mardal, K.-A. Fluid dynamics in syringomyelia cavities: Effect of heart rate, CSF velocity, CSF velocity waveform and craniovertebral decompression. Neuroradiol. J. 2018, 31, 482–489. [Google Scholar] [CrossRef]
- Isu, T.; Sasaki, H.; Takamura, H.; Kobayashi, N. Foramen magnum decompression with removal of the outer layer of the dura as treatment for syringomyelia occurring with Chiari I malformation. Neurosurgery 1993, 33, 845–849. [Google Scholar]
- Batzdorf, U.; McArthur, D.L.; Bentson, J.R. Surgical treatment of Chiari malformation with and without syringomyelia: Experience with 177 adult patients. J. Neurosurg. 2013, 118, 232–242. [Google Scholar] [CrossRef]
- Klekamp, J.; Batzdorf, U.; Samii, M.; Bothe, H.W. The surgical treatment of Chiari malformation. Acta Neurochir. Wien 1996, 138, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Beckman, J.; Naftel, R.P.; Chern, J.J.; Wellons, J.C., III; Rozzele, C.J. Institutional experience with 500 cases of pediatric patients with Chiari malformations. J. Neurosurg. Pediatr. 2011, 7, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Knafi, S.; Malcoci, M.; Morar, S.; Parker, F.; Aghakhani, N. Surgical management after Chiari decompression failure craniovetebral junction revision versus shunt strategy. J. Clin. Ned. 2022, 11, 3334. [Google Scholar]
- Cappuccio, M.; De lure, F.; Amendola, L.; Paderni, S.; Bosco, G. Occipito-cervical fusion in post-traumatic instability of the upper cervical spine and cranio-cervical junction. Eur. Spine 2013, 22, 900–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhimani, A.; Chiu, R.G.; Esfahani, D.R.; Patel, A.S.; Denyer, S.; Hobbs, J.G.; Mehta, A.I. C1-2 fusion versus occipito-cervical fusion for high cervical fractures: A multi-institutional database analysis and review of the literature. World Neurosurg. 2018, 119, e456–e459. [Google Scholar] [CrossRef]
- Sakas, D.E.; Korflas, S.I.; Wayte, S.C.; Beale, D.J.; Papapetrou, K.P.; Stranjalis, G.S.; Whittaker, K.W.; Whitwell, H.L. Chiari malformation: CSF flow dynamics in the craniocervical junction and syrinx. Acta Neurochir. Wien 2005, 147, 1223–1233. [Google Scholar] [CrossRef]
- Dolar, M.T.; Haughton, V.M.; Iskandar, B.J.; Quigley, M. Effect of craniocervical decompression on peak CSF flow velocity in symptomatic patients with Chiari I malformation. AJNR Am. J. Neuroradiol. 2004, 25, 142–1445. [Google Scholar]
- Haughton, V.M.; Iskandar, B.J. Measuring CSF flow in Chiari I malformation. Neuroradiol. J. 2006, 19, 427–432. [Google Scholar] [CrossRef]
- Williams, B. Syringomyelia. Neurosurg. Clin. N. Am. 1990, 1, 653–685. [Google Scholar] [CrossRef]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef]
- Heiss, J.D.; Patronnas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelai. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Nakagawa, H. Hypothesis on the pathophysiology of syringomyelia based on simulation of cerebrospinal fluid dynamics. J. Neurol. Neurosurg. Psychiatry 2003, 74, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Bliston, L.E.; Stoodley, M.A.; Fletcher, D.F. The influence of the relative timing of arterial and subarachnoid space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx development. J. Neurosurg. 2010, 112, 808–813. [Google Scholar] [CrossRef] [PubMed]



| CM-I Type A | CM-I Type B | CM-I Type C | CM-Borderline | |
|---|---|---|---|---|
| 36 cases | 120 cases | 115 cases | 14 cases | |
| VPCF | normal | normal | small | normal |
| VAFM | normal | small | small | small |
| VBPCF/VPCF | normal | large | large | normal/large |
| Occipital bone size | normal | small | small | small |
| Axial length of hindbrain | normal | normal | elongation | normal |
| Position of hindbrain | downward displacement | normal | downward displacement | normal |
| Cause | others | crowdedness of AFM | crowdedness of whole PCF | crowdedness of AFM |
| Intervention | others | FMD | ESCP | FMD |
| Instability of CCJ | Tethering | Hydrocephalus | Intracranial Mass | Pressure Dissociation | |
|---|---|---|---|---|---|
| VPCF | normal | normal | normal | normal | normal |
| VAFM | normal | normal | normal | normal | normal |
| VBPCV/VPCF | normal | normal | normal/large | normal/large | normal |
| Brainstem and Cerebellum | normal | elongation and downward displacement | normal | normal | |
| Cause | cranial settling | traction | pressure coning | Hypotension of intraspinal canal | |
| Intervention | CCF | untethering/SFT | VPS | resection of mass or FMD | others |
| CM-I Type A (Craniocervical Instabiity) | CM-I Type B | CM-I Type C | CM-Borderline | |||||
|---|---|---|---|---|---|---|---|---|
| 36 Cases | Improvement Rate | 120 Cases | Improvement Rate | 115 Cases | Improvement Rate | 14 Cases | Improvement Rate | |
| Symptoms and signs of brain stem and cerebellum | ||||||||
| Headache | 35 (97.2%) * | 33/35 (94.2%) † | 76 (63.3%) ** | 70/76 (92.1%) † | 91 (79.1%) * | 78/91 (85.7%) † | 7 (50.0%) ** | 16/20 (80.0%) |
| Neck pain | 34 (94.4%) * | 32/34 (94.1%) † | 74 (61.7%) ** | 68/74 (91.9%) † | 84 (73.0%) * | 77/84 (91.7%) † | 7 (50.0%) ** | 13/17 (76.4%) |
| Ataxia | 22 (61.1%) * | 14/22 (63.6%) | 55 (45.8%) ** | 45/55 (81.8%) † | 75 (65.2%) * | 65/ 75 (86.7%) † | 5 (35.7%) ** | 11/15 (73.3%) |
| Dizziness and/or vertigo | 28 (77.8%) * | 22/28 (78.5%) † | 43 (35.8%) ** | 33/43 (76.7%) | 83 (62.2%) * | 73/83 (88.0%) † | 2 (14.3%) ** | 7/10 (70.0%) |
| Dysphasia | 10 (27.8%) ** | 7/10 (70.0%) | 38 (31.7%) | 20/38 (52.6%) ⨍ | 38 (33.0%) | 20/38 (52.6%) ⨍ | 0 (0%) | 0 (0%) |
| Symptoms and signs of spinal cord myelopathy | ||||||||
| Pain of extremities, trunk | 16 (44.4%) ** | 14/16 (87.5%) † | 87 (72.5%) * | 48/70 (68.6%) ⨍ | 65 (56.5%) | 44/65 (67.7%) ⨍ | 10 (71.4%) * | 8/10 (80.0%) |
| Focal dysesthesia of extremities and/or trunk | 18 (50.0%) ** | 14/18 (77.8%) | 77 (64.2%) * | 45/67 (67.1%) ⨍ | 57 (49.6%) | 50/57 (70.2%) | 9 (64.3%) * | 6/9 (66.7%) ⨍ |
| Motor weakness | 24 (66.7%) | 12/14 (85.7%) † | 60 (50.0%) | 47/60 (78.3%) † | 51 (44.3%) | 45/51 (88.2%) † | 11 (78.6%) * | 8/11 (72.76%) |
| Hypalgesia | 12 (33.3%) ** | 6/12 (50.0%) | 55 (45.8%) | 40/55 (72.7%) | 25 (21.7%) ** | 41/55 (74.5%) | 10 (71.4%) * | 6/10 (60.0%) ⨍ |
| Dissociated sensory Sensory disturbance | 9 (25.0%) ** | 6/9 (66.7%) | 50 (41.7%) | 36/50 (72.0%) | 20 (17.4%) ** | 38/50 (76.0%) | 8 (57.1%) * | 4/8 (50.0%) ⨍ |
| Improved | JOA Score RR (%) | Re-Syringomyelia | Stabilized | Deteriorated | Transient Morbidities | |
|---|---|---|---|---|---|---|
| Neurological Symptoms/Signs | (Mean with ± SD) | Neurological Symptoms/Signs | Neurological Symptoms/Signs | |||
| Total Number: 300 surgeries | 248/300 (82.7%) | 58.5 ± 10.4 | 24/300 (8.0%) | 25/300 (8.3%) | 28/300 (9.3%) | 9/300 (3.0%) |
| (Posterior fossa decompression) | ||||||
| 12 years or older (≥12) | ||||||
| FMD: 133 cases | 115/133 (86.5%) | 58.7 ± 10.2 | 10/133 (7.5%) * | 10/133 (7.5%) | 8/130 (6.2%) * | 3/133 (1.7%) |
| (for CM-I types A, B and CM-borderline) | ||||||
| ESCP: 87 cases | 78/87 (89.7%) | 60.2 ± 10.1 | 1/87 (1.1%) ** | 8/87 (9.2%) | 2/87 (2.3%) ** | 4/87 (4.6%) * |
| (for CM-I type C) | ||||||
| Younger than 12 years (<12) | ||||||
| FMD for all types: 80 | ||||||
| CM-I type A: 12 | 7/12 (58.3%) ** | 57.2 ± 9.1 | 6/12 (50.0%) * | 0/12 (0%) | 5/12 (41.7%) * | 1/12 (8.3%) |
| CM-I type B: 33 | 25/33 (75.8%) | 58.2 ± 10.1 | 2/33 (6.1%) | 5/33 (15.1%) | 3/41 (7.3%) | 0/33 (0%) |
| CM-I type C: 28 | 18/28 (64.3%) ** | 48.6 ± 10.2 ** | 5/28 (17.9%) * | 1/28 (3.6%) | 9 /28 (32.1%) * | 1/28 (3.6%) |
| CM-borderline: 7 | 5/7 (71.4%) | 54.3 ± 10.4 | 0/7 (0%) | 1/7 (14.3%) | 1/7 (14.3%) | 0 /7 (0%) |
| Operative Procedures | Improved | Pre-Op. JOACMEQ Score | Post-Op. JOACMQ Score | JOA Score RR (%) | Bony Fusion of Joints | Stabilization of Joints |
|---|---|---|---|---|---|---|
| Neurological Symptoms/Signs | (Mean with ± One SD) | (Mean with ± One SD) | ||||
| CCF: 30 cases | 27/30 (90.0%) | 4–15 (9.7 ± 2.48) | 10–17 (15.5 ± 2.51) | 69.7% | 21/30 (70.0%) | 25/30 (83.3%) |
| C1/2 FIX 16 cases | 15/16 (93.8%) | 4–12 (9.0 ± 2.55) * | 14–17 (15.4 ± 2.58) | 78.7% * | 12/16 (75.0%) * | 15/16 (93.8%) * |
| OCF 14 cases | 12/14 (85.7%) | 3–12 (6.4 ± 2.43) ** | 10–17 (15.7 ± 2.48) | 63.5% ** | 9/14 (64.3%) ** | 10/14 (71.4%) ** |
| Controls | CM-I Type B | CM-I Type C | CM-Borderline | ||
|---|---|---|---|---|---|
| Maximum flow velocity (cm/s) | |||||
| 4th ventricle: | 0.68–2.45 (1.96) | Pre-Op | 0.86–1.72 (1.48) * | 0.55–1.72 (1.17) * | 0.56–1.47 (1.28) * |
| Post-Op | 0.43–2.68 (2.44) | 0.41–3.72 (2.18) | 0.40–3.78 (2.42) | ||
| Deteriorated cases: post-op 6 months | 0.36–0.83 (0.82) ** | 0.45–1.02 (0.85) ** | 0.56–0.97 (0.78) ** | ||
| Outlet of foramen of Magendie | 1.70–2.65 (2.24) | Pre-Op | 0.45–2.50 (1.32) * | 0.38–2.47 (1.42) * | 0.44–2.40 (1.44) * |
| Post-Op | 0.95–5.50 (3.75) | 0.84–5.70 (4.54) | 0.95 –5.50 (4.25) | ||
| Deteriorated cases: post-op 6 months | 0.45–2.50 (0.82) ** | 0.38–2.47 (0.72) ** | 0.44–2.40 (0.64) ** | ||
| Syrinx (spinal cord): | 1.25–4.50 (1.98) | Pre-Op | 2.75–6.78 (5.35) | 2.84–6.72 (4.35) | 2.80–7.80 (4.42) |
| Post-Op | N.A. | N.A. | N.A. | ||
| Deteriorated cases: post-op 6 months | 2.85–7.82 (5.35) | 243–5.53 (4.35) | 2.67–7.22 (3.25) | ||
| % Cardiac cycle (%) | |||||
| 4th ventricle | 35–48 (32) | Pre-Op | 28–50 (32) | 20–48 (31) | 23–51 (33) |
| Post-Op | 12–52 (35) | 9–48 (32) | 18–49 (34) | ||
| Deteriorated cases: post-op 6 months | 26–52 (34) | 22–50 (31) | 24–53 343) | ||
| Outlet of foramen of Magendie | 21–38 (32) | Pre-Op | 28–52 (34) | 25–50 (36) | 24–49 (35) |
| Post-Op | 14–52 (34) | 29–49 (30) | 28–47 (34) | ||
| Deteriorated cases: post-op 6 months | 28–52 (34) | 25–50 (36) | 24–49 (35) | ||
| Syrinx (spinal cord) | 25–45 (38) | Pre-Op | 27–47 (35) | 25–48 (35) | 23–49 (35) |
| Post-Op | N.A. | N.A. | N.A. | ||
| Deteriorated cases: post-op 6 months | 14–47 (35) | 29–49 (33) | 29–46 (35) | ||
| Caudal acceleration (cm/sec2) | |||||
| 4th ventricle | 5.68–12.5 (10.2) | Pre-Op | 2.8–4.7 (3.4) * | 2.1–3.2 (2.5) * | 5.6–3.7 (2.8) * |
| Post-Op | 4.4–12.6 (11.3) | 5.4–14.7 (12.8) | 5.4–13.7 (10.9) | ||
| Deteriorated cases: post-op 6 months | 2.8–4.7 (3.4) * | 2.1–3.7 (2.5) * | 5.6–3.7 (2.8) * | ||
| Outlet of foramen of Magendie | 5.70–12.6 (11.4) | Pre-Op | 3.4 –7.5 (5.3) * | 3.8–8.7 (4.4) * | 3.3–7.2 (5.4) * |
| Post-Op | 5.9–14. (12.2) | 6.8–18.7 (13.5) | 5.9–7.5 (125) | ||
| Deteriorated cases: post-op 6 months | 3.3 –7.3 (5.0) * | 3.5–8.7 (4.3) * | 3.4–7.3 (5.4) * | ||
| Syrinx (spinal cord) | 1.25–4.50 (2.80) | Pre-Op | 1.7–4.7 (4.3) | 1.8–4.2 (4.3) | 1.0–4.8 (4.4) |
| Post-Op | N.A. | N.A. | N.A. | ||
| Deteriorated cases: post-op 6 months | 1.45–3.50 (2.3) | 1.28–32.40 (21.32) | 1.44–32.33 (31.48) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, M.; Yamagata, T.; Naito, K.; Kunihiro, N.; Sakamoto, H.; Hara, M.; Ohata, K.; Goto, T. Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics. J. Clin. Med. 2022, 11, 4556. https://doi.org/10.3390/jcm11154556
Nishikawa M, Yamagata T, Naito K, Kunihiro N, Sakamoto H, Hara M, Ohata K, Goto T. Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics. Journal of Clinical Medicine. 2022; 11(15):4556. https://doi.org/10.3390/jcm11154556
Chicago/Turabian StyleNishikawa, Misao, Toru Yamagata, Kentarou Naito, Noritsugu Kunihiro, Hiroaki Sakamoto, Mistuhiro Hara, Kenji Ohata, and Takeo Goto. 2022. "Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics" Journal of Clinical Medicine 11, no. 15: 4556. https://doi.org/10.3390/jcm11154556
APA StyleNishikawa, M., Yamagata, T., Naito, K., Kunihiro, N., Sakamoto, H., Hara, M., Ohata, K., & Goto, T. (2022). Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics. Journal of Clinical Medicine, 11(15), 4556. https://doi.org/10.3390/jcm11154556






