Proton Pump Inhibitors Use and Risk of Preeclampsia: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
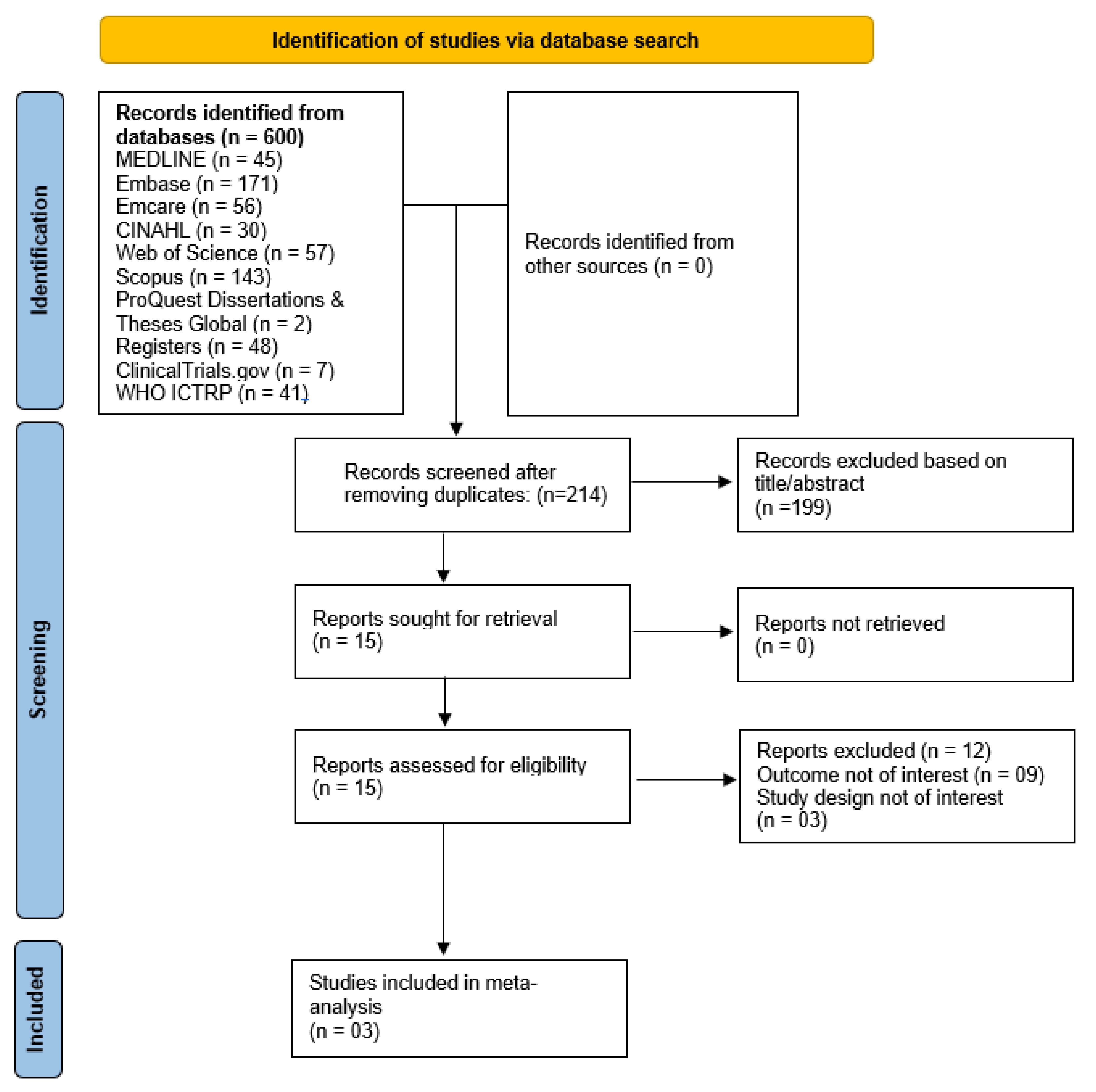
2.3. Study Selection/Inclusion Criteria
2.4. Data Extraction and Risk of Bias
2.5. Certainty of Evidence
2.6. Statistical Analysis
3. Results
3.1. Studies Characteristics
3.2. Quality Assessment and Certainty of Evidence
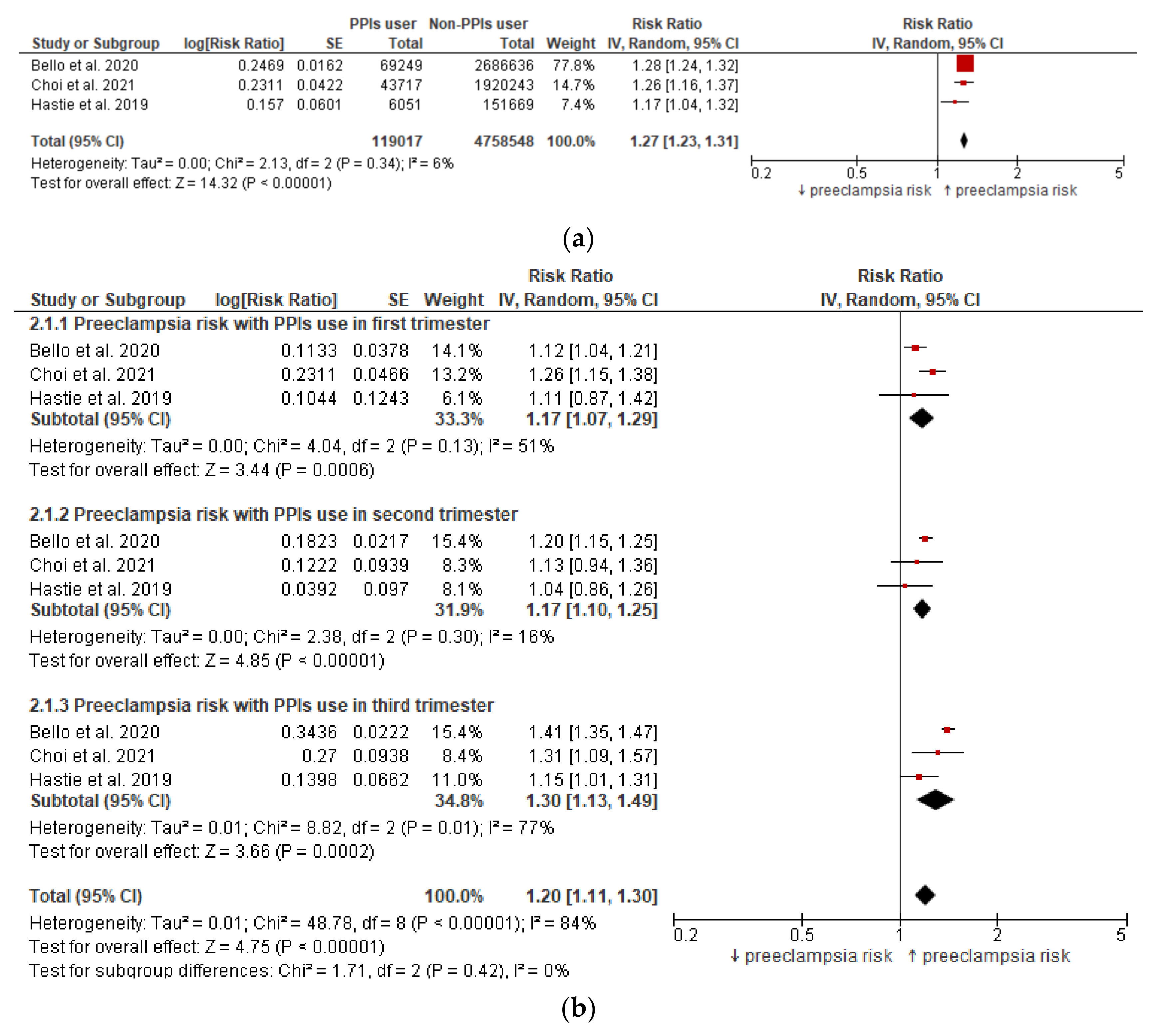
3.3. Meta-Analysis (Preeclampsia Risk)
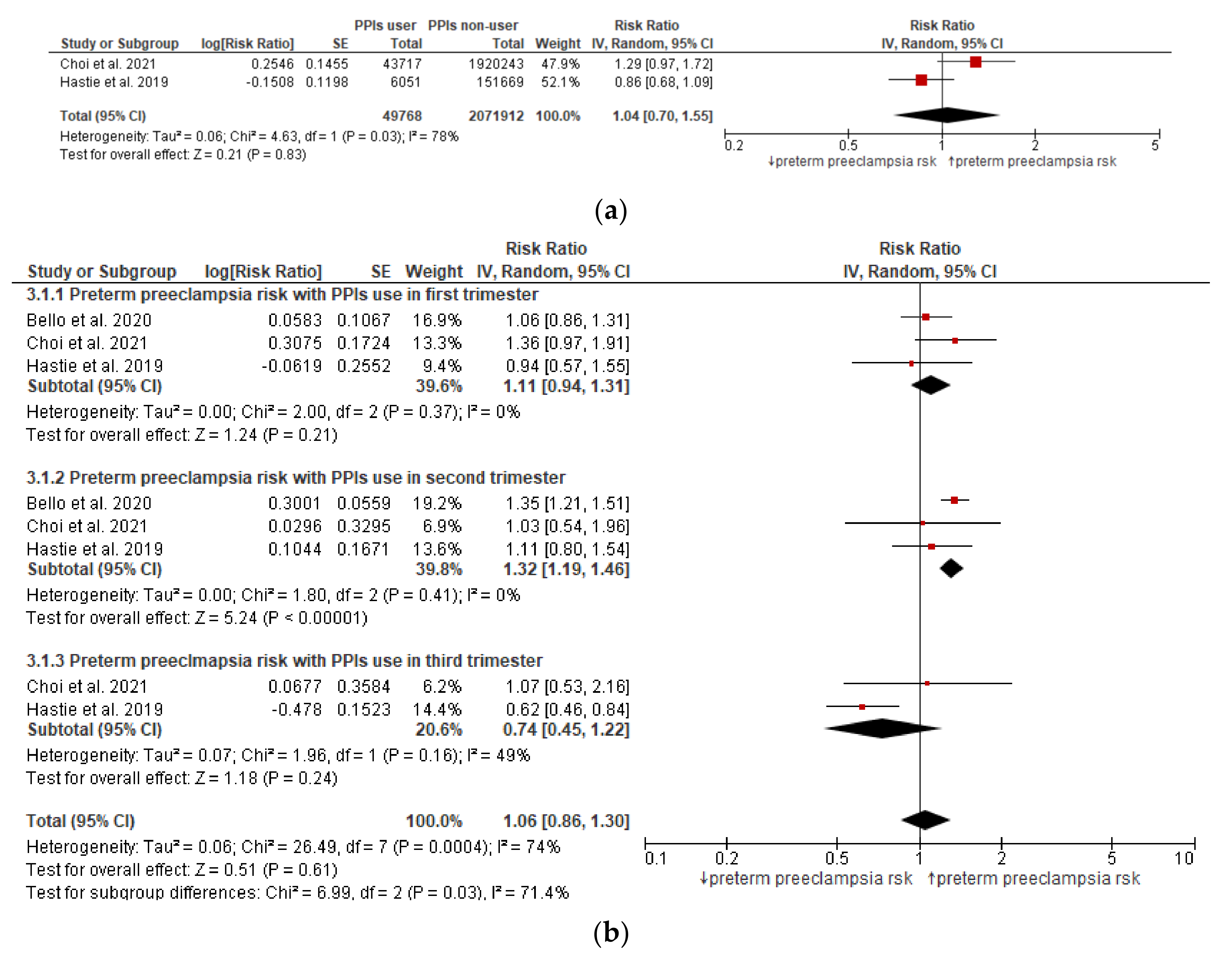
3.4. Meta-Analysis (Preterm Preeclampsia Risk)
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Mayrink, J.; Souza, R.; Feitosa, F.E.; Filho, E.A.R.; Leite, D.; Vettorazzi, J.; Calderon, I.; Sousa, M.H.; Costa, M.L.; Preterm SAMBA study group; et al. Incidence and risk factors for Preeclampsia in a cohort of healthy nulliparous pregnant women: A nested case-control study. Sci. Rep. 2019, 9, 9517. [Google Scholar] [CrossRef] [PubMed]
- García-Basteiro, A.L.; Quintó, L.; Macete, E.; Bardají, A.; González, R.; Nhacolo, A.; Sigauque, B.; Sacoor, C.; Rupérez, M.; Sicuri, E.; et al. Infant mortality and morbidity associated with preterm and small-for-gestational-age births in Southern Mozambique: A retrospective cohort study. PLoS ONE 2017, 12, e0172533. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Sundquist, K. Preterm delivery and long term mortality in women: National cohort and co-sibling study. BMJ 2020, 370, m2533. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, B.M.; Class, Q.A.; Rickert, M.E.; Larsson, H.; Långström, N.; Lichtenstein, P. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry 2013, 70, 1231–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, N.J.; Kaitu’u-Lino, T.U.; Tuohey, L.; Brownfoot, F.; Tong, S.; Onda, K. Proton Pump Inhibitors Induce Heme-Oxygenase-1 and Decrease sFlt1 and sEng Production in Primary Placental and Endothelial Cells: A Novel Candidate Therapeutic for Preeclampsia. In Reproductive Sciences; Sage Publications Inc.: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Onda, K.; Hannan, N.; Beard, S. [6-OR]: Proton pump inhibitors for treatment of preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2015, 5, 3. [Google Scholar]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- Tong, S.; Tu’uhevaha, J.; Hastie, R.; Brownfoot, F.; Cluver, C.; Hannan, N. Pravastatin, proton pump inhibitors, metformin, micronutrients and biologics: New horizons for the prevention or treatment of preeclampsia. Am. J. Obstet. Gynecol. 2020, 226, S1157–S1170. [Google Scholar] [CrossRef]
- Binder, N.K.; Brownfoot, F.C.; Beard, S.; Cannon, P.; Nguyen, T.V.; Tong, S.; Kaitu’U-Lino, T.J.; Hannan, N.J. Esomeprazole and sulfasalazine in combination additively reduce sFlt-1 secretion and diminish endothelial dysfunction: Potential for a combination treatment for preeclampsia. Pregnancy Hypertens. Health 2020, 22, 86–92. [Google Scholar] [CrossRef]
- Saleh, L.; Samantar, R.; Garrelds, I.M.; Meiracker, A.H.V.D.; Visser, W.; Danser, A.J. Low Soluble Fms-Like Tyrosine Kinase-1, Endoglin, and Endothelin-1 Levels in Women with Confirmed or Suspected Preeclampsia Using Proton Pump Inhibitors. Hypertension 2017, 70, 594–600. [Google Scholar] [CrossRef]
- Des Varannes, S.B.; Coron, E.; Galmiche, J.-P. Short and long-term PPI treatment for GERD. Do we need more-potent anti-secretory drugs? Best Pract. Res. Clin. Gastroenterol. 2010, 24, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Siddiqui, A.N.; Habib, A.; Hussain, S.; Najmi, A.K. Proton pump inhibitors’ use and risk of hip fracture: A systematic review and meta-analysis. Rheumatol. Int. 2018, 38, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Habib, A.; Najmi, A.K. Proton pump inhibitors use and risk of chronic kidney disease: Evidence-based meta-analysis of observational studies. Clin. Epidemiol. Glob. Health 2019, 7, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Singh, A.; Zameer, S.; Jamali, M.C.; Baxi, H.; Rahman, S.O.; Alam, M.; Altamish, M.; Singh, A.K.; Anil, D.; et al. No association between proton pump inhibitor use and risk of dementia: Evidence from a meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 19–28. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, S.; Jha, R.; Jayraj, A.S.; Klugar, M.; Antony, B. Proton pump inhibitor use and the risk of hepatocellular carcinoma: A systematic review of pharmacoepidemiological data. J. Evid. Based Med. 2021, 14, 278–280. [Google Scholar] [CrossRef]
- Johnson, D.A.; Katz, P.O.; Armstrong, D.; Cohen, H.; Delaney, B.C.; Howden, C.W.; Katelaris, P.; Tutuian, R.I.; Castell, D.O. The Safety of Appropriate Use of Over-the-Counter Proton Pump Inhibitors: An Evidence-Based Review and Delphi Consensus. Drugs 2017, 77, 547–561. [Google Scholar] [CrossRef]
- Pasternak, B.; Hviid, A. Use of Proton-Pump Inhibitors in Early Pregnancy and the Risk of Birth Defects. N. Engl. J. Med. 2010, 363, 2114–2123. [Google Scholar] [CrossRef]
- Li, C.M.; Zhernakova, A.; Engstrand, L.; Wijmenga, C.; Brusselaers, N. Systematic review with meta-analysis: The risks of proton pump inhibitors during pregnancy. Aliment. Pharmacol. Ther. 2020, 51, 410–420. [Google Scholar] [CrossRef]
- Hastie, R.; Bergman, L.; Cluver, C.A.; Wikman, A.; Hannan, N.J.; Walker, S.P.; Wikström, A.; Tong, S.; Hesselman, S. Proton Pump Inhibitors and Preeclampsia Risk Among 157 720 Women: A Swedish Population Register–Based Cohort Study. Hypertension 2019, 73, 1097–1103. [Google Scholar] [CrossRef]
- Choi, A.; Noh, Y.; Park, S.-H.; Choe, S.-A.; Shin, J.-Y. Exploration of Proton Pump Inhibitors Use During Pregnancy and Preeclampsia. JAMA Netw. Open 2021, 4, e2124339. [Google Scholar] [CrossRef]
- Bello, N.A.; Huang, Y.; Syeda, S.K.; Wright, J.D.; D’Alton, M.E.; Friedman, A.M. Receipt of Proton-Pump Inhibitors during Pregnancy and Risk for Preeclampsia. Am. J. Perinatol. 2020, 38, 1519–1525. [Google Scholar] [CrossRef]
- Hussain, S.; Singh, A.; Antony, B.; Klugarová, J.; Klugar, M. Proton pump inhibitors use and risk of preeclampsia. medRxiv 2021. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2008, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Antony BS, E.; Kulgarova, J.; Licenik, R.; Klugar, M. Association of acute kidney injury with the risk of dementia: A meta-analysis protocol. medRxiv 2021. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 4 October 2021).
- Viswanathan, M.; Patnode, C.D.; Berkman, N.D.; Bass, E.; Chang, S.; Hartling, L.; Murad, M.H.; Treadwell, J.R.; Kane, R.L. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J. Clin. Epidemiol. 2018, 97, 26–34. [Google Scholar] [CrossRef]
- Group, G.W. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Kwok, C.S.; Jeevanantham, V.; Dawn, B.; Loke, Y.K. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Meta-analysis. Int. J. Cardiol. 2013, 167, 965–974. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, S.; Najmi, A.K. Number of studies, heterogeneity, generalisability, and the choice of method for meta-analysis. J. Neurol. Sci. 2017, 381, 347. [Google Scholar] [CrossRef]
- McMaster University. Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. 2022. Available online: gradepro.org (accessed on 4 October 2021).
- Cluver, C.A.; Hannan, N.J.; van Papendorp, E.; Hiscock, R.; Beard, S.; Mol, B.W.; Theron, G.B.; Hall, D.R.; Decloedt, E.H.; Stander, M.; et al. Esomeprazole to treat women with preterm preeclampsia: A randomized placebo controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 388.e1–388.e17. [Google Scholar] [CrossRef]
- Abbas, A.M.; Othman, Y.M.; Abdallah, M.M.; Ellah, N.H.A.; Azim, H.G.A.; Shaamash, A.H. Effect of esomeprazole on maternal serum soluble fms-like tyrosine kinase-1 and endoglin in patients with early-onset preeclampsia. Proc. Obstet. Gynecol. 2021, 99, 1–14. [Google Scholar] [CrossRef]
- Ramu, B.; Mohan, P.; Rajasekaran, M.S.; Jayanthi, V. Prevalence and risk factors for gastroesophageal reflux in pregnancy. Indian J. Gastroenterol. 2010, 30, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Jenabi, E. The association between body mass index and preeclampsia: A meta-analysis. J. Matern. Neonatal Med. 2016, 29, 3670–3676. [Google Scholar] [CrossRef] [PubMed]
| Criterion | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Pregnant women at any stage of gestation | Non-pregnant women |
| Exposure | Exposure to any proton pump inhibitors
| Drugs other than proton pump inhibitors |
| Comparator | Nonexposure or exposure to H2RA antagonist | N/A |
| Outcomes | Studies reporting:
| Studies reporting any other outcomes:
|
| Study design | Studies assessing preeclampsia risk, including:
| Following was excluded:
|
| Time period | Studies published until September 2021 | N/A |
| Author, Year & Country | Study Design, Setting | Study Duration | Database/Source | Participants | Exposure | Comparator | Outcomes | Cohort Size | Definition of PPI Exposure | Ascertainment of PPI Use | Assessment of Outcome | Effect Estimates | Adjusted for | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||||||||||||
| Bello et al., 2020, US [22] | Cohort study | 2008 to 2014 | Truven Health MarketScan database | Women receiving PPIs during pregnancy in the Truven Health MarketScan Database | PPI user (Esomeprazole, lansoprazole, omeprazole, pantoprazole, dexlansoprazole, and rabeprazole) | No exposure to PPIs | Diagnosis of preecalmpsia | Total: 2,755,885 PPI user: 69,249 Non-PPI user: 2,686,636 | PPI exposure any time during pregnancy or individually during the 1st, 2nd, and 3rd trimesters | Outpatient pharmaceutical claims data | Idiopathic PD diagnosis confirmed by based on the presence of International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis codes for mild (642.4×), severe (642.5×), or superimposed (642.7×) preeclampsia or eclampsia (642.6×). | Preeclampsia Any time PPI use: 1.42 (1.38, 1.46) 1st trimester PPI use: 1.20 (1.11, 1.30) 2nd trimester PPI use: 1.34 (1.28, 1.41) 3rd trimester PPI use: 1.56 (1.50, 1.63) Preterm severe preeclampsia/ Eclampsia 1st trimester PPI use: 1.15 (0.93, 1.43) 2nd trimester PPI use: 1.58 (1.41, 1.77) | Preeclampsia Any time PPI use: 1.28 (1.24, 1.32) 1st trimester PPI use: 1.12 (1.04, 1.22) 2nd trimester PPI use: 1.20 (1.15, 1.26) 3rd trimester PPI use: 1.41 (1.35, 1.47) Preterm severe preeclampsia/ Eclampsia 1st trimester PPI use: 1.06 (0.86, 1.32) 2nd trimester PPI use: 1.35 (1.21, 1.52) | Maternal age, and the five clinical characteristics (chronic kidney disease, autoimmune disease, multiple gestation, Pregestational diabetes, and chronic hypertension) | PPI prescription during pregnancy was not associated with decreased risk for preeclampsia |
| Choi et al., 2021, Korea [21] | Cohort study | 2011 to 2017 | Health Insurance Review and Assessment database | Women receiving PPIs during pregnancy in Korea’s healthcare database | Use of any PPI, including omeprazole, esomeprazole, pantoprazole, rabeprazole, lansoprazole, dexlansoprazole, or ilaprazole at any point across gestation | (1). Non-PPI user, and (2). H2RA user | Diagnosis of preecalmpsia | Total: 1,963,960 PPI user: 43,717 Non-PPI user: 1,920,243 | ≥1 PPI prescription in 4 windows: any time during pregnancy, first, second, and third trimester | Database (based on drug chemical code, prescription supply, dosage, and others) | ICD-10 diagnostic code | Preeclampsia Any time PPI use: 1.55 (1.44–1.68) 1st trimester PPI use: 1.56 (1.42, 1.72) 2nd trimester PPI use: 1.43 (1.19, 1.71) 3rd trimester PPI use: 1.69 (1.42, 2.03) Preterm preeclampsia Any time PPI use: 1.55 (1.18–2.04) 1st trimester PPI use: 1.62 (1.17–2.24) 2nd trimester PPI use: 1.31 (0.68–2.52) 3rd trimester PPI use: 1.37 (0.68–2.74) | Preeclampsia Any time PPI use: 1.26 (1.16–1.36) 1st trimester PPI use: 1.26 (1.15, 1.39) 2nd trimester PPI use: 1.13 (0.94, 1.35) 3rd trimester PPI use: 1.31 (1.09, 1.56) Preterm preeclampsia Any time PPI use: 1.29 (0.97–1.71) 1st trimester PPI use: 1.36 (0.97–1.89) 2nd trimester PPI use: 1.03 (0.54–1.99) 3rd trimester PPI use: 1.07 (0.53–2.14) | Maternal age and insurance type, nulliparity, multiple gestation, CCI, indications for acid suppressive medications, including gastroesophageal reflux disease, heartburn, ulcer (e.g., various ulcers and ZES), maternal medical conditions (e.g., asthma, anxiety, diabetes, depression, and chronic hypertension), inflammatory diseases, migraine/headache, renal disease, thyroid disorder, concurrent medications, and proxies of health care utilization | PPI use during pregnancy was not associated with a reduced risk of preeclampsia |
| Hastie et al., 2019, Sweden [20] | Cohort study | 2013 to 2017 | Swedish pregnancy register | Women receiving PPIs during pregnancy in Swedish pregnancy register | Use of any PPI, including omeprazole, esomeprazole, pantoprazole, rabeprazole, or lansoprazole at any point across gestation | Non-PPI users | Diagnosis of preecalmpsia | Total: 157,720 PPI user: 6051 Non-PPI user: 151,669 | PPI use was categorized by use ever during pregnancy, first trimester (0–12 weeks of gestation), second trimester (13–27 weeks), and third trimester (from 28 weeks of gestation onward). | Based on the prescription record maintained in Swedish pregnancy register | Preeclampsia was identified by the diagnosis codes O14 or O15 according to International Classification of Diseases, Tenth Revision coding (n = 7258) | Preeclampsia Any time PPI use: 1.20 (1.07–1.35) 1st trimester PPI use: 1.20 (0.95, 1.52) 2nd trimester PPI use: 1.15 (0.97, 1.36) 3rd trimester PPI use: 1.21 (1.07, 1.37) Preterm preeclampsia Any time PPI use: 0.90 (0.71–1.13) 1st trimester PPI use: 0.95 (0.59–1.49) 2nd trimester PPI use: 1.13(0.83–1.54) 3rd trimester PPI use: 0.66 (0.40–1.07) | Preeclampsia Any time PPI use: 1.17 (1.04–1.32) 1st trimester PPI use: 1.11 (0.87–1.42) 2nd trimester PPI use: 1.04 (0.86–1.25) 3rd trimester PPI use: 1.15 (1.01–1.32) Preterm preeclampsia Any time PPI use: 0.86 (0.68–1.09) 1st trimester PPI use: 0.94 (0.57–1.54) 2nd trimester PPI use: 1.11(0.80–1.54) 3rd trimester PPI use: 0.62 (0.46–0.84) | Propensity matched (maternal age, body mass index, year of delivery, country of birth, smoking status, educational level, occupation, use of assisted reproduction, and the presence of pregestational disorders | PPIs have a potential role in preventing preterm preeclampsia |
| Cohort Studies | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study author | Representation of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of the study | Comparability of cohorts on the basis of design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Accuracy of follow-up of cohorts | Overall risk of bias |
| Bello, 2020, US [22] | * | * | * | * | ** | * | * | * | Low |
| Choi, 2021, Korea [21] | * | * | * | * | ** | * | * | * | Low |
| Hastie, 2019, Sweden [20] | * | * | * | * | ** | * | * | * | Low |
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Preeclampsia during AnyTime PPIs Use | Placebo | Relative (95% CI) | Absolute (95% CI) | |
| Preeclampsia risk | |||||||||||
| 3 | observational studies | not serious | not serious | not serious | not serious | none | 1294/119,017 (1.1%) | 31,204/4,758,548 (0.7%) | RR 1.27 (1.23 to 1.31) | 2 more per 1000 (from 2 more to 2 more) | ⨁⨁◯◯ Low |
| Certainty assessment | № of patients | Effect | Certainty | ||||||||
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Preterm preeclampsia during Anytime PPIs Use | Placebo | Relative (95% CI) | Absolute (95% CI) | |
| Preterm preeclampsia risk | |||||||||||
| 2 | observational studies | not serious | serious a | not serious | not serious | none | 129/49,768 (0.3%) | 3626/2,071,912(0.2%) | RR 1.04 (0.70–1.55) | 0 fewer per 1000 (from 1 fewer to 1 more) | ⨁◯◯◯ VERY Low |
| Trial Number | Trial Name or Title | Methods | Participants | Interventions | Outcomes | Start Date | Recruitment Status | Link to Trials |
|---|---|---|---|---|---|---|---|---|
| NCT03717740 | Esomeprazole for the Prevention of Preeclampsia | Randomized double-blinded placebo-controlled intervention trial | Pregnant women presenting prior to 17 + 0 weeks’ gestation with moderate to high risk of preeclampsia | Esomeprazole single dose of 40 mg orally once a day from 12+ and 17 weeks of pregnancy until 34 weeks of pregnancy | Primary Outcome Measures: Number of Participants With early onset Preeclampsia Secondary Outcome Measures:
| 1 December 2018 | Recruiting | https://clinicaltrials.gov/ct2/show/NCT03717740 (accessed on 4 October 2021) |
| NCT03717701 | Metformin and Esomeprazole in Treatment of Early Onset Preeclampsia | Randomized double-blinded placebo-controlled intervention trial | Pregnant women presenting at a Gestational age between 28 + 0 weeks and 32 + 0 weeks presented with preterm preeclampsia | Metformin 1000 mg orally once a day; Esmoperazole 40 mg orally once a day | Primary Outcome Measures: Prolongation of gestation measured from the time of enrollment to the time of delivery. Secondary Outcome Measures:
| 1 December 2018 | Recruiting | https://clinicaltrials.gov/ct2/show/NCT03717701 (accessed on 4 October 2021) |
| NCT03724838 | Esomeprazole With Sildenafil Citrate in Women With Early-onset Preeclampsia | Randomized, double-blind, placebo-controlled trial | Pregnant women presenting at a Gestational age between 28 + 0 weeks and 32 + 0 weeks presented with preterm preeclampsia | Patients will take esomeprazole single dose of 40 mg orally once a day; Patients will take Sildenafil Citrate 40 mg every 8 h; other comparators | Primary outcome measures: Prolongation of gestation measured from the time of enrollment to the time of delivery Secondary outcome measures:
| 1 December 2018 | Recruiting | https://clinicaltrials.gov/ct2/show/NCT03724838 (accessed on 4 October 2021) |
| EUCTR2018-000283-28-NL or Netherland Trial Register L7718 | Potential effect of proton-pump inhibitor on angiogenic markers in preeclampsia: a pilot study | Randomised controlled trial | Women with (≥18 years) with a singleton pregnancy diagnosed with PE with a gestational age of ≥20 weeks and <34 weeks | Omeprazole | Primary outcome measures: The difference in sFlt-1 levels in women who have received PPI, in comparison to women who have not received PPI, at different time points. Secondary outcome measures:
| 17 December 2018 | Ongoing | https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000283-28/NL; (accessed on 4 October 2021) https://www.trialregister.nl/trial/7718 (accessed on 4 October 2021) |
| IRCT2017082333680N2 | The evaluation of esomeprazole efficacy in treatment of early onset pre- eclampsia | Randomized, single-blind, placebo-controlled trial | Pregnant women with hypertensive Pregnancy and the gestational age between 26 to 32 weeks with single-crowned pregnanc | The intervention group received 12 mg Betamethasone in two doses every 24 h plus prescribed 40 mg osmoparazole daily. The control group received 12 mg Betamethasone in two doses every 24 h plus prescribed 40 mg placebo daily. | Primary outcome measure: Duration of admission to delivery Secondary outcome measure:
| 18 April 2017 | Ongoing | https://en.irct.ir/trial/25917 (accessed on 4 October 2021) |
| ChiCTR1900026972 | A randomized controlled trial for efficacy of esomeprazole in the treatment of early-onset preeclampsia | Randomized controlled trial | Pregnant women with gestational age between 26 + 0 weeks and 33+ 6 weeks; Diagnosis of pre-eclampsia, gestational hypertension | Forty milligrams of esomeprazole+ Standard treatment vs. control group | Primary outcome measure: Duration of admission to delivery Secondary outcome measures: The change in levels of sFlt-1, and sEndoglin | 1 January 2020 | Recruiting | https://www.chictr.org.cn/showprojen.aspx?proj=44939 (accessed on 4 October 2021) |
| ACTRN12618000690257 | A Prospective, Pre-ecLampsia/Eclampsia Prevention IntervEntion | Multi-centre, double blind, randomised, placebo-controlled trial | Nulliparous women with singleton pregnancy (12–20 weeks) | Forty milligrams of oral esomeprazole tablets once daily | Primary outcome measure: Incidence of preeclampsia Secondary outcome measure:
| 31 October 2018 | Recruiting | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374798 (accessed on 4 October 2021) |
| ACTRN12618001755224 | Can esomeprazole improve outcomes in women at high risk of pre-eclampsia? The ESPRESSO Study | Multi-centre, double blind, randomised, placebo-controlled superiority trial. | Pregnant women screened at 11 + 0 to 13 + 6 weeks gestation and at high risk (>1%) of pre-eclampsia | Esomeprazole 40 mg oral tablet once a day prior to 16 weeks gestation and continuing until delivery of pregnancy. Aspirin 150 mg oral tablet at night commencing prior to 16 weeks gestation and continuing until 36 weeks gestation as a background therapy | Primary outcome measures: Mean arterial pressure, measured by 24-h ambulatory blood pressure at 36 weeks of gestation Secondary outcome measures:
| 18 April 2019 | Recruiting | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375343 (accessed on 4 October 2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Singh, A.; Antony, B.; Klugarová, J.; Murad, M.H.; Jayraj, A.S.; Langaufová, A.; Klugar, M. Proton Pump Inhibitors Use and Risk of Preeclampsia: A Meta-Analysis. J. Clin. Med. 2022, 11, 4675. https://doi.org/10.3390/jcm11164675
Hussain S, Singh A, Antony B, Klugarová J, Murad MH, Jayraj AS, Langaufová A, Klugar M. Proton Pump Inhibitors Use and Risk of Preeclampsia: A Meta-Analysis. Journal of Clinical Medicine. 2022; 11(16):4675. https://doi.org/10.3390/jcm11164675
Chicago/Turabian StyleHussain, Salman, Ambrish Singh, Benny Antony, Jitka Klugarová, M. Hassan Murad, Aarthi S. Jayraj, Alena Langaufová, and Miloslav Klugar. 2022. "Proton Pump Inhibitors Use and Risk of Preeclampsia: A Meta-Analysis" Journal of Clinical Medicine 11, no. 16: 4675. https://doi.org/10.3390/jcm11164675
APA StyleHussain, S., Singh, A., Antony, B., Klugarová, J., Murad, M. H., Jayraj, A. S., Langaufová, A., & Klugar, M. (2022). Proton Pump Inhibitors Use and Risk of Preeclampsia: A Meta-Analysis. Journal of Clinical Medicine, 11(16), 4675. https://doi.org/10.3390/jcm11164675









