Monoclonal Antibodies in Treating Chronic Spontaneous Urticaria: New Drugs for an Old Disease
Abstract
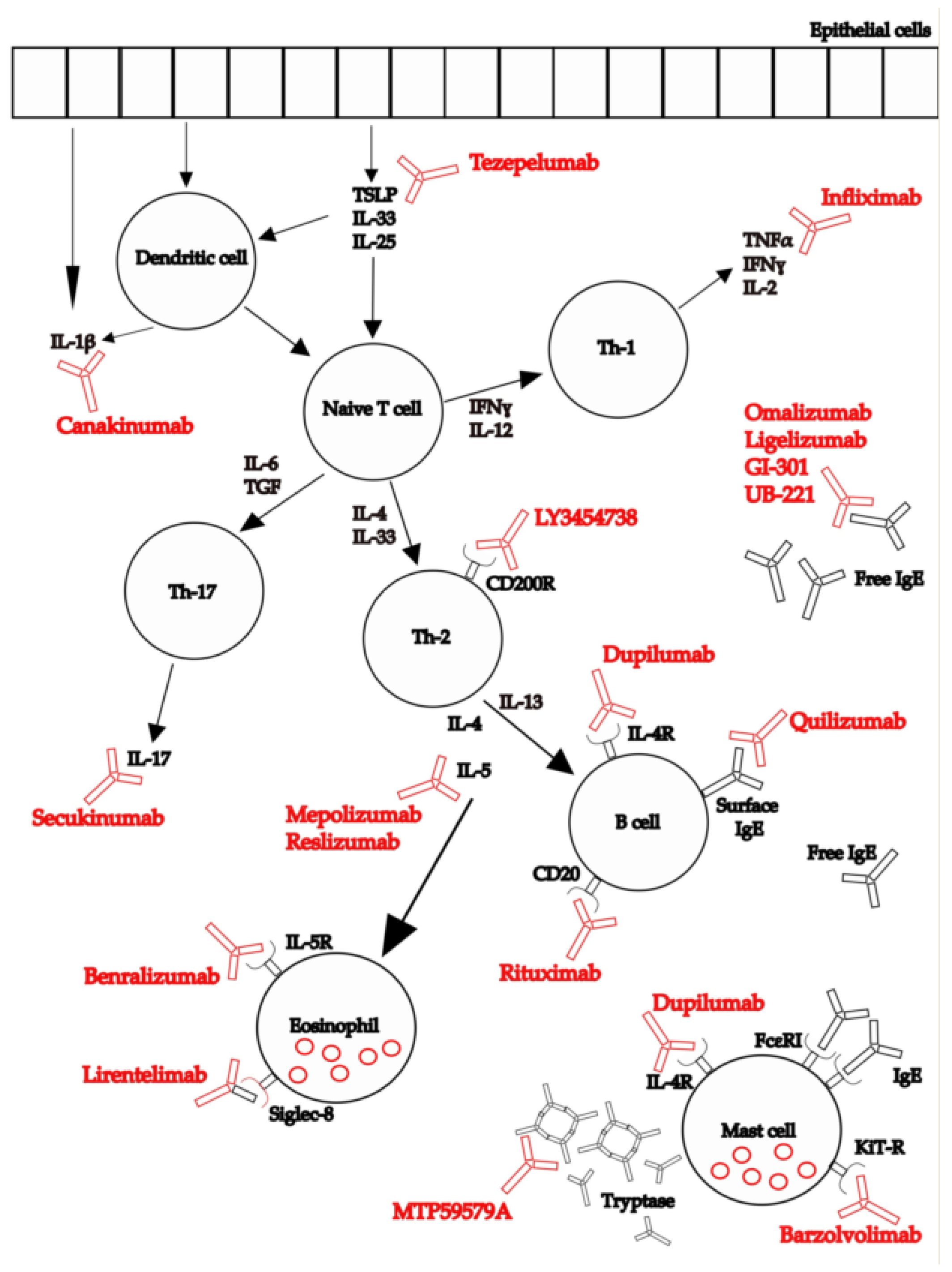
:1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Eligibility Criteria
2.3. Guideline Review
3. Results
3.1. Anti-IgE
3.1.1. Omalizumab
- QoL
- Safety
3.1.2. Ligelizumab
3.1.3. Quilizumab
3.1.4. Other Anti-IgE
3.2. Anti-IL-5
3.2.1. Mepolizumab
3.2.2. Reslizumab
3.2.3. Benralizumab
3.3. Anti-IL-4 Receptor
Dupilumab
3.4. Anti-CD20
Rituximab
3.5. Anti-IL-17
Secukinumab
3.6. Anti-IL-1
Canakinumab
3.7. Anti-Tumor Necrosis Factore Alfa (TNF-α)
Infliximab
3.8. Anti-TSLP
Tezepelumab
3.9. Other Biologics
3.9.1. Barzolvolimab
3.9.2. MTPS9579A
3.9.3. LY3454738
3.9.4. Lirentelimab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| AE-QoL | angioedema quality of life questionnaire |
| BTK | Bruton’s tyrosine kinase |
| CI | confidence interval |
| CRS | chronic rhinosinusitis |
| CU | chronic urticaria |
| CU-Q2oL | chronic urticaria quality of life questionnaire |
| CSU | chronic spontaneous urticaria |
| FDA | Food and Drug Administration |
| EMA | European Medicine Agency |
| FcεRI | high affinity IgE receptor |
| DLQI | dermatology life quality index |
| H1AH | H1 antihistamines |
| H2AH | H2 antihistamines |
| HRQoL | health-related quality of life |
| HSS7 | weekly hives severity score |
| IgE | immunoglobulin E |
| IgG | immunoglobulin G |
| ISI | insomnia severity index |
| ISS7 | weekly itch severity score |
| LTRAs | leukotriene receptor antagonists |
| mAbs | monoclonal antibodies |
| OR | odds ratio |
| RDBPCT | randomized double-blind placebo controlled trial |
| RCTs | randomized controlled trials |
| SAEs | serious adverse events |
| TPO | thyroperoxidase |
| TSLP | anti-thymic stromal lymphopoietin |
| UAS7 | weekly urticaria activity score |
| Versus | vs. |
| WPAI | work productivity and activity impairment |
Appendix A
- Biologic drugs
- Biological
- Monoclonal antibody
- Treatment
- Omalizumab
- Anti-IgE
- Mepolizumab
- Anti-IL-5
- Dupilumab
- Tezepelumab
- Anti-thymic stromal lymphopoietin
- Chronic spontaneous urticaria
- Chronic urticaria
- Child
- Children
- Adolescent
- Adult
- 1 or 2 or 3 or 4
- 18 and 5 and/or 6
- 18 and 7 and/or 8
- 18 and 9
- 18 and 12 and/or 13
- 22 and 14 and/or 15 and/or 16
- 22 and 17
- Guideline/or practice guideline/as topic/or practice guidelines as topic/
- (guideline* or algorithm* or standard*).ti.ab.
- “best practice”.ti.ab.
- Meta-analysis, systematic review, review, original article, case series, case report, letter
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Ansotegui, I.J.; Baiardini, I.; Bernstein, J.; Canonica, G.W.; Ebisawa, M.; Gomez, M.; Gonzalez-Diaz, S.N.; Martin, B.; Morais-Almeida, M.; et al. The challenges of chronic urticaria part 1: Epidemiology, immunopathogenesis, comorbidities, quality of life, and management. World Allergy Organ. J. 2021, 14, 100533. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Ávila, G.; Keller, T.; Weller, K.; Lau, S.; Maurer, M.; Zuberbier, T.; Keil, T. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy 2020, 75, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.K. Management of chronic urticaria in Asia: 2010 AADV consensus guidelines. Asia Pac. Allergy 2012, 2, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Kay, A.B.; Clark, P.; Maurer, M.; Ying, S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br. J. Dermatol. 2015, 172, 1294–1302. [Google Scholar] [CrossRef]
- Bracken, S.J.; Abraham, S.; MacLeod, A.S. Autoimmune Theories of Chronic Spontaneous Urticaria. Front. Immunol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Hide, M.; Francis, D.M.; Grattan, C.E.; Hakimi, J.; Kochan, J.P.; Greaves, M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993, 328, 1599–1604. [Google Scholar] [CrossRef]
- Tong, L.J.; Balakrishnan, G.; Kochan, J.P.; Kinét, J.P.; Kaplan, A. Assessment of autoimmunity in patients with chronic urticaria. J. Allergy Clin. Immunol. 1997, 99, 461–465. [Google Scholar] [CrossRef]
- Kolkhir, P.; Metz, M.; Altrichter, S.; Maurer, M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: A systematic review. Allergy 2017, 72, 1440–1460. [Google Scholar] [CrossRef]
- Altrichter, S.; Peter, H.J.; Pisarevskaja, D.; Metz, M.; Martus, P.; Maurer, M. IgE mediated autoallergy against thyroid peroxidase—A novel pathomechanism of chronic spontaneous urticaria? PLoS ONE 2011, 6, e14794. [Google Scholar] [CrossRef]
- Çildağ, S.; Yenisey, Ç.; Ünübol, M.; Şentürk, T. Comparison of immunoglobulin E anti-thyroid peroxidase antibodies in patients with Hashimoto thyroiditis and chronic spontaneous urticaria. Med. Pharm. Rep. 2021, 94, 53–57. [Google Scholar] [CrossRef]
- Tienforti, D.; Di Giulio, F.; Spagnolo, L.; Castellini, C.; Totaro, M.; Muselli, M.; Francavilla, S.; Baroni, M.G.; Barbonetti, A. Chronic urticaria and thyroid autoimmunity: A meta-analysis of case-control studies. J. Endocrinol. Investig. 2022, 45, 45, 1317–1326. [Google Scholar] [CrossRef]
- Cugno, M.; Marzano, A.V.; Asero, R.; Tedeschi, A. Activation of blood coagulation in chronic urticaria: Pathophysiological and clinical implications. Intern. Emerg. Med. 2010, 5, 97–101. [Google Scholar] [CrossRef]
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.A.; Ensina, L.F.; Fomina, D.; Galvàn, C.A.; Godse, K.; Grattan, C.; et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef]
- Maurer, M.; Abuzakouk, M.; Bérard, F.; Canonica, W.; Oude Elberink, H.; Giménez-Arnau, A.; Grattan, C.; Hollis, K.; Knulst, A.; Lacour, J.P.; et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017, 72, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Aguinaga, S.; Jáuregui Presa, I.; Aguinaga-Ontoso, E.; Guillén-Grima, F.; Ferrer, M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: A systematic review and meta-analysis. Br. J. Dermatol. 2016, 175, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Church, M.K.; Gonçalo, M.; Sussman, G.; Sánchez-Borges, M. Management and treatment of chronic urticaria (CU). J. Eur. Acad. Dermatol. Venereol. 2015, 29 (Suppl. S3), 16–32. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Salpietro, C.; Cuppari, C. Antihistamines: Recommended Dosage–Divergence between Clinical Practice and Guideline Recommendations. Int. Arch. Allergy Immunol. 2019, 178, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P. Chronic Spontaneous Urticaria: Pathogenesis and Treatment Considerations. Allergy Asthma Immunol. Res. 2017, 9, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Taylor, P.C.; Feldmann, M. Monoclonal antibodies in immune and inflammatory diseases. Curr. Opin. Biotechnol. 2002, 13, 615–620. [Google Scholar] [CrossRef]
- Licari, A.; Manti, S.; Castagnoli, R.; Marseglia, A.; Foiadelli, T.; Brambilla, I.; Marseglia, G.L. Immunomodulation in Pediatric Asthma. Front. Pediatrics 2019, 7, 289. [Google Scholar] [CrossRef]
- Licari, A.; Manti, S.; Marseglia, A.; De Filippo, M.; De Sando, E.; Foiadelli, T.; Marseglia, G.L. Biologics in Children with Allergic Diseases. Curr. Pediatr. Rev. 2020, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency|(europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xolair (accessed on 28 May 2022).
- Saini, S.; Rosen, K.E.; Hsieh, H.J.; Wong, D.A.; Conner, E.; Kaplan, A.; Spector, S.; Maurer, M. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J. Allergy Clin. Immunol. 2011, 128, 567–573.e1. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Altrichter, S.; Bieber, T.; Biedermann, T.; Bräutigam, M.; Seyfried, S.; Brehler, R.; Grabbe, J.; Hunzelmann, N.; Jakob, T.; et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J. Allergy Clin. Immunol. 2011, 128, 202–209.e5. [Google Scholar] [CrossRef]
- Kaplan, A.; Ledford, D.; Ashby, M.; Canvin, J.; Zazzali, J.L.; Conner, E.; Veith, J.; Kamath, N.; Staubach, P.; Jakob, T.; et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J. Allergy Clin. Immunol. 2013, 132, 101–109. [Google Scholar] [CrossRef]
- Maurer, M.; Rosén, K.; Hsieh, H.J.; Saini, S.; Grattan, C.; Gimenéz-Arnau, A.; Agarwal, S.; Doyle, R.; Canvin, J.; Kaplan, A.; et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N. Engl. J. Med. 2013, 368, 924–935. [Google Scholar] [CrossRef]
- Saini, S.S.; Bindslev-Jensen, C.; Maurer, M.; Grob, J.J.; Bülbül Baskan, E.; Bradley, M.S.; Canvin, J.; Rahmaoui, A.; Georgiou, P.; Alpan, O.; et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: A randomized, placebo-controlled study. J. Investig. Dermatol. 2015, 135, 67–75. [Google Scholar] [CrossRef]
- Staubach, P.; Metz, M.; Chapman-Rothe, N.; Sieder, C.; Bräutigam, M.; Canvin, J.; Maurer, M. Effect of omalizumab on angioedema in H1-antihistamine-resistant chronic spontaneous urticaria patients: Results from X-ACT, a randomized controlled trial. Allergy 2016, 71, 1135–1144. [Google Scholar] [CrossRef]
- Staubach, P.; Metz, M.; Chapman-Rothe, N.; Sieder, C.; Bräutigam, M.; Maurer, M.; Weller, K. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy 2018, 73, 576–584. [Google Scholar] [CrossRef]
- Hide, M.; Park, H.S.; Igarashi, A.; Ye, Y.M.; Kim, T.B.; Yagami, A.; Roh, J.; Lee, J.H.; Chinuki, Y.; Youn, S.W.; et al. Efficacy and safety of omalizumab in Japanese and Korean patients with refractory chronic spontaneous urticaria. J. Dermatol. Sci. 2017, 87, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Kaplan, A.; Rosén, K.; Holden, M.; Iqbal, A.; Trzaskoma, B.L.; Yang, M.; Casale, T.B. The XTEND-CIU study: Long-term use of omalizumab in chronic idiopathic urticaria. J. Allergy Clin. Immunol. 2018, 141, 1138–1139.e7. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Murphy, T.R.; Holden, M.; Rajput, Y.; Yoo, B.; Bernstein, J.A. Impact of omalizumab on patient-reported outcomes in chronic idiopathic urticaria: Results from a randomized study (XTEND-CIU). J Allergy Clin. Immunol. Pract. 2019, 7, 2487–2490.e1. [Google Scholar] [CrossRef] [PubMed]
- Sussman, G.; Hébert, J.; Gulliver, W.; Lynde, C.; Yang, W.H.; Papp, K.; Gooderham, M.; Chambenoit, O.; Khalil, S.; DeTakacsy, F.; et al. Omalizumab Re-Treatment and Step-Up in Patients with Chronic Spontaneous Urticaria: OPTIMA Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 2372–2378. [Google Scholar] [CrossRef]
- Yuan, W.; Hu, S.; Li, M.; Yang, L.; Liu, L.; Zheng, M.; Guo, Z.; Song, Z.; Zhang, C.; Diao, Q.; et al. Efficacy and safety of omalizumab in Chinese patients with anti-histamine refractory chronic spontaneous urticaria. Dermatol. Ther. 2022, 35, e15303. [Google Scholar] [CrossRef]
- Maurer, M.; Giménez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.Y.; Chung, W.-H.; et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.; Maurer, M.; Bernstein, J.; Staubach, P.; Barbier, N.; Hua, E.; Severin, T.; Joubert, Y.; Janocha, R.; Balp, M.M. Ligelizumab improves sleep interference and disease burden in patients with chronic spontaneous urticaria. Clin. Transl. Allergy 2022, 12, e12121. [Google Scholar] [CrossRef]
- Maurer, M.; Giménez-Arnau, A.; Bernstein, J.A.; Chu, C.Y.; Danilycheva, I.; Hide, M.; Makris, M.; Metz, M.; Savic, S.; Sitz, K.; et al. Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: A one-year extension study. Allergy 2021, 77, 2175–2184. [Google Scholar] [CrossRef]
- Study to Investigate the Efficacy and Safety of QGE031 in Adolescent Patients with Chronic Spontaneous Urticaria (CSU)–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03437278 (accessed on 11 July 2022).
- Harris, J.M.; Cabanski, C.R.; Scheerens, H.; Samineni, D.; Bradley, M.S.; Cochran, C.; Staubach, P.; Metz, M.; Sussman, G.; Maurer, M. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2016, 138, 1730–1732. [Google Scholar] [CrossRef]
- Magerl, M.; Terhorst, D.; Metz, M.; Altrichter, S.; Zuberbier, T.; Maurer, M.; Bergmann, K.C. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J. Dtsch. Dermatol. Ges. 2018, 16, 477–478. [Google Scholar] [CrossRef]
- Maurer, M.; Altrichter, S.; Metz, M.; Zuberbier, T.; Church, M.K.; Bergmann, K.C. Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e112–e113. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Benralizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2020, 383, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Simpson, R.S. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J. Allergy Clin. Immunol. Pract. 2019, 7, 1659–1661.e1. [Google Scholar] [CrossRef] [PubMed]
- Staubach, P.; Peveling-Oberhag, A.; Lang, B.M.; Zimmer, S.; Sohn, A.; Mann, C. Severe chronic spontaneous urticaria in children—treatment options according to the guidelines and beyond—A 10 years review. J. Dermatol. Treat. 2022, 33, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Stinco, G. Recalcitrant chronic urticaria treated with dupilumab: Report of two instances refractory to H1-antihistamines, omalizumab and cyclosporine and brief literature review. Dermatol. Ther. 2021, 34, e14821. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, P.D. Anti-CD20 or anti-IgE therapy for severe chronic autoimmune urticaria. J. Allergy Clin. Immunol. 2009, 123, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.D.; Yee, A.F.; Paget, S.A. Rituximab successfully treats refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J. Allergy Clin. Immunol. 2011, 128, 1354–1355. [Google Scholar] [CrossRef]
- Steinweg, S.A.; Gaspari, A.A. Rituximab for the Treatment of Recalcitrant Chronic Autoimmune Urticaria. J. Drugs Dermatol. 2015, 14, 1387. [Google Scholar]
- Combalia, A.; Losno, R.A.; Prieto-González, S.; Mascaró, J.M. Rituximab in Refractory Chronic Spontaneous Urticaria: An Encouraging Therapeutic Approach. Skin Pharmacol. Physiol. 2018, 31, 184–187. [Google Scholar] [CrossRef]
- Sabag, D.A.; Matanes, L.; Bejar, J.; Sheffer, H.; Barzilai, A.; Church, M.K.; Toubi, E.; Maurer, M.; Vadasz, Z. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin. Exp. Allergy 2020, 50, 799–804. [Google Scholar] [CrossRef]
- Maul, J.T.; Distler, M.; Kolios, A.; Maul, L.V.; Guillet, C.; Graf, N.; Imhof, L.; Lang, C.; Navarini, A.A.; Schmid-Grendelmeier, P. Canakinumab Lacks Efficacy in Treating Adult Patients with Moderate to Severe Chronic Spontaneous Urticaria in a Phase II Randomized Double-Blind Placebo-Controlled Single-Center Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 463–468.e3. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.H.; Eliason, M.J.; Leiferman, K.M.; Hull, C.M.; Powell, D.L. Treatment of refractory chronic urticaria with tumor necrosis factor-alfa inhibitors. J. Am. Acad. Dermatol. 2011, 64, 1221–1222. [Google Scholar] [CrossRef] [PubMed]
- A Safety and Efficacy Study of Ligelizumab in the Treatment of CSU in Japanese Patients Inadequately Controlled with H1-Antihistamines–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03907878 (accessed on 15 July 2022).
- Study of Efficacy and Safety of Ligelizumab in Chronic Spontaneous Urticaria Patients Who Completed a Previous Study with Ligelizumab–Tabular View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04210843 (accessed on 15 July 2022).
- Study of Mechanism of Action of Ligelizumab (QGE031) in Patients with Chronic Urticaria–Tabular View–ClinicalTri-als.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04513548 (accessed on 15 July 2022).
- A Phase III Study of Efficacy and Safety of Ligelizumab in the Treatment of CSU in Adolescents and Adults Inadequately Controlled With H1-Antihistamines–Tabular View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03580369?term=ligelizumab&draw=2&rank=8 (accessed on 15 July 2022).
- A Phase III Study of Efficacy and Safety of Ligelizumab in the Treatment of CSU in Adolescents and Adults Inadequately Controlled With H1-Antihistamines–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03580356 (accessed on 15 July 2022).
- Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of UB-221 as an Add-on Therapy in CSU Patients–Tabular View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03632291?term=ub-221&draw=2&rank=3 (accessed on 15 July 2022).
- Mepolizumab for the Treatment of Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03494881 (accessed on 15 July 2022).
- A Study to Investigate the Use of Benralizumab in Patients with Chronic Spontaneous Urticaria Who Are Symptomatic De-spite the Use of Antihistamines (ARROYO)–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04612725 (accessed on 15 July 2022).
- Dupilumab in Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03749135 (accessed on 15 July 2022).
- Dupilumab for the Treatment of Chronic Spontaneous Urticaria in Patients Who Remain Symptomatic Despite the Use of H1 Antihistamine and Who Are Naïve to, Intolerant of, or Incomplete Responders to Omalizumab (LIBERTY-CSU CUPID)–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04180488 (accessed on 15 July 2022).
- Safety Study of Rituximab (Rituxan®) in Chronic Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00216762 (accessed on 15 July 2022).
- Study to Evaluate Tezepelumab in Adults with Chronic Spontaneous Urticaria (INCEPTION). Available online: https://clinicaltrials.gov/ct2/show/NCT04833855 (accessed on 15 July 2022).
- A Study of CDX-0159 in Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04538794 (accessed on 15 July 2022).
- A Phase 2 Study of CDX-0159 in Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05368285 (accessed on 15 July 2022).
- A Study of MTPS9579A in Participants with Refractory Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05129423 (accessed on 15 July 2022).
- A Study of LY3454738 in Adults with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04159701 (accessed on 15 July 2022).
- A Study to Assess the Efficacy and Safety of AK002 in Subjects with Antihistamine-Resistant Chronic Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03436797?term=Siglec-8&draw=2&rank=1 (accessed on 15 July 2022).
- Holgate, S.; Casale, T.; Wenzel, S.; Bousquet, J.; Deniz, Y.; Reisner, C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Vadasz, Z.; Kocatürk, E.; Giménez-Arnau, A.M. Omalizumab Updosing in Chronic Spontaneous Urticaria: An Overview of Real-World Evidence. Clin. Rev. Allergy Immunol. 2020, 59, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Spector, S.L.; Tan, R.A. Effect of omalizumab on patients with chronic urticaria. Ann. Allergy Asthma Immunol. 2007, 99, 190–193. [Google Scholar] [CrossRef]
- Kaplan, A.; Ferrer, M.; Bernstein, J.A.; Antonova, E.; Trzaskoma, B.; Raimundo, K.; Rosén, K.; Omachi, T.A.; Khalil, S.; Zazzali, J.L. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J. Allergy Clin. Immunol. 2016, 137, 474–481. [Google Scholar] [CrossRef]
- Stull, D.E.; McBride, D.; Houghton, K.; Finlay, A.Y.; Gnanasakthy, A.; Balp, M.M. Assessing Changes in Chronic Spontaneous/Idiopathic Urticaria: Comparisons of Patient-Reported Outcomes Using Latent Growth Modeling. Adv. Ther. 2016, 33, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Kaplan, A.P.; Beck, L.A.; Antonova, E.N.; Balp, M.M.; Zazzali, J.; Khalil, S.; Maurer, M. Omalizumab substantially improves dermatology-related quality of life in patients with chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1715–1721. [Google Scholar] [CrossRef]
- Maurer, M.; Sofen, H.; Ortiz, B.; Kianifard, F.; Gabriel, S.; Bernstein, J.A. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: Analyses according to the presence or absence of angioedema. J. Eur. Acad Dermatol. Venereol. 2017, 31, 1056–1063. [Google Scholar] [CrossRef]
- Arm, J.P.; Bottoli, I.; Skerjanec, A.; Floch, D.; Groenewegen, A.; Maahs, S.; Owen, C.E.; Jones, I.; Lowe, P.J. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy 2014, 44, 1371–1385. [Google Scholar] [CrossRef]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbären, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Wedi, B. Ligelizumab for the treatment of chronic spontaneous urticaria. Expert. Opin. Biol. Ther. 2020, 20, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, D.; Hans, L.L.; Yuwen, L.Z.; Tan, M.; Meng, G.; Gloria, Y.; Balazs, M.; Chung, S.; Wu, C. Lawren, Quilizumab is an Afucosylated Humanized Anti-M1 Prime Therapeutic Antibody. Clin. Anti-Inflamm. Anti-Allergy Drugs (Discontin.) 2014, 1, 24–31. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Harris, J.M.; Boulet, L.P.; Scheerens, H.; Fitzgerald, J.M.; Putnam, W.S.; Cockcroft, D.W.; Davis, B.E.; Leigh, R.; Zheng, Y.; et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci. Transl. Med. 2014, 6, 243ra85. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Evaluating the Safety and Tolerability and Determining the PK and PD of Single Dose UB-221 in Chronic Spontaneous Urti-caria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04175704 (accessed on 12 July 2022).
- A Study to Evaluate the Pharmacodynamics, Pharmacokinetics, Safety, and Efficacy of UB-221 IV Infusion as an Add-on Therapy in Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05298215?term=ub-221&draw=2&rank=1 (accessed on 12 July 2022).
- Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/WikiPathways:WP127 (accessed on 12 July 2022).
- Adachi, T.; Alam, R. The mechanism of IL-5 signal transduction. Am. J. Physiol. 1998, 275, C623–C633. [Google Scholar] [CrossRef]
- Jackson, D.J.; Akuthota, P.; Roufosse, F. Eosinophils and eosinophilic immune dysfunction in health and disease. Eur. Respir. Rev. 2022, 31, 210150. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017, 9, CD010834. [Google Scholar] [CrossRef]
- Harvima, I.T.; Horsmanheimo, L.; Naukkarinen, A.; Horsmanheimo, M. Mast cell proteinases and cytokines in skin inflammation. Arch. Dermatol. Res. 1994, 287, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef]
- Kay, A.B.; Ying, S.; Ardelean, E.; Mlynek, A.; Kita, H.; Clark, P.; Maurer, M. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br. J. Dermatol. 2014, 171, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nucala (accessed on 24 June 2022).
- Antonicelli, L.; Tontini, C.; Garritani, M.S.; Piga, M.A.; Bilò, M.B. Efficacy of mepolizumab in patients with concomitant severe eosinophilic asthma and severe chronic urticaria: An example of personalized medicine? J. Investig. Allergol. Clin. Immunol. 2021, 32. [Google Scholar] [CrossRef]
- Cinqaero|European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cinqaero (accessed on 16 July 2022).
- Fasenra|European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf (accessed on 24 June 2022).
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. 2020, 69, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dupixent|European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent (accessed on 24 June 2022).
- Dupixent (Dupilumab) FDA Approval History–Drugs.com. Available online: https://www.drugs.com/history/dupixent.html (accessed on 15 July 2022).
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.P.; Lindorfer, M.A. Drug insight: The mechanism of action of rituximab in autoimmune disease--the immune complex decoy hypothesis. Nat. Clin. Pract. Rheumatol. 2007, 3, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rituxan (Genentech, Inc.). FDA Package Insert (medlibrary.org). Available online: https://medlibrary.org/lib/rx/meds/rituxan/ (accessed on 24 June 2022).
- Delate, T.; Hansen, M.L.; Gutierrez, A.C.; Le, K.N. Indications for Rituximab Use in an Integrated Health Care Delivery System. J. Manag. Care Spec. Pharm. 2020, 26, 832–838. [Google Scholar] [CrossRef]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef]
- McAtee, C.L.; Lubega, J.; Underbrink, K.; Curry, K.; Msaouel, P.; Barrow, M.; Muscal, E.; Lotze, T.; Srivaths, P.; Forbes, L.R.; et al. Association of Rituximab Use With Adverse Events in Children, Adolescents, and Young Adults. JAMA Netw. Open 2021, 4, e2036321. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Q.; Liu, C.; Ying, M.; Xu, S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci. Rep. 2017, 7, 17797. [Google Scholar] [CrossRef]
- Cosentyx|European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyx (accessed on 25 June 2022).
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Hermes, B.; Prochazka, A.K.; Haas, N.; Jurgovsky, K.; Sticherling, M.; Henz, B.M. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J. Allergy Clin. Immunol. 1999, 103 Pt 1, 307–314. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, P.K.; Chitkara, A.; Rani, S. To Evaluate the Role and Relevance of Cytokines IL-17, IL-18, IL-23 and TNF-α and Their Correlation with Disease Severity in Chronic Urticaria. Indian Dermatol. Online J. 2020, 11, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-Activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response Through OX40 Ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Wang, S.H.; Zuo, Y.G. Thymic Stromal Lymphopoietin in Cutaneous Immune-Mediated Diseases. Front. Immunol. 2021, 12, 698522. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy 2022. [Google Scholar] [CrossRef]
- Maun, H.R.; Jackman, J.K.; Choy, D.F.; Loyet, K.M.; Staton, T.L.; Jia, G.; Dressen, A.; Hackney, J.A.; Bremer, M.; Walters, B.T.; et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell 2020, 180, 406. [Google Scholar] [CrossRef]
- Payne, V.; Kam, P.C. Mast cell tryptase: A review of its physiology and clinical significance. Anaesthesia 2004, 59, 695–703. [Google Scholar] [CrossRef]
- Blom, L.H.; Martel, B.C.; Larsen, L.F.; Hansen, C.V.; Christensen, M.P.; Juel-Berg, N.; Litman, T.; Poulsen, L.K. The immunoglobulin superfamily member CD200R identifies cells involved in type 2 immune responses. Allergy 2017, 72, 1081–1090. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef]
- Ocak, M.; Soyer, O.; Buyuktiryaki, B.; Sekerel, B.E.; Sahiner, U.M. Omalizumab treatment in adolescents with chronic spontaneous urticaria: Efficacy and safety. Allergol. Immunopathol. 2020, 48, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaikhly, T.; Rosenthal, J.A.; Ayars, A.G.; Petroni, D.H. Omalizumab for chronic urticaria in children younger than 12 years. Ann. Allergy Asthma Immunol. 2019, 123, 208–210.e2. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Rocha, C.; Pereira, A.; Song, Y.; Alonso-Coello, P.; Solà, I.; Beltran, J.; Posso, M.; Akdis, C.A.; Akdis, M.; et al. Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: A systematic review for the EAACI Biologicals Guidelines. Allergy 2021, 76, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Tharp, M.D.; Bernstein, J.A.; Kavati, A.; Ortiz, B.; MacDonald, K.; Denhaerynck, K.; Abraham, I.; Lee, C.S. Benefits and Harms of Omalizumab Treatment in Adolescent and Adult Patients With Chronic Idiopathic (Spontaneous) Urticaria: A Meta-analysis of “Real-world” Evidence. JAMA Dermatol. 2019, 155, 29–38. [Google Scholar] [CrossRef]
- Jia, H.X.; He, Y.L. Efficacy and Safety of Omalizumab for Chronic Spontaneous Urticaria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Ther. 2020, 27, e455–e467. [Google Scholar] [CrossRef]
- Bérard, F.; Ferrier Le Bouedec, M.; Bouillet, L.; Reguiai, Z.; Barbaud, A.; Cambazard, F.; Milpied, B.; Pelvet, B.; Kasujee, I.; Gharbi, H.; et al. Omalizumab in patients with chronic spontaneous urticaria nonresponsive to H1-antihistamine treatment: Results of the phase IV open-label SUNRISE study. Br. J. Dermatol. 2019, 180, 56–66. [Google Scholar] [CrossRef]
- Salman, A.; Comert, E. The Real-Life Effectiveness and Safety of Omalizumab Updosing in Patients with Chronic Spontaneous Urticaria. J. Cutan. Med. Surg. 2019, 23, 496–500. [Google Scholar] [CrossRef]
- Curto-Barredo, L.; Spertino, J.; Figueras-Nart, I.; Expósito-Serrano, V.; Guilabert, A.; Melé-Ninot, G.; Cubiró, X.; Bonfill-Ortí, M.; Garcias-Ladaria, J.; Villar, M.; et al. Omalizumab updosing allows disease activity control in patients with refractory chronic spontaneous urticaria. Br. J. Dermatol. 2018, 179, 210–212. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Fierro, M.; Dapavo, P.; Crimi, N.; Ferrucci, S.; Pepe, P.; Liberati, S.; Pigatto, P.D.; et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: A study of 470 patients. J. Eur. Acad Dermatol. Venereol. 2019, 33, 918–924. [Google Scholar] [CrossRef]
- Ertas, R.; Ozyurt, K.; Atasoy, M.; Hawro, T.; Maurer, M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018, 73, 705–712. [Google Scholar] [CrossRef]
- Saini, S.S.; Omachi, T.A.; Trzaskoma, B.; Hulter, H.N.; Rosén, K.; Sterba, P.M.; Courneya, J.P.; Lackey, A.; Chen, H. Effect of Omalizumab on Blood Basophil Counts in Patients with Chronic Idiopathic/Spontaneous Urticaria. J. Investig. Dermatol. 2019, 139, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kang, S.H.; Park, J.O.; Park, G.H.; Choi, J.H. Serum transglutaminase 2 activity as a potential biomarker of disease severity and response to omalizumab in chronic spontaneous urticaria. Allergol. Int. 2020, 69, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Novartis Provides an Update on Phase III Ligelizumab (QGE031) Studies in Chronic Spontaneous Urticaria (CSU)|Novartis. Available online: https://www.novartis.com/news/media-releases/novartis-provides-update-phase-iii-ligelizumab-qge031-studies-chronic-spontaneous-urticaria-csu (accessed on 20 July 2022).
- Gabizon, R.; London, N. A Fast and Clean BTK Inhibitor. J. Med. Chem. 2020, 63, 5100–5101. [Google Scholar] [CrossRef] [PubMed]
- This Was a Dose-Finding Study to Evaluate Efficacy and Safety of LOU064 in Patients with CSU Inadequately Controlled by H1-Antihistamines–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03926611 (accessed on 20 July 2022).
- Open-Label, Multicenter, Extension Study to Evaluate Long-Term Safety and Tolerability of LOU064 in Subjects with CSU–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04109313 (accessed on 20 July 2022).
- A Safety and Efficacy Study of Remibrutinib in the Treatment of CSU in Japanese Adults Inadequately Controlled by H1-Antihistamines–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05048342 (accessed on 20 July 2022).
- A Phase 3 Study of Efficacy and Safety of Remibrutinib in the Treatment of CSU in Adults Inadequately Controlled by H1-Antihistamines–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05032157?cond=chronic+spontaneous+urticaria&draw=5&rank=32 (accessed on 20 July 2022).
- Global Managed Access Program Cohort for Remibrutinib in Adult Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05170724 (accessed on 20 July 2022).
- Metz, M.; Sussman, G.; Gagnon, R.; Staubach, P.; Tanus, T.; Yang, W.H.; Lim, J.J.; Clarke, H.J.; Galanter, J.; Chinn, L.W.; et al. Fenebrutinib in H1 antihistamine-refractory chronic spontaneous urticaria: A randomized phase 2 trial. Nat. Med. 2021, 27, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate the Long-Term Safety and Efficacy of Fenebrutinib in Participants Previously Enrolled in a Fenebrutinib Chronic Spontaneous Urticaria (CSU) Study–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03693625 (accessed on 20 July 2022).
- Study to Evaluate the Efficacy, Safety, and Tolerability of Tirabrutinib in Participants with Antihistamine-Resistant Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04827589 (accessed on 20 July 2022).
- Rilzabrutinib for the Treatment of Chronic Spontaneous Urticaria in Patients Who Remain Symptomatic Despite the Use of H1 Antihistamine–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05107115 (accessed on 20 July 2022).
- A Phase 2a Study of TAS5315 in Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05335499 (accessed on 20 July 2022).
- Etanercept for the Treatment of Chronic Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01030120 (accessed on 20 July 2022).
- A Study of GDC-0853 in Participants with Refractory Chronic Spontaneous Urticaria (CSU). Available online: https://clinicaltrials.gov/ct2/show/NCT03137069 (accessed on 20 July 2022).
- Safety and Efficacy of TLL018 in Patients with Chronic Spontaneous Urticaria–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05373355 (accessed on 20 July 2022).
- Oliver, E.T.; Chichester, K.; Devine, K.; Sterba, P.M.; Wegner, C.; Vonakis, B.M.; Saini, S.S. Effects of an Oral CRTh2 Antagonist (AZD1981) on Eosinophil Activity and Symptoms in Chronic Spontaneous Urticaria. Int. Arch. Allergy Immunol. 2019, 179, 21–30, Correction in Int. Arch. Allergy Immunol. 2019, 179, 320. [Google Scholar] [CrossRef]
| Authors | Type of Study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse Events | Beneficial |
|---|---|---|---|---|---|---|---|---|---|---|
| Saini et al. 2011 [25] | Phase 2 RDBPCT MYSTIQUE | 90 | 40.8 ± 14.7 <18 (5.6%) | Moderate-to-severe CSU despite H1AH UAS7 ≥ 12 | 75, 300, 600 mg or placebo combined with H1AH as needed | 4 weeks | 12 weeks | At week 4 ↓ ΔUAS7 −6.9 (placebo), −9.8 (75 mg) −19.9 (300 mg), −14.6 (600 mg) | 44% ≥ 1 AE ≅AEs vs. placebo URTI, Pharyngitis Headache 2 hypersensitivity | Yes |
| Maurer et al. 2011 [26] | RDBPCT X-QUISITE | 49 | 40.5 (18–70) | CSU refractory to H1AH UAS7 ≥ 10 Total IgE 30–700 IU/mL anti-TPO IgE ≥ 5.0 IU/mL | 75–375 mg Q2W or Q4W or placebo | 24 weeks | Not reported | At week 24 ↓ UAS7 (−17.8 vs. −7.9) symptoms free (67 vs. 4%) ↓ concomitant medication use | ≅AEs vs. placebo Diarrhea Pharyngitis Headache | Yes |
| Kaplan et al. 2013 [27] | Phase 3 RDBPCT GLACIAL | 335 | 43 ± 14 | CSU refractory to H1AH (up to x4) + H2AH or LTRAs, or both UAS7 ≥ 16 | 300 mg or placebo Q4W as add-on | 24 weeks | 16 weeks | No safety concern (w 40 w) At week 12 ↓ ISS7 (−8.6 vs. −4) ↓ UAS7 (−19 vs. −8.5) | ≅ incidence of drug related AEs vs. placebo (11 vs. 13%) | Yes |
| Maurer et al. 2013 [28] | RDBPCT ASTERIA II | 323 | 42.5 ± 13.7 (≥12) | Moderate-to-severe CSU symptomatic despite H1AH UAS7 ≥ 16 | 75 mg, 150 mg, 300 mg or placebo Q4W + H1AH | 12 weeks | 16 weeks | At week 12 ↓ ISS7 (−5.9 vs. −8.1 vs. −9.8) ↓ UAS7 ↑ QoL | ≅rate of AEs Higher rate of SAEs (6%) in 300 mg group | Yes |
| Saini et al. 2015 [29] | Phase 3 RDBPCT ASTERIA I | 319 | 41 (12–75) 12–17 (2.5%) | CSU refractory to H1AH UAS7 ≥ 16 | 75 mg, 150 mg, or 300 mg or placebo Q4W | 24 weeks | 16 weeks | At week 12 ↓ ISS7 −9.4 (300 mg), −6.6 (150 mg) −6.4 (75 mg), −3.6 (placebo) ↓ UAS7 ↓ rescue medicine | Mild dose-dependent AEs Headache Arthralgia Injection-site reactions | Yes |
| Staubach et al. 2016 [30] | Phase 3 RDBPCT X-ACT | 91 | 42 ± 12 (18–75) | CSU with angioedema refractory to 2–4x H1AH UAS7 ≥ 14 CU-Q2oL score ≥ 30 | 300 mg or placebo Q4W | 28 weeks | 8 weeks | At week 28 ↓ AE-QoL and AAS7 At week 12 ↓ UAS7 (−16 vs. −4) | ≅AEs vs. placebo SEAs not drug-related | Yes |
| Staubach et al. 2017 [31] | RDBPCT X-ACT | 91 | 42 ± 12 (18–75) | CSU with angioedema (≥4 episodes in 6 months) refractory to 2–4x H1AH UAS7 ≥ 14 | 300 mg or placebo Q4W | 28 weeks | 8 weeks | ↓ AE-QoL ↓ DLQI ↓ AAS7 | NR | Yes |
| Hide et al. 2017 [32] | Phase 3 RDBPCT POLARIS | 218 | 43.5 | CSU refractory to standard H1AH UAS7 ≥ 16 | 300 mg, 150 mg, or placebo Q4W With H1AH | 12 weeks | 12 weeks | At week 12 ↓ ISS 7 −10.2 (300 mg), −8.8 (150 mg) −6.5 (placebo) ↓ UAS7 | ≅AEs vs. placebo Pharyngitis Headache, Eczema 1 pharyngeal edema | Yes |
| Maurer et al. 2017 [33] | RDBPCT XTEND-CIU | 205 (open label) 134 (double blind) | 44 ± 14 (open label) 45 (double blind) (12–75) | CSU refractory to H1AH UAS7 ≥ 16 | 300 mg Q4W for 24 weeks then randomization if UAS7 ≤ 6 If UAS7 ≥ 12 at week 24–48 -> Re-trt with omalizumab | 48 weeks | 12 weeks | ↑ UAS7 and DLQI after discontinuation or placebo UAS7 ≥ 12 week 24–48 (21% omalizumab vs. 60% placebo) ↓ UAS7 after re-treatment | 16 drug-related AEs 6 SAEs not drug-related 1 anaphylaxis | Yes |
| Casale et al. 2019 [34] | Open-label + RDBPCT XTEND-CIU | 205 (open label) 134 (double blind) | 44 ± 14 (open label) 45 (double blind) (12–75) | CSU refractory to H1AH 48% CSS | 300 mg Q4W for 24 weeks then randomization if UAS7 ≤ 6 If UAS7 ≥ 12 at week 24–48 -> open label omalizumab | 48 weeks | 12 weeks | At week 12 and 24 ↓ HRQoL scores At week 48 Sustained improvement of HRQoL scores | NR | Yes |
| Sussman et al. 2020 [35] | Phase 3 RCT OPTIMA trial | 314 | 46.3 | CSU refractory to H1AH, H2AH, LTRA | 150 mg or 300 mg Q4W Step-up to 300 mg if UAS7 ≥ 6 before week 24 | 24 weeks (+12 weeks if UAS7 ≥ 6) 12 weeks re-trt if UAS ≥ 16 | 4–24 weeks | At week 24 Step-up to 300 mg (79% in 150 mg) UAS7 ≥ 6 (31% in 300 mg) UAS ≤ 6 (37%) -> UAS7 ≥ 6 after discontinuation (48%) -> re-trt -> UAS7 ≤ 6 (88%) | 13% ≥ 1 AEs Headache, Pharyngitis Nausea Fatigue 8 SAEs not drug-related | Yes |
| Yuan et al. 2022 [36] | RDBPCT | 418 | ≥18 | CSU refractory to H1AH for ≥6 months | 150 or 300 or placebo mg Q4W | 20 weeks | NR | At week 12 ↓ ISS 7 (LSM) −4.2 (300 mg), −3.8 (150 mg) −2.3 (placebo) | A little higher AEs in 300 mg (71 vs. 64%) | Yes |
| Ligelizumab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Type of Study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse Events | Beneficial |
| Maurer et al. 2019 [37] | Phase 2b RDBPCT NCT02477332 | 382 | 43.3 ± 12.5 (18–75) | CSU refractory to H1AH ± H2AH ± LTRA UAS7 ≥ 16 HSS7 ≥ 8 | Ligelizumab 240 or 72 or 24 mg Q4W, or omalizumab 300 mg Q4W; placebo Q4W; 120 mg ligelizumab followed by placebo Q4W Combined with standard trt | 20 weeks | 24 weeks | dose–response curve plateau at 72 mg dose ligelizumab HSS7 = 0 at week 12 72 mg ligeliz > omaliz (51 vs. 26%) 240 mg ligeliz > omaliz (42 vs. 26%) UAS7 = 0 at week 12 72 mg ligeliz > omaliz (44 vs. 26%) 240 mg ligeliz > omaliz (40 vs. 26%) at week 20 240 mg ligeliz > omalizumab | ≅incidence of AEs ↑ injection site reactions in 72 mg and 240 mg ligelizumab groups Headache | Yes |
| Giménez-Arnau et al. 2022 [38] | Open-label extension study of NCT02477332 | 226 | 44.5 ± 12.7 (≥18) | UAS7 ≥ 12 at week 32 in NCT02477332 | 240 mg Q4W | 52 weeks | 48 weeks | ↓ SIS7 ↓ AIS7 ↓ work impairment | NR | Yes |
| Maurer M et al. 2021 [39] | Open-label extension study of NCT02477332 | 226 | 44.5 ± 12.7 (≥18) | UAS7 ≥ 12 at week 32 in NCT02477332 | 240 mg Q4W | 52 weeks | 48 weeks | 46% UAS7 = 0 at week 12 53% UAS7 = 0 at week 52 | 84% ≥ 1 AE 77% mild/moderate and mostly drug unrelated | Yes |
| NCT03437278 [40] | Phase 2 RDBPCT | 49 | 12–17 | UAS7 ≥ 16 HSS7 ≥ 8 | 24 mg or 120 mg Q4W, or 8 weeks placebo followed by 120 mg | 24 weeks | 16 weeks | ↓ UAS7, HSS7, ISS7 UAS7 = 0 at week 24 (33% vs. 62% vs. 33%) | 77% AEs 4% SAEs Nasopharyngitis Headache, Nausea | Yes |
| Quilizumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Harris et al. 2016 [41] | RDBPCT QUAIL study | 32 | 18–75 | CSU refractory to H1AH ± LTRAs UAS7 ≥ 16 | 450 mg or placebo Q4W | 20 weeks | 8 weeks | At week 20 ΔISS7 (−12.9, NS, p = 0.17) ΔUAS7 (−6, NS, p = 0.24) | No | No |
| Mepolizumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Magerl et al. 2018 [42] | Case report | 1 | 27 | Severe refractory eosinophilic asthma and refractory CSU | 100 mg Q4W | 16 weeks | NR | ↑ UCT CSU remission Relapse after discontinuation | Discontinuation because of immune-complex reaction | Yes |
| Reslizumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Maurer et al. 2017 [43] | Case report | 1 | 43 | Severe refractory eosinophilic asthma and refractory CSU and cold urticaria | 300 mg monthly | 5 months | No | ↑ UCT | NR | Yes |
| Benralizumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Bernstein A. et al. 2020 [44] | Single- blind trial | 12 | 47.3 ± 1.3 | CSU refractory to H1AH UAS ≥ 16 | 30 mg monthly after a dose of placebo | 3 months | 2 months | At week 20 ↓ UAS7 (−15.7) 3 (25%) withdrew (1 non-response) | No | Yes |
| Dupilumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Lee et al. 2019 [45] | Case series | 6 | 36.2 (18–50) | CSU refractory to omalizumab up to 600 mg and H1AH (comorbidities: all AD, 1 asthma, 1 AH, 1 joint pain) | 600 mg loading dose, then 300 mg Q2W Combined with H1AH | 3 months | NR | Symptom resolution (3) ↓ UAS7 ≤ 6 (2) NR (1) | NR | Yes |
| Staubach et al. 2022 [46] | Case series | 2 | 6–17 | Inadequate response to H1AH, omalizumab (450 or 600 mg), and cyclosporine | 300 mg Q2W | 3 months | 2–3 months | P1 UAS7 = 0 at week 8 P2 improvement at month 3 | NR | Yes |
| Errichetti et al. 2021 [47] | Case series | 2 | 52–63 | CSU refractory to H1AH, LTRA, methotrexate, omalizumab, cyclosporine (comorbidities: Graves and atopic dermatitis) | 600 mg, followed by 300 mg weekly | 8 weeks | 5–23 months | Complete response at week 8 and symptom free at follow-up | No | Yes |
| Rituximab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Arkwright et al. 2009 [48] | Case report | 1 | 12 | CSU refractory to H1AH CSS dependence and side effects | 375 mg/m2 weekly | 4 doses | 12 months | Symptom resolution for 12 months | NR | Yes |
| Chakravarty et al. 2011 [49] | Case report | 1 | 51 | CSU refractory to H1AH, H2AH, CSS, cyclosporine, mycophenolate mofetil | 375 mg/m2 weekly Plus methotrexate | 4 weeks | 9 months | Symptom resolution for 8 months | NR | Yes |
| Steinweg et al. 2015 [50] | Case report | 1 | 38 | CSU refractory to H1AH and CSS | 1000 mg QW2 | 2 weeks | 10 months | Symptom resolution for 10 months | Fatigue Arthralgia Injection site-reaction | Yes |
| Combalia et al. 2018 [51] | Case report | 1 | 44 | Antisynthetase syndrome and CSU refractory to H1AH and immunosuppressants | 1000 mg QW2 Plus one-week CSS | 2 weeks | 8 months | Early symptom resolution Mild controlled flares during follow-up | No | Yes |
| Secukinumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Sabag et al. 2020 [52] | Case series | 8 | NR | CSU refractory to H1AH, omalizumab, CSS, and cyclosporine UAS 32–40 | 150 mg weekly for 4 weeks then Q2W | 3 months | NR | At day 30 ↓ 55% in UAS7 (−19.6) At day 90 ↓ 82% in UAS7 (−29.5) | Mild injection site reactions (3) | Yes |
| Canakinumab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Maul et al. 2021 [53] | Phase 2 RDPCT | 20 | 40.4 (18–70) | CSU refractory to H1AH ± CSS or LTRAs | 150 mg | 1 dose | 8 weeks | Δ UAS7 (NS) Δ DLQI (NS) | No | No |
| Infliximab | ||||||||||
| Authors | Type of study | N. | Age (Yrs) | Indication | Dosage | Duration | Follow-Up | Results | Adverse events | Beneficial |
| Wilson et al. 2011 [54] | Case report | 1 | 35 | CSU refractory to H1AH and immunosuppressants | 5 mg/kg Q6W | NR | NR | Symptom free for 3 years, then flares controlled by cyclosporine | NR | Yes |
| Authors | Type of Study | N. | Dosage | End Point | Outcome |
|---|---|---|---|---|---|
| Omalizumab | |||||
| Saini et al. 2011 [25] | Phase 2 RDBPCT MYSTIQUE | 90 | 75, 300, 600 mg combined with H1AH as needed | Week 4 | ↓ UAS7 (−9.8 vs. −19.9 vs. −14.6) |
| Maurer et al. 2011 [26] | RDBPCT X-QUISITE | 49 | 75–375 mg Q2W or Q4W | Week 24 | ↓ UAS7 (−17.8) |
| Kaplan et al. 2013 [27] | Phase 3 RDBPCT GLACIAL | 335 | 300 mg Q4W as add-on | Week 12 | ↓ ISS7 (−8.6) ↓ UAS7 (−19) |
| Maurer et al. 2013 [28] | RDBPCT ASTERIA II | 323 | 75 mg, 150 mg, 300 mg Q4W | Week 12 | ↓ ISS7 (−5.9 vs. −8.1 vs. −9.8) |
| Saini et al. 2015 [29] | Phase 3 RDBPCT ASTERIA I | 319 | 75 mg, 150 mg, or 300 mg Q4W | Week 12 | ↓ ISS7 (−6.4 vs. −6.6 vs. −9.4) ↓ UAS7 (−13.8 vs. −14.4 vs. −20.8) |
| Staubach et al. 2016 [30] | Phase 3 RDBPCT X-ACT | 91 | 300 mg Q4W | Week 12 | ↓ UAS7 (−16) |
| Staubach et al. 2017 [31] | RDBPCT X-ACT | 91 | 300 mg Q4W | Week 12 | ↓ AAS7 (−14.1) |
| Hide et al. 2017 [32] | Phase 3 RDBPCT POLARIS | 218 | 150 mg, 300 mg Q4W With H1AH | Week 12 | ↓ ISS 7 (LSM) (−8.8 vs. −10.2) ↓ UAS 7 (LSM) (−18.8 vs. −22.4) |
| Yuan et al. 2022 [36] | RDBPCT | 418 | 150 or 300 mg Q4W | Week 12 | ↓ ISS 7 (LSM) (−3.8 vs. −4.2) |
| Ligelizumab | |||||
| Maurer et al. 2019 [37] | Phase 2b RDBPCT NCT02477332 | 382 | Ligelizumab 240 or 72 or 24 mg Q4W or omalizumab 300 mg Q4W; placebo Q4W; 120 mg ligelizumab followed by placebo Q4W | Week 12 | HSS7 = 0 72 mg ligeliz > omaliz (51 vs. 26%) 240 mg ligeliz > omaliz (42 vs. 26%) UAS7 = 0 72 mg ligeliz > omaliz (44 vs. 26%) 240 mg ligeliz > omaliz (40 vs. 26%) |
| Maurer M et al. 2021 [39] | Open-label extension study of NCT02477332 | 226 | 240 mg Q4W | Week 12 | UAS7 = 0 (41.6%) |
| NCT03437278 [40] | Phase 2 RDBPCT | 49 | 24 mg or 120 mg Q4W, or 8 weeks placebo followed by 120 mg | Week 24 | ↓ UAS7 (−20.4 vs. −22.5 vs. −21.3) |
| Quilizumab | |||||
| Harris et al. 2016 [41] | RDBPCT QUAIL study | 32 | 450 mg Q4W | Week 20 | ISS7 (−12.9, NS) |
| Mepolizumab | |||||
| Magerl et al. 2018 [42] | Case report | 1 | 100 mg Q4W | Week 12 | ↑ UCT |
| Reslizumab | |||||
| Maurer et al. 2017 [43] | Case report | 1 | 300 mg monthly | Week 4 | ↑ UCT (+10) |
| Benralizumab | |||||
| Bernstein et al. 2020 [44] | Single- blind trial | 12 | 30 mg monthly after a dose of placebo | Week 20 | ↓ UAS7 (−15.7) |
| Dupilumab | |||||
| Lee et al. 2019 [45] | Case series | 6 | 600 mg loading dose, then 300 mg Q2W Combined with H1AH | Month 3 post-dupilumab | Symptom resolution (3) ↓ UAS7 ≤ 6 (2) NA (1) |
| Staubach et al. 2020 [46] | Case series | 2 | 300 mg Q2W | NA | P1 UAS7 = 0 at week 8 P2 improvement at month 3 |
| Errichetti et al. 2021 [47] | Case series | 2 | 600 mg, followed by 300 mg weekly | NA | Complete response at week 8 and symptom free at follow-up |
| Rituximab | |||||
| Arkwright et al. 2009 [48] | Case report | 1 | 375 mg/m2 Weekly | NA | Symptom resolution for 12 months |
| Chakravarty et al. 2011 [49] | Case report | 1 | 375 mg/m2 weekly Plus mtx | NA | Symptom resolution for 8 months |
| Steinweg et al. 2015 [50] | Case report | 1 | 1000 mg QW2 | NA | Symptom resolution for 10 months |
| Combalia et al. 2018 [51] | Case report | 1 | 1000 mg QW2 Plus one-week CSS | NA | Early symptom resolution Mild controlled flares during follow-up |
| Secukinumab | |||||
| Sabag et al. 2020 [52] | Case series | 8 | 150 mg weekly for 4 weeks then Q2W | Day 90 | ↓ 82% in UAS7 (−29.5) |
| Canakinumab | |||||
| Maul et al. 2021 [53] | Phase 2 RDPCT | 20 | 150 mg single dose | Week 4 | Δ UAS7 (NS) |
| Infliximab | |||||
| Wilson et al. 2011 [54] | Case report | 1 | 5 mg/kg Q6W | NA | Symptom free for 3 years, then flares controlled by cyclosporine |
| Authors | Type of Study | N. | Dosage | End Point | Outcome |
|---|---|---|---|---|---|
| Omalizumab | |||||
| Maurer et al. 2011 [26] | RDBPCT X-QUISITE | 49 | 75–375 mg Q2W or Q4W | Week 24 | ↓ DLQI ↓ Cu-Q2oL |
| Kaplan et al. 2013 [27] | Phase 3 RDBPCT GLACIAL | 335 | 300 mg Q4W as add-on | Week 12 | ↓ DLQI (−9.7) ↓ CU-Q2OL (−29.3) |
| Maurer et al. 2013 [28] | RDBPCT ASTERIA II | 323 | 75 mg, 150 mg, 300 mg Q4W + H1AH | Week 12 | ↓ DLQI (−7.5 vs. −8.3 vs. −10.2) |
| Saini et al. 2015 [29] | Phase 3 RDBPCT ASTERIA I | 319 | 300 mg Q4W | Week 12 | ↓ DLQI (−10.3) |
| Staubach et al. 2016 [30] | Phase 3 RDBPCT X-ACT | 91 | 300 mg or placebo Q4W | Week 28 | ↓ CU-Q2oL (LSM) (−21.5) ↓ DLQI (−10.5) |
| Staubach et al. 2017 [31] | RDBPCT X-ACT | 91 | 300 mg or placebo Q4W | Week 4 | ↓ DLQI (LSM) (−7.6) |
| Hide et al. 2017 [32] | Phase 3 RDBPCT POLARIS | 218 | 150 mg, 300 mg Q4W With H1AH | Week 12 | ↓ DLQI (−7.2 vs. −8.4) |
| Casale et al. 2019 [34] | Open-label + RDBPCT XTEND-CIU | 205 | 300 mg Q4W for 24 weeks then randomization if UAS7 ≤ 6 | Week 24 | ↓ DLQI (−12.6) |
| Ligelizumab | |||||
| Maurer et al. 2019 [37] | Phase 2b RDBPCT NCT02477332 | 382 | Ligelizumab 240 or 72 or 24 mg Q4W or omalizumab 300 mg Q4W; placebo Q4W; 120 mg ligelizumab followed by placebo Q4W | Week 20 | ↓ DLQI (LSM) (−9.79 vs. −9.93 vs. −8.35 vs. −6.99) |
| Giménez-Arnau et al. 2022 [38] | Open-label extension study of NCT02477332 | 226 | 240 mg Q4W | Week 52 | ↓ DLQI (−9.52) |
| NCT03437278 [40] | Phase 2 RDBPCT | 49 | 24 mg or 120 mg Q4W, or 8 weeks placebo followed by 120 mg | Week 12 | ↓ DLQI (−10.1 vs. −6.6 vs. −5) |
| Canakinumab | |||||
| Maul et al. 2021 [53] | Phase 2 RDPCT | 20 | 150 mg single dose | Week 4 | Δ DLQI (NS) |
| Ligelizumab | ||||||||
| Trial Number | Type of Study | Status | N. | Age (Yrs) | Inclusion Criteria | Dosage | Duration | Follow-Up |
| NCT03907878 [55] | Phase 3 Multi-center, Open-label | Completed | 66 | ≥18 | CSU refractory to H1AH at approved doses UAS7 ≥ 16 and HSS7 ≥ 8 | NR | 52 weeks | 12 weeks |
| NCT04210843 [56] | Phase 3 Double-blinded and open-label extension study Re-treatment with ligelizumab | Active, not recruiting | 1041 | ≥12 | CSU patients who successfully completed studies CQGE031C2302, CQGE031C2303, CQGE031C2202, or CQGE031C1301 | 72 mg followed by 120 mg Q4W or 120 mg Q4W | NR | NR |
| NCT04513548 [57] | Phase 1 RDBPCT (Part 2) | Active, not recruiting | 68 | 18–79 | CSU refractory to H1-AH UAS7 ≥ 16 and HSS7 ≥ 8 or cholinergic urticaria or cold urticaria | 120 mg Q4W | 16 weeks | 12 weeks |
| NCT03580369 [58] | Phase 3 Multi-center RDBPCT | Active, not recruiting | 1073 | ≥12 | CSU refractory to H1-AH at approved doses UAS7 ≥ 16 and HSS7 ≥ 8 | Ligelizumab Q4W or omalizumab 300 mg Q4W or placebo till week 20 followed by ligelizumab | 52 weeks | 12 weeks |
| NCT03580356 [59] | Phase 3 Multi-center RDBPCT | Active, not recruiting | 1078 | ≥12 | CSU refractory to approved doses of H1-AH UAS7 ≥ 16 and HSS7 ≥ 8 | Ligelizumab Q4W or omalizumab 300 mg Q4W or placebo till week 20 followed by ligelizumab | 52 weeks | 12 weeks |
| UB-221 | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT03632291 [60] | Phase 1 Open-label study | Completed | 15 | 20–65 | CSU | 0.2 or 0.6 or 2 or 6 or 10 mg/kg | NR | NR |
| Mepolizumab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT03494881 [61] | Phase 1 Open-label study | Recruiting | 20 | ≥18 | CSU refractory to H1AH | 100 mg Q2W | 8 weeks | NR |
| Benralizumab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT04612725 [62] | Phase 2 RDBPCT | Active, not recruiting | 155 | ≥18 | CSU refractory to H1AH UAS7 ≥ 16 and ISS7 ≥ 8 | NR | 24 weeks with 28-week extension | NR |
| Dupilumab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT03749135 [63] | Phase 2a RDBPCT | Completed | 72 | 18–75 | CSU refractory to standard trt UAS7 ≥ 16 | NR | 16 weeks | 16 weeks |
| NCT04180488 [64] | Phase 3 Multi-center RDBPCT | Active, not recruiting | 384 | 6–80 | CSU refractory to H1AH UAS7 ≥ 16 and ISS7 ≥ 8 Study A: omalizumab naïve Study B: intolerant or incomplete responder to omalizumab | NR | 24 weeks | 12 weeks |
| Rituximab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT00216762 [65] | Phase 1–2 Open-label | Terminated | 15 | 18–70 | CSU refractory to high dose H1AH and immuno suppressants | 1000 mg Q2W | 2 weeks | NR |
| Tezepelumab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT04833855 [66] | Phase 2b RDBPCT | Recruiting | 159 | 18–80 | CSU refractory to H1AH and 6-months omalizumab UAS7 ≥ 16 and HSS7 ≥ 8 | NR | 16 weeks | NR |
| Barzolvolimab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT04538794 [67] | Phase 1 RDBPCT | Recruiting | 159 | 18–75 | CSU refractory to H1AH ± H2AH or LTRAs UAS7 ≥ 16 and ISS7 ≥ 8 | NR | 12 weeks | 12 weeks |
| NCT05368285 [68] | Phase 2 RDBPCT | Recruiting | 168 | ≥18 | CSU refractory to H1AH UAS7 ≥ 16 and ISS7 ≥ 8 | A. 75 mg for 16 weeks then 150 mg Q4W B. 75 mg for 16 weeks then 300 mg Q4W C. 150 mg Q4W D. 300 mg Q8W E. 16-weeks placebo then 150 mg Q4W F. 16-weeks placebo then 300 mg Q4W | 52 weeks | NR |
| MTPS9579A | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT05129423 [69] | Phase 2 Multi-center RDBPCT | Recruiting | 240 | 18–75 | CSU refractory to H1AH | Part 1 (12 weeks): Dose A vs. placebo Q4W Part 2 (12 weeks): Dose A, B, C, D vs. placebo Q4W | 24 weeks | NR |
| LY3454738 | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT04159701 [70] | Phase 2 RDBPCT | Terminated for lack of efficacy | 52 | 18–65 | CSU refractory to H1AH | A 500 mg Q2W for 12 weeks followed by placebo B Placebo for 12 weeks followed by 500 mg Q2W | 24 weeks | NR |
| Lirentelimab | ||||||||
| Trial number | Type of study | Status | N. | Age (Yrs) | Inclusion criteria | Dosage | Duration | Follow-Up |
| NCT03436797 [71] | Phase 2 Open-label study | Completed | 47 | 18–85 | CU refractory to H1AH | Up to 3 mg/kg Q4W | 6 months | 8 weeks |
| Biological Drugs | Target |
| Bruton’s tyrosine kinase (BTK) inhibitors | |
| Remibrutinib (LOU064) | BTK |
| Rilzabrutinib | BTK |
| Tirabrutinib | BTK |
| Fenebrutinib (GDC-0853) | BTK |
| TAS5315 | BTK |
| Others | |
| Etanercept | TNF-α |
| TLL018 | JAK1/TYK2 |
| AZD1981 | Prostaglandin D2 receptor 2 (DP2 or CRTH2) |
| Trial Number | Type of Study | Status | N. | Age (Yrs) | Inclusion Criteria | Duration |
|---|---|---|---|---|---|---|
| Remibrutinib | ||||||
| NCT03926611 [134] | Phase 2 RDBPCT | Completed | 311 | ≥18 | CSU refractory to H1AH UAS ≥ 16 | 12 weeks |
| NCT04109313 [135] | Open label | Active, not recruiting | 195 | 18–99 | Completed CLOU064A2201 or other preceding studies with LOU064 | 52 weeks |
| NCT05048342 [136] | Phase 3 Open label | Recruiting | 70 | ≥18 | CSU refractory to H1AH UAS ≥ 16 | 52 weeks |
| NCT05032157 NCT05030311 [137,138] | Phase 3 RDBPCT | Recruiting | 450 | ≥18 | CSU refractory to H1AH UAS ≥ 16 | 24 weeks +28 weeks |
| NCT05170724 [139] | Cohort | Available | NR | 18–99 | CSU refractory to H1AH | NR |
| Fenebrutinib (GDC-0853) | ||||||
| Metz et al. 2021 [140] | Phase 2 RDBPCT | Completed | 134 | 18–75 | CSU refractory to H1AH | 8 weeks |
| NCT03693625 [141] | Phase 2 Open label | Terminated | 31 | 18–75 | CSU refractory to H1AH | NR |
| Tirabrutinib | ||||||
| NCT04827589 [142] | Phase 2 RDBPCT | Withdrawn | NR | 18–75 | CSU refractory to H1AH UAS ≥ 16 | 8 weeks +16 weeks |
| Rilzabrutinib | ||||||
| NCT05107115 [143] | Phase 2 RDBPCT + OL | Recruiting | 152 | 18–80 | CSU refractory to H1AH | 12 weeks +40 weeks |
| TAS5315 | ||||||
| NCT05335499 [144] | Phase 2 RDBPCT | Not yet recruiting | 120 | 18–75 | CSU refractory to H1AH UAS ≥ 16 | 12 weeks |
| Etanercept | ||||||
| NCT01030120 [145] | RDBPCT Open label | Withdrawn | 0 | 18–70 | CSU refractory to H1AH | 6 weeks +6 weeks |
| TLL018 | ||||||
| NCT05373355 [146] | Phase 1 RDBPCT | Not yet recruiting | 36 | 18–70 | CSU and UAS ≥ 16 | 12 weeks |
| AZD1981 | ||||||
| Oliver et al. 2019 [147] | Phase 2 RDBPCT | Completed | 26 | 18–65 | CSU refractory to H1AH | 8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manti, S.; Giallongo, A.; Papale, M.; Parisi, G.F.; Leonardi, S. Monoclonal Antibodies in Treating Chronic Spontaneous Urticaria: New Drugs for an Old Disease. J. Clin. Med. 2022, 11, 4453. https://doi.org/10.3390/jcm11154453
Manti S, Giallongo A, Papale M, Parisi GF, Leonardi S. Monoclonal Antibodies in Treating Chronic Spontaneous Urticaria: New Drugs for an Old Disease. Journal of Clinical Medicine. 2022; 11(15):4453. https://doi.org/10.3390/jcm11154453
Chicago/Turabian StyleManti, Sara, Alessandro Giallongo, Maria Papale, Giuseppe Fabio Parisi, and Salvatore Leonardi. 2022. "Monoclonal Antibodies in Treating Chronic Spontaneous Urticaria: New Drugs for an Old Disease" Journal of Clinical Medicine 11, no. 15: 4453. https://doi.org/10.3390/jcm11154453
APA StyleManti, S., Giallongo, A., Papale, M., Parisi, G. F., & Leonardi, S. (2022). Monoclonal Antibodies in Treating Chronic Spontaneous Urticaria: New Drugs for an Old Disease. Journal of Clinical Medicine, 11(15), 4453. https://doi.org/10.3390/jcm11154453







