Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis
Abstract
1. Introduction
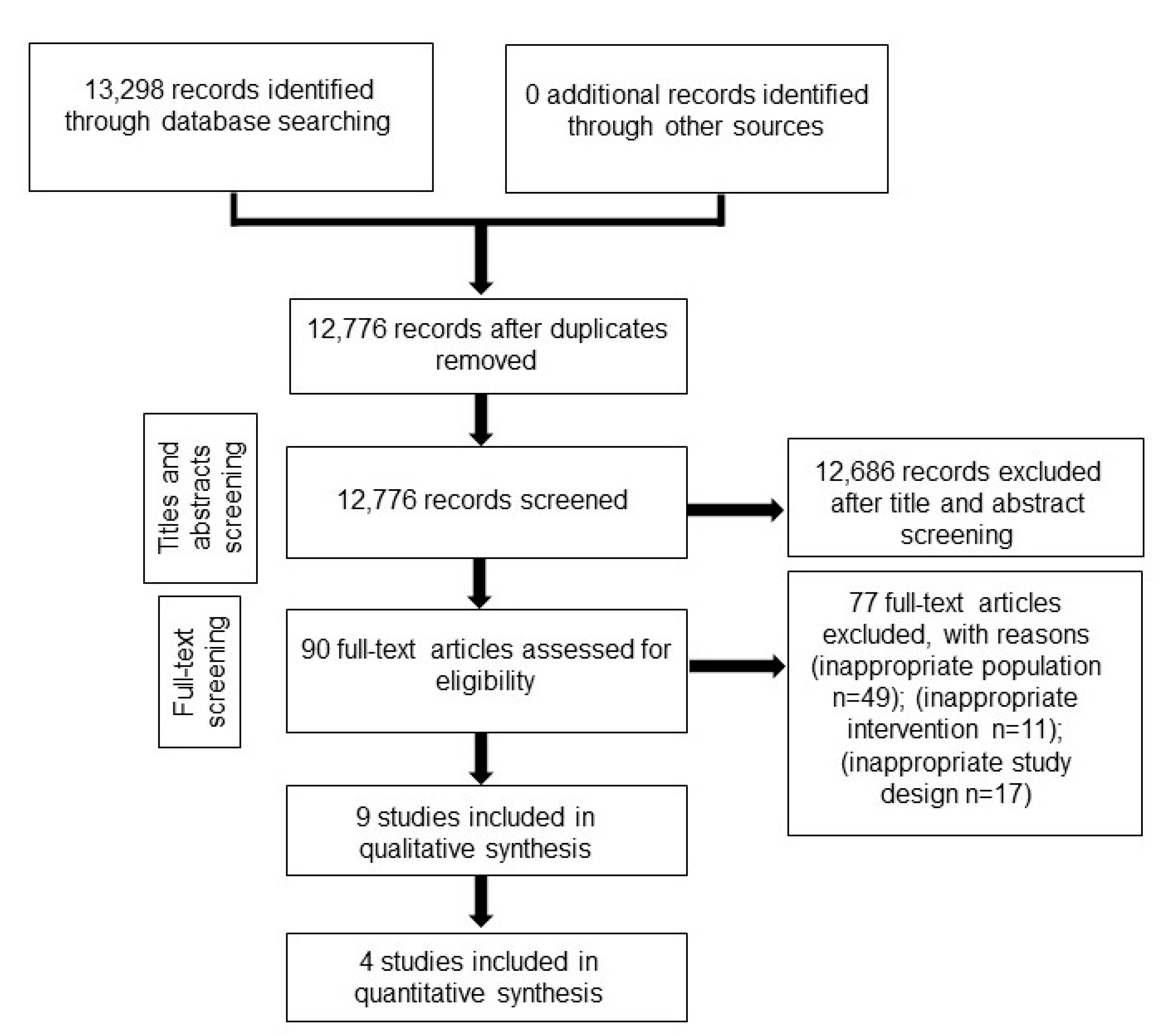
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Risk of Bias Assessment
2.3. Data Synthesis and Assessment of Heterogeneity Sensitivity Analysis
2.4. Summary of Findings
3. Results
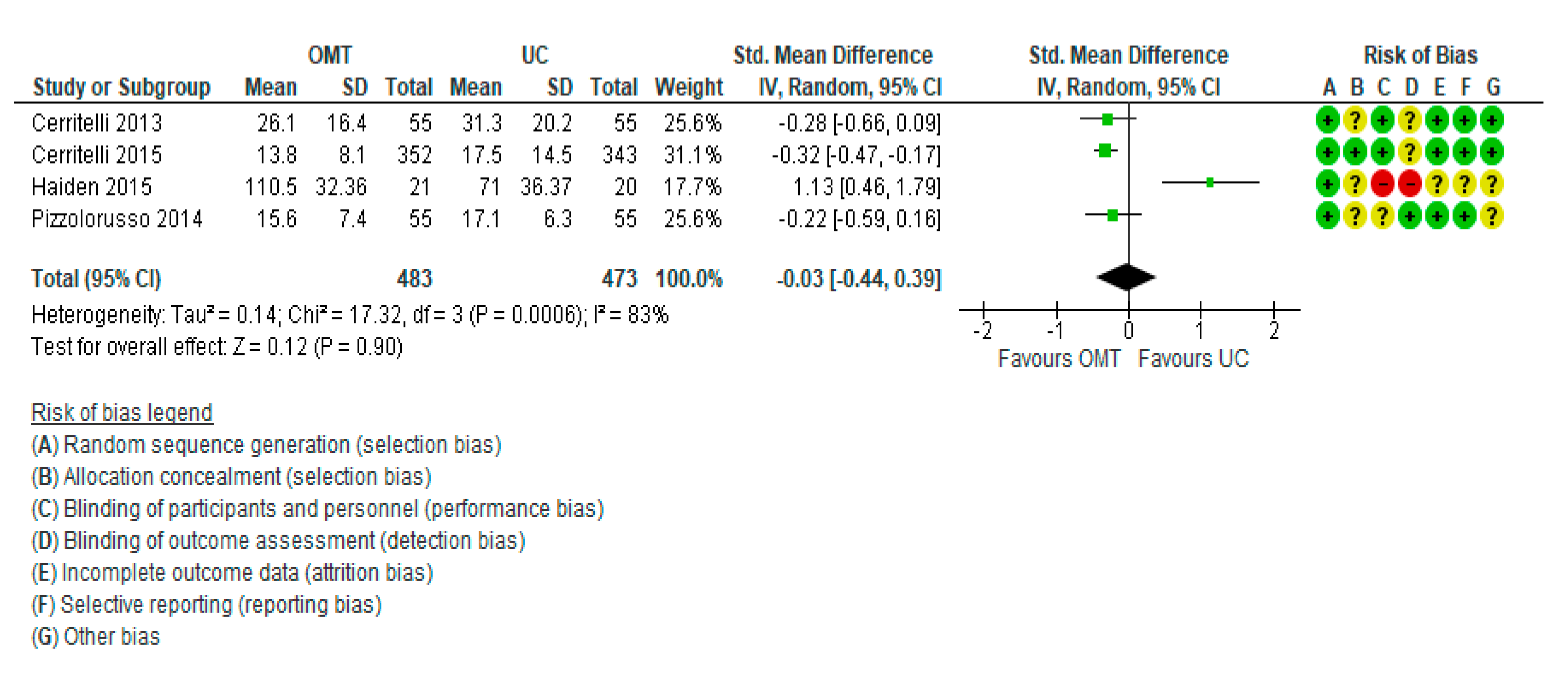
3.1. Meta-Analysis Results: Length of Hospital Stay
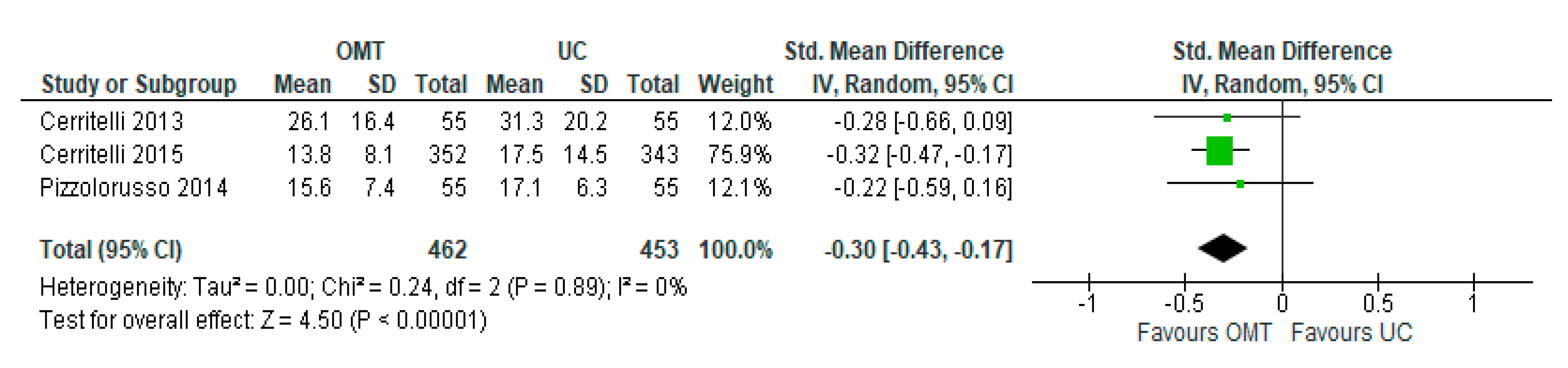
3.2. Sensitivity Analysis
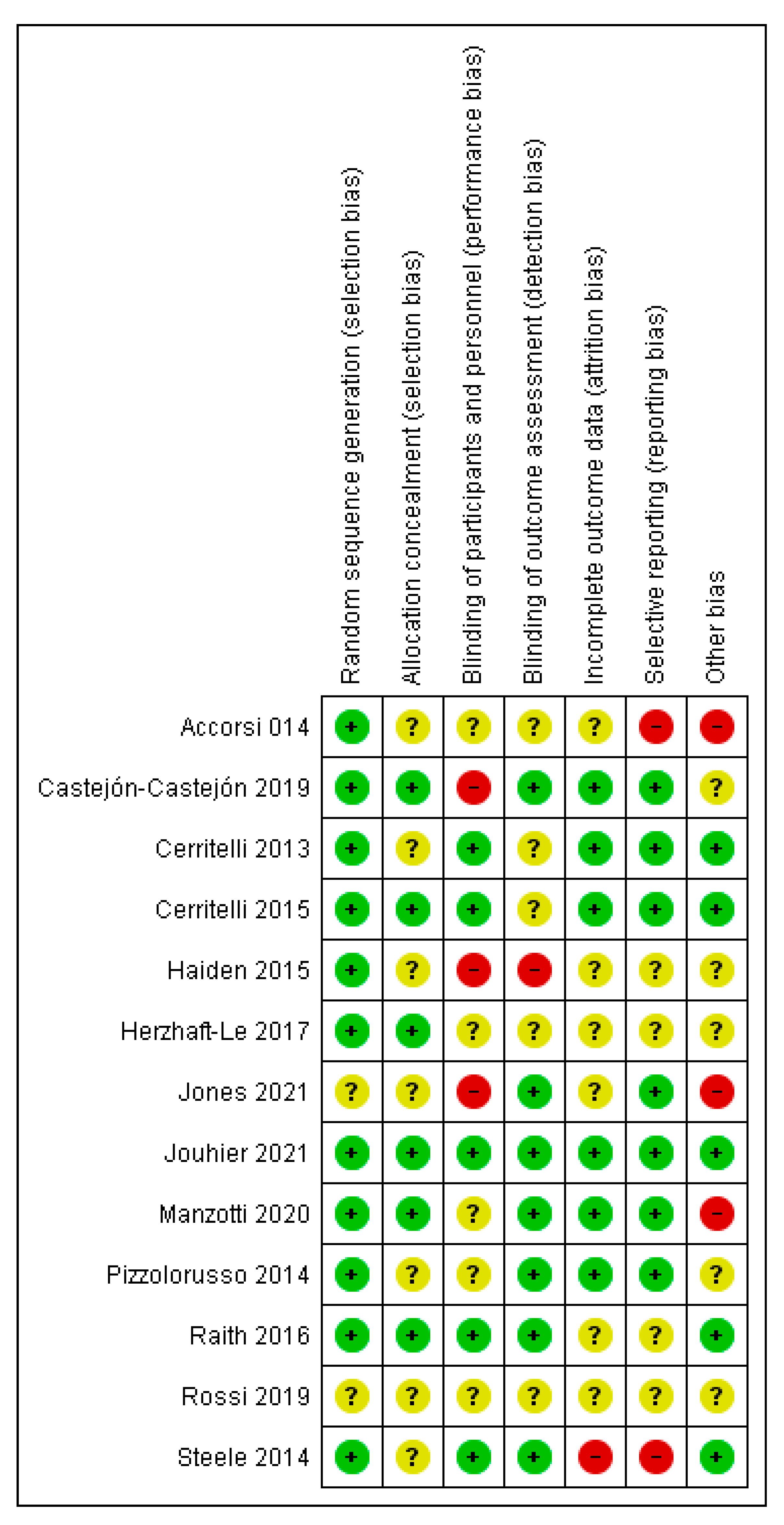
3.3. Risk of Bias
GRADE Assessments
4. Discussion
4.1. Origin of the Evidence
4.2. Agreements and Disagreements with Other Reviews
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Association, A.O. 2020–2021 Annual Report. 2021. Available online: https://osteopathic.org/wp-content/uploads/AOA-Annual-Report-2020-21.pdf (accessed on 30 May 2022).
- Gevitz, N. Center or Periphery? The Future of Osteopathic Principles and Practices. J. Osteopath. Med. 2006, 106, 121–129. [Google Scholar]
- Johnson, S.M.; Kurtz, M.E. Diminished use of osteopathic manipulative treatment and its impact on the uniqueness of the osteopathic profession. Acad. Med. 2001, 76, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.J.; Brockway, M.D.; Wilde, B.B. Osteopathic manipulative treatment (OMT) use among osteopathic physicians in the United States. J. Osteopath. Med. 2021, 121, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bagagiolo, D.; Didio, A.; Sbarbaro, M.; Priolo, C.G.; Borro, T.; Farina, D. Osteopathic Manipulative Treatment in Pediatric and Neonatal Patients and Disorders: Clinical Considerations and Updated Review of the Existing Literature. Am. J. Perinatol. 2016, 33, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Lee, M.S.; Ernst, E. Osteopathic manipulative treatment for pediatric conditions: A systematic review. Pediatrics 2013, 132, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Parnell Prevost, C.; Gleberzon, B.; Carleo, B.; Anderson, K.; Cark, M.; Pohlman, K.A. Manual therapy for the pediatric population: A systematic review. BMC Complement. Altern. Med. 2019, 19, 60. [Google Scholar] [CrossRef]
- DeMarsh, S.; Huntzinger, A.; Gehred, A.; Stanek, J.R.; Kemper, K.J.; Belsky, J.A. Pediatric Osteopathic Manipulative Medicine: A Scoping Review. Pediatrics 2021, 147, e2020016162. [Google Scholar] [CrossRef]
- Lanaro, D.; Ruffini, N.; Manzotti, A.; Lista, G. Osteopathic manipulative treatment showed reduction of length of stay and costs in preterm infants: A systematic review and meta-analysis. Medicine 2017, 96, e6408. [Google Scholar] [CrossRef]
- Yu, H.; Shearer, H.; Taylor-Vaisey, A.; Mior, S.; Verville, L.; Connell, G.; Côté, P. Methodological flaws on “manual therapy for the pediatric population: A systematic review” by Prevost et al. BMC Complement. Med. Ther. 2021, 21, 4. [Google Scholar] [CrossRef]
- Garner, P.; Hopewell, S.; Chandler, J.; MacLehose, H.; Akl, E.A.; Beyene, J.; Chang, S.; Churchill, R.; Dearness, K.; Guyatt, G.; et al. When and how to update systematic reviews: Consensus and checklist. BMJ 2016, 354, i3507. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019) [Internet]: Cochrane. 2019. Available online: https://training.cochrane.org/handbook (accessed on 30 May 2022).
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health; University of York: York, UK, 2009; Available online: http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed on 30 May 2022).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [PubMed]
- Accorsi, A.; Lucci, C.; Di Mattia, L.; Granchelli, C.; Barlafante, G.; Fini, F.; Pizzolorusso, G.; Cerritelli, F.; Pincherle, M. Effect of osteopathic manipulative therapy in the attentive performance of children with attention-deficit/hyperactivity disorder. J. Am. Osteopath. Assoc. 2014, 114, 374–381. [Google Scholar]
- Cerritelli, F.; Pizzolorusso, G.; Ciardelli, F.; La Mola, E.; Cozzolino, V.; Renzetti, C.; D’Incecco, C.; Fusilli, P.; Sabatino, G.; Barlafante, G. Effect of osteopathic manipulative treatment on length of stay in a population of preterm infants: A randomized controlled trial. BMC Pediatr. 2013, 13, 65. [Google Scholar] [CrossRef]
- Cerritelli, F.; Pizzolorusso, G.; Renzetti, C.; Cozzolino, V.; D’Orazio, M.; Lupacchini, M.; Marinelli, B.; Accorsi, A.; Lucci, C.; Lancellotti, J.; et al. A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms. PLoS ONE 2015, 10, e0127370. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Pimpel, B.; Kreissl, A.; Jilma, B.; Berger, A. Does visceral osteopathic treatment accelerate meconium passage in very low birth weight infants?—A prospective randomized controlled trial. PLoS ONE 2015, 10, e0123530. [Google Scholar]
- Herzhaft-Le Roy, J.; Xhignesse, M.; Gaboury, I. Efficacy of an Osteopathic Treatment Coupled With Lactation Consultations for Infants’ Biomechanical Sucking Difficulties: A Randomized Controlled Trial. J. Hum. Lact. 2017, 33, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Regan, C.; Wolf, K.; Bryant, J.; Rakowsky, A.; Pe, M.; Snyder, D.A. Effect of osteopathic manipulative treatment on pulmonary function testing in children with asthma. J. Osteopath. Med. 2021, 121, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Jouhier, M.D.; Boscher, C.; Roze, J.C.; Cailleau, N.; Chaligne, F.; Legrand, A.; Flamant, C.; Muller, J.B. Osteopathic manipulative treatment to improve exclusive breast feeding at 1 month. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, F591–F595. [Google Scholar] [CrossRef] [PubMed]
- Manzotti, A.; Cerritelli, F.; Lombardi, E.; La Rocca, S.; Chiera, M.; Galli, M.; Lista, G. Effects of osteopathic treatment versus static touch on heart rate and oxygen saturation in premature babies: A randomized controlled trial. Complement. Ther. Clin. Pract. 2020, 39, 101116. [Google Scholar] [CrossRef] [PubMed]
- Pizzolorusso, G.; Cerritelli, F.; Accorsi, A.; Lucci, C.; Tubaldi, L.; Lancellotti, J.; Barlafante, G.; Renzetti, C.; D’Incecco, C.; Perri, F.P. The Effect of Optimally Timed Osteopathic Manipulative Treatment on Length of Hospital Stay in Moderate and Late Preterm Infants: Results from a RCT. Evid. Based Complement. Altern. Med. 2014, 2014, 243539. [Google Scholar] [CrossRef] [PubMed]
- Raith, W.; Marschik, P.B.; Sommer, C.; Maurer-Fellbaum, U.; Amhofer, C.; Avian, A.; Löwenstein, E.; Soral, S.; Müller, W.; Einspieler, C.; et al. General Movements in preterm infants undergoing craniosacral therapy: A randomised controlled pilot-trial. BMC Complement. Altern. Med. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Versace, A.; Lauria, B. The role of osteopathic complementary treatment in high frequency paediatric headache: A randomised controlled study. Neurol. Sci. 2019, 40, S231–S232. [Google Scholar]
- Steele, K.M.; Carreiro, J.E.; Viola, J.H.; Conte, J.A.; Ridpath, L.C. Effect of osteopathic manipulative treatment on middle ear effusion following acute otitis media in young children: A pilot study. J. Am. Osteopath. Assoc. 2014, 114, 436–447. [Google Scholar] [CrossRef][Green Version]
- Castejón-Castejón, M.; Murcia-González, M.A.; Gil, J.M.; Todri, J.; Rancel, M.S.; Lena, O.; Chillón-Martínez, R. Effectiveness of craniosacral therapy in the treatment of infantile colic. A randomized controlled trial. Complement. Ther. Med. 2019, 47, 102164. [Google Scholar] [CrossRef]
- Pizzolorusso, G.; Turi, P.; Barlafante, G.; Cerritelli, F.; Renzetti, C.; Cozzolino, V.; D’orazio, M.; Fusilli, P.; Carinci, F.; D’incecco, C. Effect of osteopathic manipulative treatment on gastrointestinal function and length of stay of preterm infants: An exploratory study. Chiropr. Man. Ther. 2011, 19, 15. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]

| Author Year (Ref.) | n/Characteristics of Participants/ Age or Age Range | Experimental Intervention (Duration/Frequency/Intensity) | Control | Outcome Measure | Main Result (Between-Group Differences) | Effect Estimate | Authors’ Conclusions | AEs/COI | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Accorsi 2014 [18] | 28/Children aged 5 to 15 years with attention-deficit/hyperactivity disorder | OMT + UC (6 sessions, 40 min each) | UC only (drug therapy and psychosocial intervention) | Biancardi-Stroppa Modified Bell Cancellation Test: a. accuracy and b. rapidity scores | a. p = 0.04 §§ b. p = 0.03 §§ | 1.a. β = 7.948, 95% CI 0.18 to 15.71; 1.b. β = 9.090, 95% CI 0.82 to 17.35 | “Participants who received OMT had greater improvement in Biancardi- Stroppa Test scores than participants who received conventional care only” | None reported/not reported | Univariate analyses for post-interventions are missing; very small sample, significant baseline differences; possible confounding effects of UC |
| Castejón-Castejón 2019 [30] | 58/infants aged 0–84 days/infantile colic | OMT (craniosacral therapy) (1–3 sessions, 30–40 min each) | No treatment | 1. Crying hours 2. Sleep hours 3. Colic (pain) severity | 1. p < 0.0005) 2. p < 0.0005) 3. p < 0.0005) | 1. MD = −3.2 (95% CI −3.7, −2.6) at day 24 2. MD = 3.13 (95% CI 2.2, 3.9) at Day 24 3. −18.55 95% CI 21.4, −15.6) at day 24 | “Craniosacral therapy appears to be effective and safe for infantile colic by reducing the number of crying hours, the colic severity and increasing the total hours of sleep.” | None reported/not reported | Small sample, no control for placebo effects, no blinding of parents |
| Cerritelli 2013 [19] | 110/preterm infants (34 weeks) * | OMT + UC (20 min) | UC only | 1. Length of stay 2. Daily weight gain 3. Costs | 1. p < 0.03 2. p = 0.06§ 3. p < 0.001§ | MD = −5.20; 95% CI −12.08 to 1.68 (in days) ^ | “The present study suggests that OMT may have an important role in the management of preterm infants hospitalization.” | None reported/none declared | Unequal distribution of loss to follow-up; unclear why newborns transferred from another hospital were ineligible |
| Cerritelli 2015 [20] | 695/preterm infants (range: 29 to 37 weeks) * | OMT + UC (30 min/for the entire hospitalization, twice a week) | UC only (20 min) | 1. Length of stay 2. Daily weight gain 3. Costs | 1. p < 0.001 2. n.s. 3. p < 0.001 | ES = 0.31 | “Osteopathic treatment reduced significantly the number of days of hospitalization and is cost-effective on a large cohort of preterm infants” | None reported/none declared | Well-designed and adequately powered, unequal distribution of loss to follow-up, missing details of the OMT |
| Danielo Jouhier 2021 [24] | 128/infants (range 38–42 weeks) | OMT (two sessions) | No OMT | Exclusive breast milk feeding at 1 month | 1. n.s. | OR = 0.55; 95% CI 0.26 to 1.17 | “OMT did not improve exclusive breast feeding at 1 month.” | None reported/none declared | No control for placebo effects |
| Haiden 2015 [21] | 41/preterm infants (32 weeks) * | Visceral OMT (3 times during their first week of life) | No treatment | 1. Time to enteral feedings 2. Length of hospital stay | 1. p = 0.02 2. n.s. | n.r. | “Infants in the OMT group had a longer time to full enteral feedings and a longer hospital stay what must be interpreted as negative side effect. | None reported/none reported | Small sample, no control for placebo effects, no blinding |
| Herzhaft-Le Roy 2017 [22] | 97/infants with biomechanical impairments to suckling (mean = 15 days) | OMT + UC (4 treatments, once a week for 4 weeks) | UC | LATCH score | p = 0.001 | MD = 1.04 | “Findings support the hypothesis that the addition of osteopathy to regular lactation Consultations is beneficial and safe” | None reported/none declared | Lack of objective outcome measures, treatment protocol not standardized, small sample, underpowered |
| Jones 2021 [23] | 58/children with asthma (mean = 10.8 years) | OMT + UC (single session 15–20 min) | UC | 1. FEF 25–75% 2. FVC 3. FEV1 4. FEV-1/FVC ^^ | 1. p = 0.05 2. p = 0.26 3. p = 0.06 4. p = 0.51 | 1. Mean change + 4.4% 2. Mean change + 2.4% 3. Mean change 2.4% 4. Mean change = 0% | “The benefits of OMT on short term spirometry results in pediatric asthma patients remain unclear” | Not reported/none declared | Small sample, lack of follow-up, long-term benefits/harms unknown, selection bias, baseline differences in pulmonary function |
| Manzotti 2020 [25] | 96/preterm infants (mean (SD) 33.5 (4.3) weeks)) | OMT + UC (single session 20 min) | Static touch + UC | 1. Heart rate 2. Oxygen saturation | 1. n.s. 2. p = 0.04 | 1. Mean change (SD) = 1.2 (13.1) 2. Mean change (SD) = 0.3 (2.4) | “Results from the present study suggest that a single osteopathic intervention may induce beneficial effects on preterm physiological parameters.” | Not reported/none declared | Lack of follow-up; poor biological plausibility, underpowered |
| Pizzolorusso 2014 [26] | 110/preterm infants (range 33.8 and 34.3 weeks) * | OMT (twice per week, 20 min sessions) + UC | UC | Length of stay | p < 0.01 | Mean = −2.03; 95% CI −3.15 to −0.91 | “This study shows evidence that the sooner OMT is provided, the shorter their hospital stay is.” | None reported/none declared | Selection bias; lack of standardized treatment, poor generalizability |
| Raith 2016 [27] | 30/preterm infants (range: 25 and 33 weeks) * | OMT (20 min/twice a week over three weeks) | UC | General movements | p > 0.05 | n.r. | The primary outcome showed no difference between groups. Craniosacral therapy seems to be safe in preterm infants. | Not reported/none declared | Very small sample, insufficiently powered, high drop-out rate |
| Rossi 2019 [28] | 18/teenagers with pediatric headache | OMT (5 sessions over 2 months) | Light Touch Therapy | Headache frequency, analgesic use, quality of life and adverse events | n.r. | n.r. | “The results are still partial and we need to recruit more patients to have a statistical significance. | Not reported/not declared | Abstract only; no results |
| Steele 2014 [29] | 52/young children with otitis media (range: 6 months to 2 years) | OMT (3 weekly visits) | UC | Change in middle ear effusion over four weeks | n.r. ** | OR = 2.98; 95% CI 1.16 to 7.62 | “A standardized OMT protocol administered adjunctively with standard care for patients with acute otitis media may result in faster resolution of middle ear effusion […] than UC alone” | None reported/none declared | 17.3% drop-out rate; small sample, lack of power calculation, high risk of reporting bias, no control for placebo effects |
| Author Year (Ref) | Details of Treatment (Quote Where Appropriate) |
|---|---|
| Accorsi 2014 [18] | “Manipulative techniques used included myofascial release, craniosacral, balanced ligamentous tension, and balanced membranous tension”. |
| Castejón-Castejón 2019 [30] | “The craniosacral treatments were implemented by the main author of the study, a professional craniosacral therapist with 7 years of experience as a paediatric craniosacral therapist and osteopath, and 12 years of experience as a child physiotherapist. The babies received a 30–40 min session once a week (experimental group) or no treatment (control group). Babies in the OMT group received either 1, 2 or 3 CST sessions over a 14-day period.” |
| Cerritelli 2013 [19] | “The OMT techniques of choice in treating preterm infants are myofascial release, balanced ligamentous/membranous tension, indirect fluidic and v-spread”. |
| Cerritelli, 2015 [20] | “The treatment included the application of a selected range of manipulative techniques aimed at relieving the somatic dysfunctions. Techniques used were in line with the benchmarks on osteopathic treatment available in the medical literature and were limited to indirect techniques such as: myofascial release and balanced ligamentous/membranous tension.” |
| Haiden 2015 [21] | “Infants in the intervention group received an osteopathic treatment algorithm within their first 48 h of life according the following protocol adapted from visceral treatment of adults by Barral and Finet”. |
| Herzhaft-Le Roy 2017 [22] | “[…] after assessing somatic dysfunctions and cranial strains based on tissue texture, tone, asymmetry, and quality of motion, active treatment was carried out, most commonly using techniques such as balanced membranous tension, cranial sutures, and myofascial release.” |
| Jones 2021 [23] | “Two techniques were used […] Rib raising was performed in the seated position with the physician treating the rib cage bilaterally. […] Suboccipital release was performed for 45 s on a supine patient with the physician’s finger pads contacting the suboccipital musculature”. |
| Danielo Jouhier 2021 [24] | “The practitioner performed interventions on the part of the body considered appropriate, that is, muscles, bones or viscera […]”. |
| Manzotti 2020 [25] | “[…] treatment, which is based on the palpatory findings of the initial assessment. It lasted approximately 9 min and aimed at releasing detected changes in the tension and mobility of the tissue. The techniques chosen were those already used in previous studies and demonstrated to be safe in the context of preterm infants.” |
| Pizzolorusso 2014 [26] | A range of osteopathic techniques were used, including: indirect myofascial release, balanced ligamentous tension or balanced membranous tension. |
| Raith 2016 [27] | “The 10 step-program was modified as follows: exploration of the cranial system (step 1), treatment of asymmetry (step 2), evaluation of the overlapping of the cranial bones (step 4), exploration of the balance of the membranes of the cranial and spinal dura mater (step 7), exploration and treatment of the sacrum (step 8), and exploration and treatment of the chest (step 9). After the evaluation craniosacral therapy was initiated to achieve the greatest relaxation.” |
| Rossi 2019 [28] | Abstract only (no details of OMT treatment). |
| Steele 2014 [29] | “Standardized osteopathic manipulative treatment protocol used in the present study. Adapted from Steele et al. 2010”, which involved 9 techniques. |
| Patient or population: Premature infants | ||||||
| Setting: Neonatology clinics | ||||||
| Intervention: OMT (various techniques) | ||||||
| Comparison: UC | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect | №. of participants | Certainty of the evidence | Comments | |
| Risk with UC | Risk with OMT | (95% CI) | (studies) | (GRADE) | ||
| Length of hospital stay | The mean length of stay was 0 | SMD 0.03 lower | - | 956 | Downgraded for inconsistency, as studies showed contradictory results (I2 = 83%). Risk of bias was very high in Haiden 2015. Downgraded for indirectness, as different OMT protocols were used. | |
| (0.44 lower to 0.39 higher) | (4 RCTs) | |||||
| GRADE Working Group grades of evidence | ||||||
| Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posadzki, P.; Kyaw, B.M.; Dziedzic, A.; Ernst, E. Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4455. https://doi.org/10.3390/jcm11154455
Posadzki P, Kyaw BM, Dziedzic A, Ernst E. Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(15):4455. https://doi.org/10.3390/jcm11154455
Chicago/Turabian StylePosadzki, Pawel, Bhone Myint Kyaw, Arkadiusz Dziedzic, and Edzard Ernst. 2022. "Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 15: 4455. https://doi.org/10.3390/jcm11154455
APA StylePosadzki, P., Kyaw, B. M., Dziedzic, A., & Ernst, E. (2022). Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(15), 4455. https://doi.org/10.3390/jcm11154455