Ectopic Prostate Tissue in the Uterine Cervix of a Female with Non-Classic Congenital Adrenal Hyperplasia—A Case Report
Abstract
:1. Background
2. Patient Information
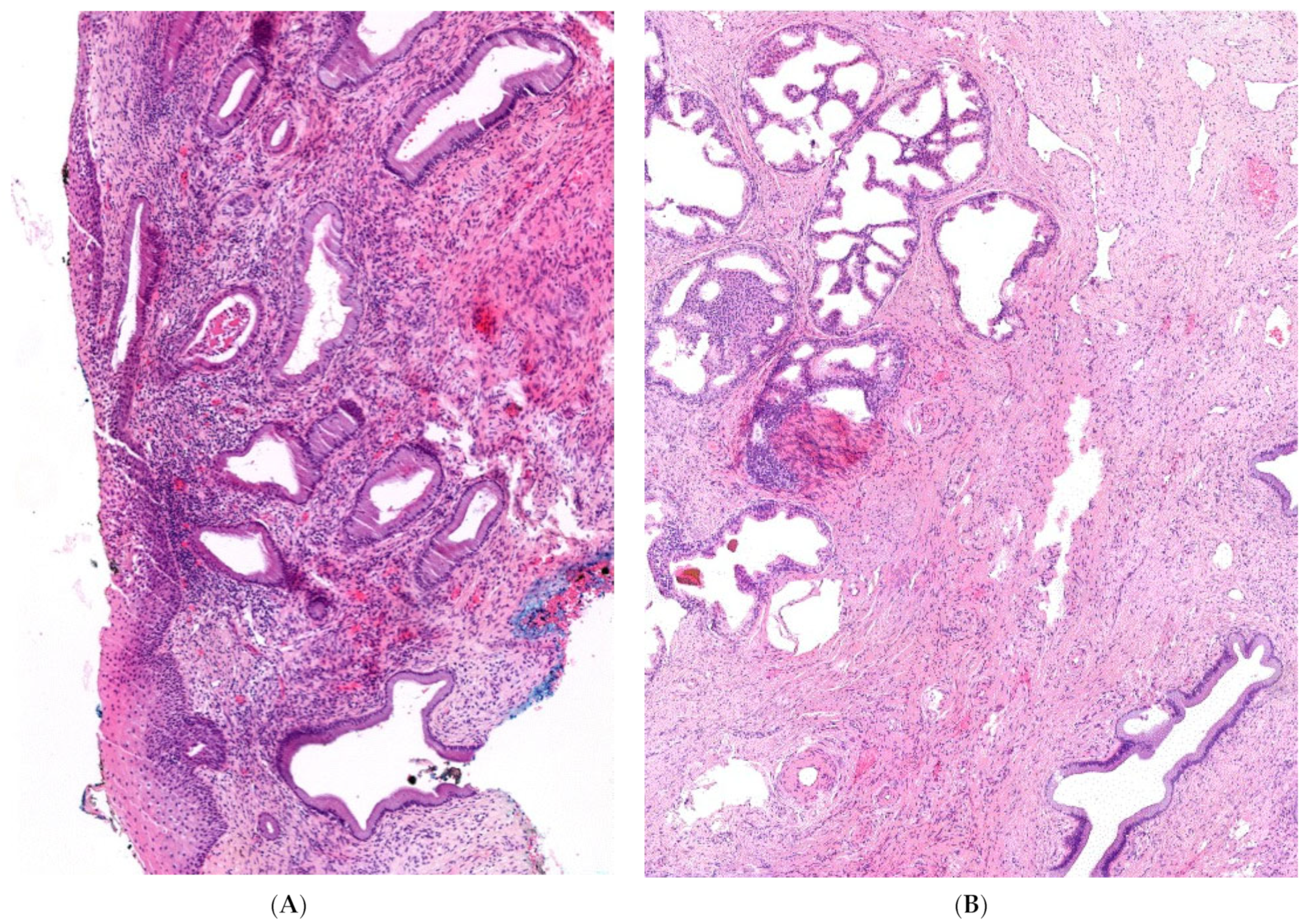
3. Clinical Findings
4. Diagnostic Assessment
5. Therapeutic Intervention and Follow-Up
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anderson, W.J.; Kolin, D.L.; Neville, G.; Diamond, D.A.; Crum, C.P.; Hirsch, M.S.; Vargas, S.O. Prostatic Metaplasia of the Vagina and Uterine Cervix: An Androgen-associated Glandular Lesion of Surface Squamous Epithelium. Am. J. Surg. Pathol. 2020, 44, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Ganesan, R.; Hirschowitz, L.; Miller, K.; Rollason, T.P. Ectopic prostatic tissue in the uterine cervix and vagina: Report of a series with a detailed immunohistochemical analysis. Am. J. Surg. Pathol. 2006, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wesselius, R.; Schotman, M.; Schotman, M.; Pereira, A.M. A Patient (46XX) With Congenital Adrenal Hyperplasia and Prostate Cancer: A Case Report. J. Endocr. Soc. 2017, 1, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfekih, H.; Ben Abdelkrim, A.; Marzouk, H.; Saad, G.; Gasmi, A.; Gribaa, M.; Zaghouani, H.; Hasni, Y.; Maaroufi, A. Prostatic tissue in 46XX congenital adrenal hyperplasia: Case report and literature review. Clin. Case Rep. 2021, 9, 1655–1662. [Google Scholar] [CrossRef]
- Fang, B.; Cho, F.; Lam, W. Prostate gland development and adrenal tumor in a female with congenital adrenal hyperplasia: A case report and review from radiology perspective. J. Radiol. Case Rep. 2013, 7, 21–34. [Google Scholar] [CrossRef]
- Finkielstain, G.P.; Vieites, A.; Bergadá, I.; Rey, R.A. Disorders of Sex Development of Adrenal Origin. Front. Endocrinol. (Lausann) 2021, 12, 770782. [Google Scholar] [CrossRef]
- Merke, D.P.; Auchus, R.J. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N. Engl. J. Med. 2020, 383, 1248–1261. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Montori, V.M.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef]
- Wedell, A. Molecular genetics of 21-hydroxylase deficiency. Endocr. Dev. 2011, 20, 80–87. [Google Scholar]
- Hannah-Shmouni, F.; Morissette, R.; Sinaii, N.; Elman, M.; Prezant, T.R.; Chen, W.; Pulver, A.; Merke, D.P. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Nordenström, A.; Falhammar, H. Management of endocrine disease: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 2019, 180, R127–R145. [Google Scholar] [CrossRef] [PubMed]
- New, M.I.; Abraham, M.; Gonzalez, B.; Dumic, M.; Razzaghy-Azar, M.; Chitayat, D.; Sun, L.; Zaidi, M.; Wilson, R.C.; Yuen, T. Genotype-phenotype correlation in 1507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc. Natl. Acad. Sci. USA 2013, 110, 2611–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiviat, M.D.; Leonard, J.M. True prostatic tissue in 46,XX female with adrenogenital syndrome. Urology 1978, 12, 75–78. [Google Scholar] [CrossRef]
- Subramanian, S.; Gamanagatti, S.; Sharma, R.M.R. demonstration of a prostate gland in a female pseudohermaphrodite. Pediatr. Radiol. 2006, 36, 1194–1196. [Google Scholar] [CrossRef]
- Lázaro, A.P.; de Lacerda, A.M.; Ghiaroni, J.; de Miranda, L.C.; Vidal, A.P.; Collett-Solberg, P.F.; Michelatto, D.P.; Mello, M.P.; Guimarães, M.M. Leydig cell tumour in a 46,XX child with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm. Res. Paediatr. 2013, 79, 179–184. [Google Scholar] [CrossRef]
- Nucci, M.R.; Ferry, J.A.; Young, R.H. Ectopic prostatic tissue in the uterine cervix: A report of four cases and review of ectopic prostatic tissue. Am. J. Surg. Pathol. 2000, 24, 1224–1230. [Google Scholar] [CrossRef]
- Winters, J.L.; Chapman, P.H.; Powell, D.E.; Banks, E.R.; Allen, W.R.; Wood, D.P., Jr. Female pseudohermaphroditism due to congenital adrenal hyperplasia complicated by adenocarcinoma of the prostate and clear cell carcinoma of the endometrium. Am. J. Clin. Pathol. 1996, 106, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Boutin, E.L.; Battle, E.; Cunha, G.R. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: Prostatic inductions. Differentiation 1991, 48, 99–105. [Google Scholar] [CrossRef]
- Singh, K.; Sung, C.J.; Lawrence, W.D.; Quddus, M.R. Testosterone-induced “Virilisation” of Mesonephric Duct Remnants and Cervical Squamous Epithelium in Female-to-Male Transgenders: A Report of 3 Cases. Int. J. Gynecol. Pathol. 2017, 36, 328–333. [Google Scholar] [CrossRef]
- Paulino Mda, C.; Steinmetz, L.; Menezes Filho, H.C.; Kuperman, H.; Della Manna, T.; Vieira, J.G.; Blasbalg, R.; Baroni, R.; Setian, N.; Damiani, D. [Search of prostatic tissue in 46, XX congenital adrenal hyperplasia]. Arq. Bras. Endocrinol. Metabol. 2009, 53, 716–720. [Google Scholar]
- Yeşilkaya, E.; Cinaz, P.; Bideci, A.; Camurdan, O.; Boyraz, M.A. 46XX disorder of sex development with a prostate gland and increased level of prostate-specific antigen. J. Natl. Med. Assoc. 2008, 100, 256–258. [Google Scholar] [CrossRef]
- Delle Piane, L.; Rinaudo, P.F.; Miller, W.L. 150 years of congenital adrenal hyperplasia: Translation and commentary of De Crecchio’s classic paper from 1865. Endocrinology 2015, 156, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Heyns, C.F.; Rimington, P.D.; Kurger, T.F.; Falck, V.G. Benign prostatic hyperplasia and uterine leiomyomas in a female pseudohermaphrodite: A case report. J. Urol. 1987, 137, 1245–1247. [Google Scholar] [CrossRef]
- Klessen, C.; Asbach, P.; Hein, P.A.; Beyersdorff, D.; Hamm, B.; Taupitz, M. Complex genital malformation in a female with congenital adrenal hyperplasia: Evaluation with magnetic resonance imaging. Acta Radiol. 2005, 46, 891–894. [Google Scholar] [CrossRef]
- Kim, K.R.; Park, K.H.; Kim, J.W.; Cho, K.J.; Ro, J.Y. Transitional cell metaplasia and ectopic prostatic tissue in the uterine cervix and vagina in a patient with adrenogenital syndrome: Report of a case suggesting a possible role of androgen in the histogenesis. Int. J. Gynecol. Pathol. 2004, 23, 182–187. [Google Scholar] [CrossRef]


| Patient Visits | Patient Age | Clinical Presentation | Diagnostic Findings | Consequences and Procedure |
|---|---|---|---|---|
| Visit 1 10/1997 | 10 |
|
|
|
| Visit 2 05/2000 | 12 |
|
|
|
| Visit 3 07/2006 | 18 |
|
|
|
| Visit 4 05/2016 | 28 |
|
|
|
| Visit 5 10/2021 | 34 |
|
|
|
| Visit 6 01/2022 Post-conzation follow-up | 34 |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschaidse, L.; Auer, M.K.; Dubinski, I.; Lottspeich, C.; Nowotny, H.; Schmidt, H.; Gut, N.; Reisch, N. Ectopic Prostate Tissue in the Uterine Cervix of a Female with Non-Classic Congenital Adrenal Hyperplasia—A Case Report. J. Clin. Med. 2022, 11, 4307. https://doi.org/10.3390/jcm11154307
Tschaidse L, Auer MK, Dubinski I, Lottspeich C, Nowotny H, Schmidt H, Gut N, Reisch N. Ectopic Prostate Tissue in the Uterine Cervix of a Female with Non-Classic Congenital Adrenal Hyperplasia—A Case Report. Journal of Clinical Medicine. 2022; 11(15):4307. https://doi.org/10.3390/jcm11154307
Chicago/Turabian StyleTschaidse, Lea, Matthias K. Auer, Ilja Dubinski, Christian Lottspeich, Hanna Nowotny, Heinrich Schmidt, Nadezda Gut, and Nicole Reisch. 2022. "Ectopic Prostate Tissue in the Uterine Cervix of a Female with Non-Classic Congenital Adrenal Hyperplasia—A Case Report" Journal of Clinical Medicine 11, no. 15: 4307. https://doi.org/10.3390/jcm11154307
APA StyleTschaidse, L., Auer, M. K., Dubinski, I., Lottspeich, C., Nowotny, H., Schmidt, H., Gut, N., & Reisch, N. (2022). Ectopic Prostate Tissue in the Uterine Cervix of a Female with Non-Classic Congenital Adrenal Hyperplasia—A Case Report. Journal of Clinical Medicine, 11(15), 4307. https://doi.org/10.3390/jcm11154307






