Risk of Cancer in Connective Tissue Diseases in Northeastern Italy over 15 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
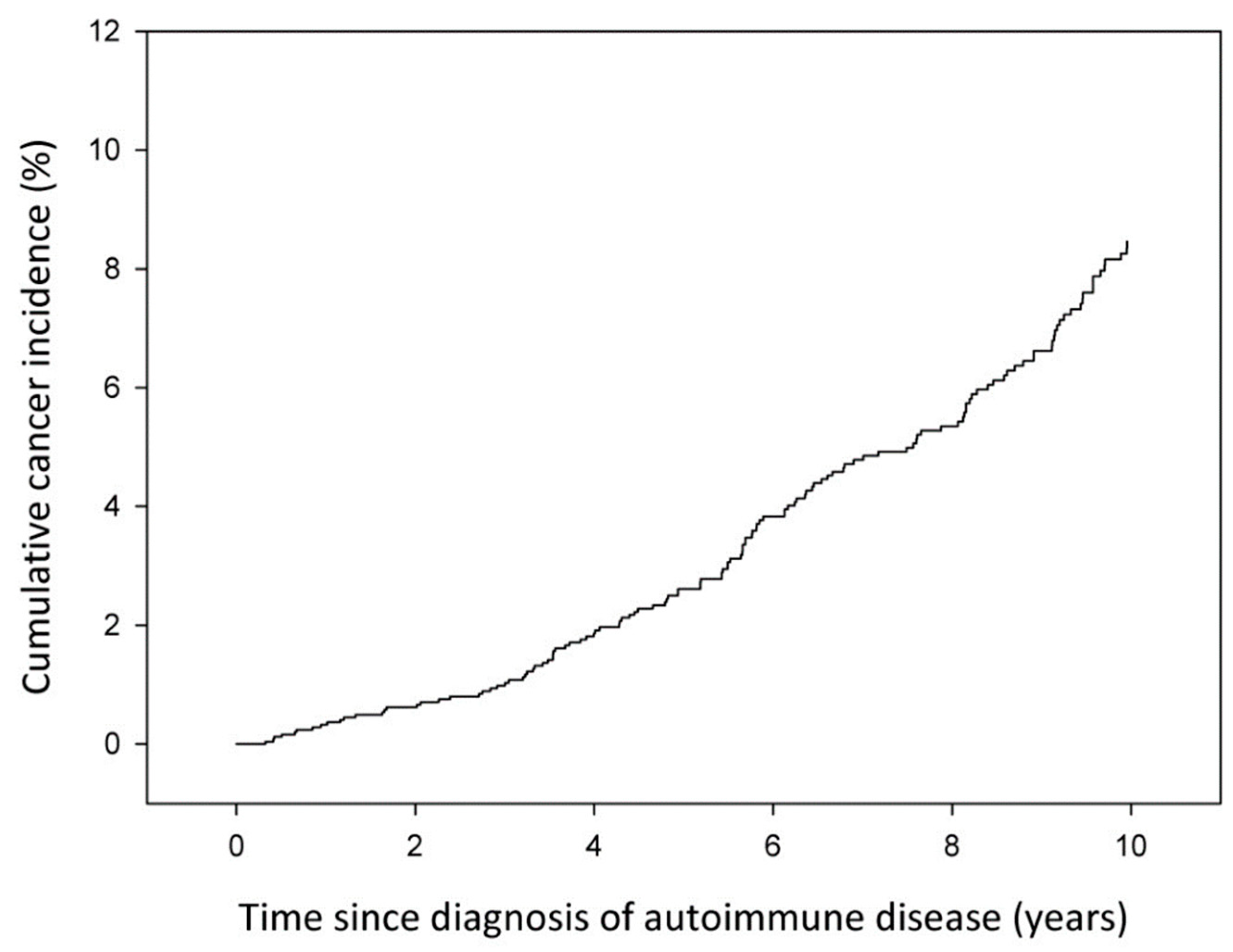
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human Autoimmune Diseases: A Comprehensive Update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Autoimmune Diseases and Hematological Malignancies: Exploring the Underlying Mechanisms from Epidemiological Evidence. Semin. Cancer Biol. 2020, 64, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ekström Smedby, K.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Seniori Costantini, A.; Bracci, P.M.; et al. Autoimmune Disorders and Risk of Non-Hodgkin Lymphoma Subtypes: A Pooled Analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giat, E.; Ehrenfeld, M.; Shoenfeld, Y. Cancer and Autoimmune Diseases. Autoimmun. Rev. 2017, 16, 1049–1057. [Google Scholar] [CrossRef]
- Goules, A.V.; Argyropoulou, O.D.; Pezoulas, V.C.; Chatzis, L.; Critselis, E.; Gandolfo, S.; Ferro, F.; Binutti, M.; Donati, V.; Zandonella Callegher, S.; et al. Primary Sjögren’s Syndrome of Early and Late Onset: Distinct Clinical Phenotypes and Lymphoma Development. Front. Immunol. 2020, 11, 594096. [Google Scholar] [CrossRef]
- Björnådal, L.; Löfström, B.; Yin, L.; Lundberg, I.E.; Ekbom, A. Increased Cancer Incidence in a Swedish Cohort of Patients with Systemic Lupus Erythematosus. Scand. J. Rheumatol. 2002, 31, 66–71. [Google Scholar] [CrossRef]
- Baecklund, E.; Iliadou, A.; Askling, J.; Ekbom, A.; Backlin, C.; Granath, F.; Catrina, A.I.; Rosenquist, R.; Feltelius, N.; Sundström, C.; et al. Association of Chronic Inflammation, Not Its Treatment, with Increased Lymphoma Risk in Rheumatoid Arthritis. Arthritis Rheum. 2006, 54, 692–701. [Google Scholar] [CrossRef]
- Bernatsky, S.; Ramsey-Goldman, R.; Joseph, L.; Boivin, J.-F.; Costenbader, K.H.; Urowitz, M.B.; Gladman, D.D.; Fortin, P.R.; Nived, O.; Petri, M.A.; et al. Lymphoma Risk in Systemic Lupus: Effects of Disease Activity versus Treatment. Ann. Rheum. Dis. 2014, 73, 138–142. [Google Scholar] [CrossRef]
- Shah, A.A.; Rosen, A. Cancer and Systemic Sclerosis: Novel Insights into Pathogenesis and Clinical Implications. Curr. Opin. Rheumatol. 2011, 23, 530–535. [Google Scholar] [CrossRef]
- Oldroyd, A.G.S.; Allard, A.B.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; George, M.D.; Gordon, P.; Kolstad, K.; Kurtzman, D.J.B.; et al. A Systematic Review and Meta-Analysis to Inform Cancer Screening Guidelines in Idiopathic Inflammatory Myopathies. Rheumatol. Oxf. Engl. 2021, 60, 2615–2628. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Shah, A.A. The Relationships between Cancer and Autoimmune Rheumatic Diseases. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101472. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lin, F.; Qin, B.; Liang, Y.; Zhong, R. Polymyositis/Dermatomyositis and Malignancy Risk: A Metaanalysis Study. J. Rheumatol. 2015, 42, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Sorensen, I.J.; Mellemkjaer, L.; Loft, A.G.R.; Thomsen, B.S.; Tvede, N.; Baslund, B. Malignancies in Wegener’s Granulomatosis: Incidence and Relation to Cyclophosphamide Therapy in a Cohort of 293 Patients. J. Rheumatol. 2008, 35, 100–105. [Google Scholar] [PubMed]
- Raaschou, P.; Simard, J.F.; Holmqvist, M.; Askling, J. ARTIS Study Group Rheumatoid Arthritis, Anti-Tumour Necrosis Factor Therapy, and Risk of Malignant Melanoma: Nationwide Population Based Prospective Cohort Study from Sweden. BMJ 2013, 346, f1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vita, S.; Quartuccio, L.; Salvin, S.; Corazza, L.; Zabotti, A.; Fabris, M. Cryoglobulinaemia Related to Sjogren’s Syndrome or HCV Infection: Differences Based on the Pattern of Bone Marrow Involvement, Lymphoma Evolution and Laboratory Tests after Parotidectomy. Rheumatol. Oxf. Engl. 2012, 51, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Quartuccio, L.; Baldini, C.; Bartoloni, E.; Priori, R.; Carubbi, F.; Corazza, L.; Alunno, A.; Colafrancesco, S.; Luciano, N.; Giacomelli, R.; et al. Anti-SSA/SSB-Negative Sjögren’s Syndrome Shows a Lower Prevalence of Lymphoproliferative Manifestations, and a Lower Risk of Lymphoma Evolution. Autoimmun. Rev. 2015, 14, 1019–1022. [Google Scholar] [CrossRef]
- De Vita, S.; Gandolfo, S.; Zandonella Callegher, S.; Zabotti, A.; Quartuccio, L. The Evaluation of Disease Activity in Sjögren’s Syndrome Based on the Degree of MALT Involvement: Glandular Swelling and Cryoglobulinaemia Compared to ESSDAI in a Cohort Study. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S112), 150–156. [Google Scholar]
- Ansell, P.; Simpson, J.; Lightfoot, T.; Smith, A.; Kane, E.; Howell, D.; Newton, R.; McGonagle, D.; Jack, A.; Roman, E. Non-Hodgkin Lymphoma and Autoimmunity: Does Gender Matter? Int. J. Cancer 2011, 129, 460–466. [Google Scholar] [CrossRef]
- Chatzis, L.; Pezoulas, V.C.; Ferro, F.; Gandolfo, S.; Donati, V.; Binutti, M.; Callegher, S.Z.; Venetsanopoulou, A.; Zampeli, E.; Mavrommati, M.; et al. Sjögren’s Syndrome: The Clinical Spectrum of Male Patients. J. Clin. Med. 2020, 9, 2620. [Google Scholar] [CrossRef]
- Zard, E.; Arnaud, L.; Mathian, A.; Chakhtoura, Z.; Hie, M.; Touraine, P.; Heard, I.; Amoura, Z. Increased Risk of High Grade Cervical Squamous Intraepithelial Lesions in Systemic Lupus Erythematosus: A Meta-Analysis of the Literature. Autoimmun. Rev. 2014, 13, 730–735. [Google Scholar] [CrossRef]
- Santana, I.U.; Gomes, A.D.N.; Lyrio, L.D.C.; Rios Grassi, M.F.; Santiago, M.B. Systemic Lupus Erythematosus, Human Papillomavirus Infection, Cervical Pre-Malignant and Malignant Lesions: A Systematic Review. Clin. Rheumatol. 2011, 30, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zatuchni, J.; Campbell, W.N.; Zarafonetis, C.J. Pulmonary Fibrosis and Terminal Bronchiolar (Alveolar-Cell) Carcinoma in Scleroderma. Cancer 1953, 6, 1147–1158. [Google Scholar] [CrossRef]
- Shah, A.A.; Hummers, L.K.; Casciola-Rosen, L.; Visvanathan, K.; Rosen, A.; Wigley, F.M. Examination of Autoantibody Status and Clinical Features Associated with Cancer Risk and Cancer-Associated Scleroderma. Arthritis Rheumatol. 2015, 67, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Morrisroe, K.; Hansen, D.; Huq, M.; Stevens, W.; Sahhar, J.; Ngian, G.-S.; Ferdowsi, N.; Hill, C.; Roddy, J.; Walker, J.; et al. Incidence, Risk Factors, and Outcomes of Cancer in Systemic Sclerosis. Arthritis Care Res. 2020, 72, 1625–1635. [Google Scholar] [CrossRef]
- Watad, A.; McGonagle, D.; Bragazzi, N.L.; Tiosano, S.; Comaneshter, D.; Shoenfeld, Y.; Cohen, A.D.; Amital, H. Autoantibody Status in Systemic Sclerosis Patients Defines Both Cancer Risk and Survival with ANA Negativity in Cases with Concomitant Cancer Having a Worse Survival. Oncoimmunology 2019, 8, e1588084. [Google Scholar] [CrossRef] [PubMed]
- Calatroni, M.; Buzio, C.; Vaglio, A. The Evolving Paradigm of Cancer Risk Related to Cyclophosphamide Therapy in Granulomatosis with Polyangiitis. Rheumatol. Oxf. Engl. 2015, 54, 1339–1341. [Google Scholar] [CrossRef] [Green Version]
- Tessier Cloutier, B.; Clarke, A.E.; Ramsey-Goldman, R.; Wang, Y.; Foulkes, W.; Gordon, C.; Hansen, J.E.; Yelin, E.; Urowitz, M.B.; Gladman, D.; et al. Breast Cancer in Systemic Lupus Erythematosus. Oncology 2013, 85, 117–121. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | All Patients n (%) |
|---|---|
| Sex: | |
| Men | 396 (15.8) |
| Women | 2108 (84.2) |
| Age at diagnosis: | |
| 0–29 | 198 (7.9) |
| 30–39 | 314 (12.5) |
| 40–49 | 414 (16.5) |
| 50–59 | 489 (19.5) |
| 60–69 | 522 (20.8) |
| 70–79 | 410 (16.4) |
| 80+ | 157 (6.2) |
| Median (IQR *) | 57 (43–69) |
| Period of diagnosis: | |
| 2002–2005 | 640 (25.6) |
| 2006–2009 | 676 (27.0) |
| 2010–2013 | 644 (25.7) |
| 2014–2017 | 544 (21.7) |
| Type of autoimmune condition: | |
| Systemic Lupus Erythematosus | 866 (34.6) |
| Systemic sclerosis | 455 (18.2) |
| Sjogren’s syndrome | 791 (31.6) |
| Dermatomyositis or polymyositis | 191 (7.6) |
| Multiple diagnoses | 201 (8.0) |
| Median follow-up time (years) (IQR *) | 6.8 (3.3–10.8) |
| Total person-years | 18,006 |
| Cancer Site/Type | All Patients | |
|---|---|---|
| obs./exp | SIR (95% CI) | |
| All, excluding non-melanoma skin cancers 1 | 187/215.4 | 0.87 (0.75–1.00) |
| Lip, oral cavity and pharynx (C00–C14) | 6/4.6 | 1.31 (0.48–2.85) |
| Esophagus (C15) | 3/1.8 | 1.63 (0.34–4.75) |
| Stomach (C16) | 7/9.2 | 0.76 (0.31–1.57) |
| Small intestine (C17) | 2/0.7 | 2.81 (0.34–10.2) |
| Colon, rectum and anus (C18–C21) | 20/28.2 | 0.71 (0.43–1.09) |
| Liver and gallbladder (C22–C23) | 5/6.7 | 0.75 (0.24–1.75) |
| Pancreas (C25) | 13/8.3 | 1.57 (0.83–2.68) |
| Bronchus and lung (C34) | 24/18.8 | 1.27 (0.82–1.90) |
| Melanoma (C43) | 7/7.2 | 0.98 (0.39–2.01) |
| Breast (C50) | 34/56.0 | 0.61 (0.42–0.85) |
| Corpus uteri (C54–C55) | 2/9.4 | 0.21 (0.03–0.77) |
| Prostate (C61) | 4/9.9 | 0.41 (0.11–1.04) |
| Kidney and urinary tract (C64–C66, C68) | 5/6.5 | 0.76 (0.25–1.78) |
| Bladder (C67, D09.9, D41.4) | 10/9.7 | 1.03 (0.49–1.90) |
| Brain (C71) | 2/2.8 | 0.73 (0.09–2.62) |
| Thyroid (C73) | 7/4.9 | 1.43 (0.57–2.95) |
| Non-Hodgkin lymphoma (C82–C85, C96) | 20/7.9 | 2.52 (1.54–3.89) |
| Multiple myeloma (C90) | 5/3.1 | 1.61 (0.52–3.75) |
| Leukemia (C91–C95) | 3/4.3 | 0.70 (0.14–2.04) |
| Cancer Site/Type | Type of Autoimmune Condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Systemic Lupus Erythematosus | Systemic Sclerosis | Sjögren’s Syndrome | Dermatomyositis or Polymyositis | |||||
| obs./exp | SIR (95% CI) | obs./exp | SIR (95% CI) | obs./exp | SIR (95% CI) | obs./exp | SIR (95% CI) | |
| All, excluding non-melanoma skin cancers | 54/62.3 | 0.87 (0.65–1.13) | 35/42.2 | 0.83 (0.58–1.15) | 64/75.5 | 0.85 (0.65–1.08) | 23/17.4 | 1.32 (0.84–1.98) |
| Lip, oral cavity and pharynx (C00–C14) | 3/1.4 | 2.13 (0.44–6.22) | 2/0.9 | 2.25 (0.27–8.12) | 1/1.5 | 0.68 (0.02–3.76) | 0/0.4 | 0.00 (0.00–6.94) |
| Stomach (C16) | 3/2.5 | 1.22 (0.25–3.55) | 2/1.9 | 1.06 (0.13–3.84) | 2/3.2 | 0.62 (0.07–2.23) | 0/0.9 | 0.00 (0.00–3.48) |
| Colon rectum and anus (C18–C21) | 3/7.7 | 0.39 (0.08–1.14) | 6/5.7 | 1.05 (0.39–2.29) | 5/10.1 | 0.49 (0.16–1.15) | 4/2.5 | 1.63 (0.44–4.17) |
| Liver and gallbladder (C22–C23) | 1/1.9 | 0.54 (0.01–3.01) | 0/1.4 | 0.00 (0.00–2.16) | 2/2.2 | 0.89 (0.11–3.23) | 2/0.7 | 3.02 (0.37–10.9) |
| Pancreas (C25) | 3/2.1 | 1.42 (0.29–4.16) | 3/1.7 | 1.75 (0.36–5.11) | 4/3.1 | 1.29 (0.35–3.31) | 3/0.7 | 4.16 (0.86–12.2) |
| Bronchus and lung (C34) | 7/5.4 | 1.29 (0.52–2.67) | 7/3.9 | 1.82 (0.73–3.74) | 6/6.3 | 0.96 (0.35–2.09) | 1/1.9 | 0.54 (0.01–3.01) |
| Melanoma (C43) | 2/2.4 | 0.84 (0.10–3.03) | 1/1.3 | 0.79 (0.02–4.40) | 3/2.4 | 1.26 (0.26–3.68) | 1/0.5 | 1.97 (0.05–11.0) |
| Breast (C50) | 11/16.1 | 0.68 (0.34–1.23) | 7/10.2 | 0.69 (0.28–1.42) | 14/21.7 | 0.65 (0.35–1.08) | 1/3.2 | 0.32 (0.01–1.77) |
| Prostate (C61) | 3/3.7 | 0.80 (0.17–2.35) | 0/2.1 | 0.00 (0.00–1.41) | 1/1.7 | 0.58 (0.01–3.24) | 0/1.6 | 0.00 (0.00–1.90) |
| Kidney and urinary tract (C64–C66, C68) | 1/1.9 | 0.53 (0.01–2.97) | 2/1.3 | 1.53 (0.19–5.54) | 0/2.3 | 0.00 (0.00–1.32) | 2/0.6 | 3.42 (0.41–12.4) |
| Bladder (C67, D09.9, D41.4) | 3/2.9 | 1.05 (0.22–3.06) | 2/2.0 | 1.00 (0.12–3.62) | 1/3.0 | 0.33 (0.01–1.83) | 3/1.0 | 2.88 (0.59–8.43) |
| Brain (C71) | 2/0.8 | 2.49 (0.30–8.98) | 0/0.5 | 0.00 (0.00–5.61) | 0/1.0 | 0.00 (0.00–3.10) | 0/0.2 | 0.00 (0.00–13.3) |
| Thyroid (C73) | 1/1.8 | 0.57 (0.01–3.16) | 1/0.8 | 1.29 (0.03–7.18) | 5/1.7 | 3.02 (0.98–7.06) | 0/0.2 | 0.00 (0.00–12.3) |
| Non-Hodgkin lymphoma (C82–C85, C96) | 6/2.2 | 2.69 (0.99–5.84) | 0/1.6 | 0.00 (0.00–1.92) | 11/2.9 | 3.84 (1.92–6.87) | 1/0.6 | 1.58 (0.04–8.82) |
| Multiple myeloma (C90) | 0/0.8 | 0.00 (0.00–3.69) | 1/0.6 | 1.57 (0.04–8.76) | 3/1.1 | 2.61 (0.54–7.64) | 1/0.3 | 3.77 (0.10–21.0) |
| Leukemia (C91–C95) | 1/1.2 | 0.83 (0.02–4.61) | 0/0.9 | 0.00 (0.00–3.51) | 2/1.5 | 1.33 (0.16–4.79) | 0/0.4 | 0.00 (0.00–7.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treppo, E.; Toffolutti, F.; Manfrè, V.; Taborelli, M.; De Marchi, G.; De Vita, S.; Serraino, D.; Quartuccio, L. Risk of Cancer in Connective Tissue Diseases in Northeastern Italy over 15 Years. J. Clin. Med. 2022, 11, 4272. https://doi.org/10.3390/jcm11154272
Treppo E, Toffolutti F, Manfrè V, Taborelli M, De Marchi G, De Vita S, Serraino D, Quartuccio L. Risk of Cancer in Connective Tissue Diseases in Northeastern Italy over 15 Years. Journal of Clinical Medicine. 2022; 11(15):4272. https://doi.org/10.3390/jcm11154272
Chicago/Turabian StyleTreppo, Elena, Federica Toffolutti, Valeria Manfrè, Martina Taborelli, Ginevra De Marchi, Salvatore De Vita, Diego Serraino, and Luca Quartuccio. 2022. "Risk of Cancer in Connective Tissue Diseases in Northeastern Italy over 15 Years" Journal of Clinical Medicine 11, no. 15: 4272. https://doi.org/10.3390/jcm11154272
APA StyleTreppo, E., Toffolutti, F., Manfrè, V., Taborelli, M., De Marchi, G., De Vita, S., Serraino, D., & Quartuccio, L. (2022). Risk of Cancer in Connective Tissue Diseases in Northeastern Italy over 15 Years. Journal of Clinical Medicine, 11(15), 4272. https://doi.org/10.3390/jcm11154272







