Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
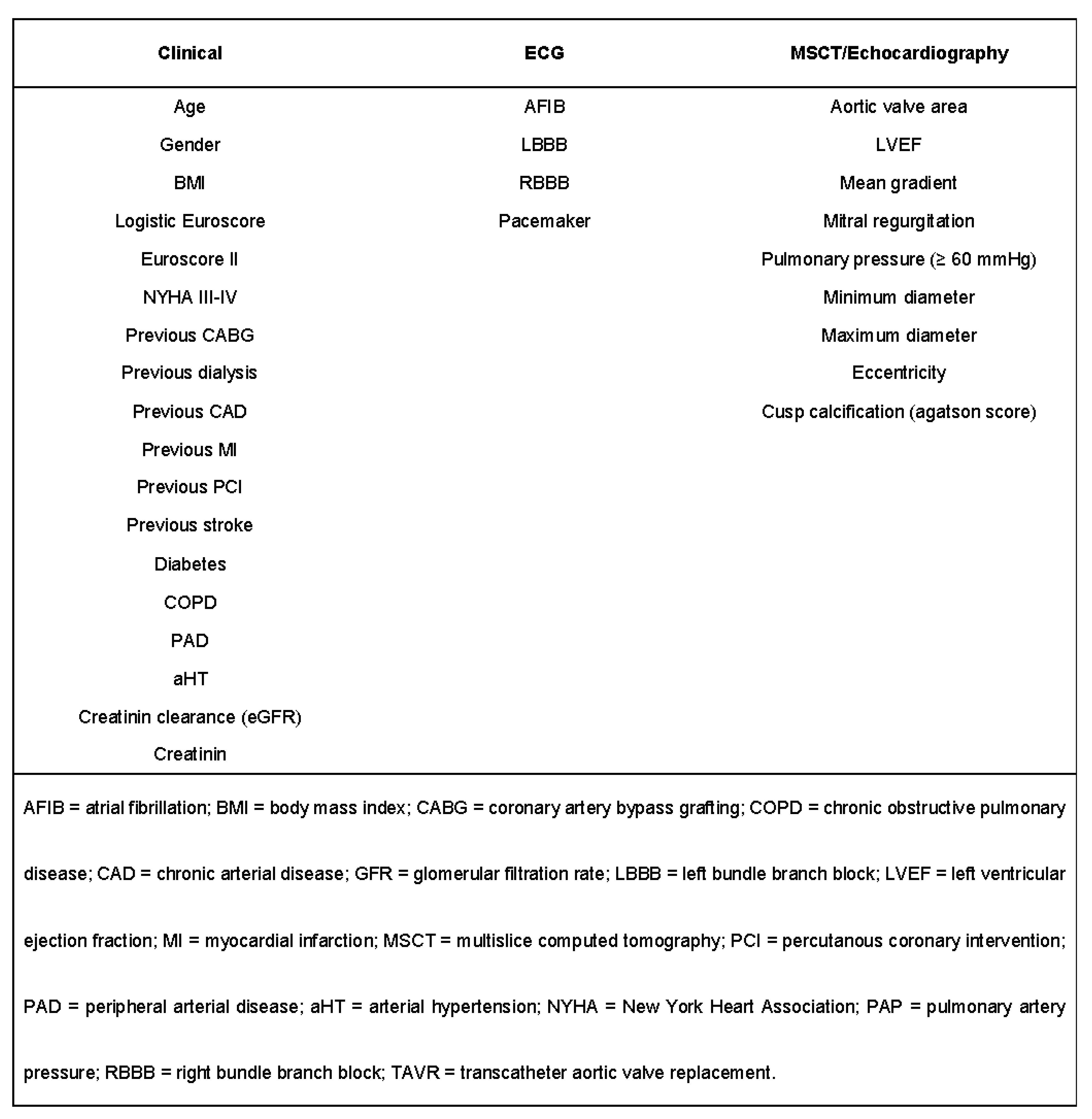
2.2. Multislice Computed Tomography Data Analysis
2.3. Device Description
2.4. Definition of Endpoints and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Propensity Matching
3.2. Procedural Data and In-Hospital Outcome of the Matched Cohort
3.3. Device Failure According to VARC-2
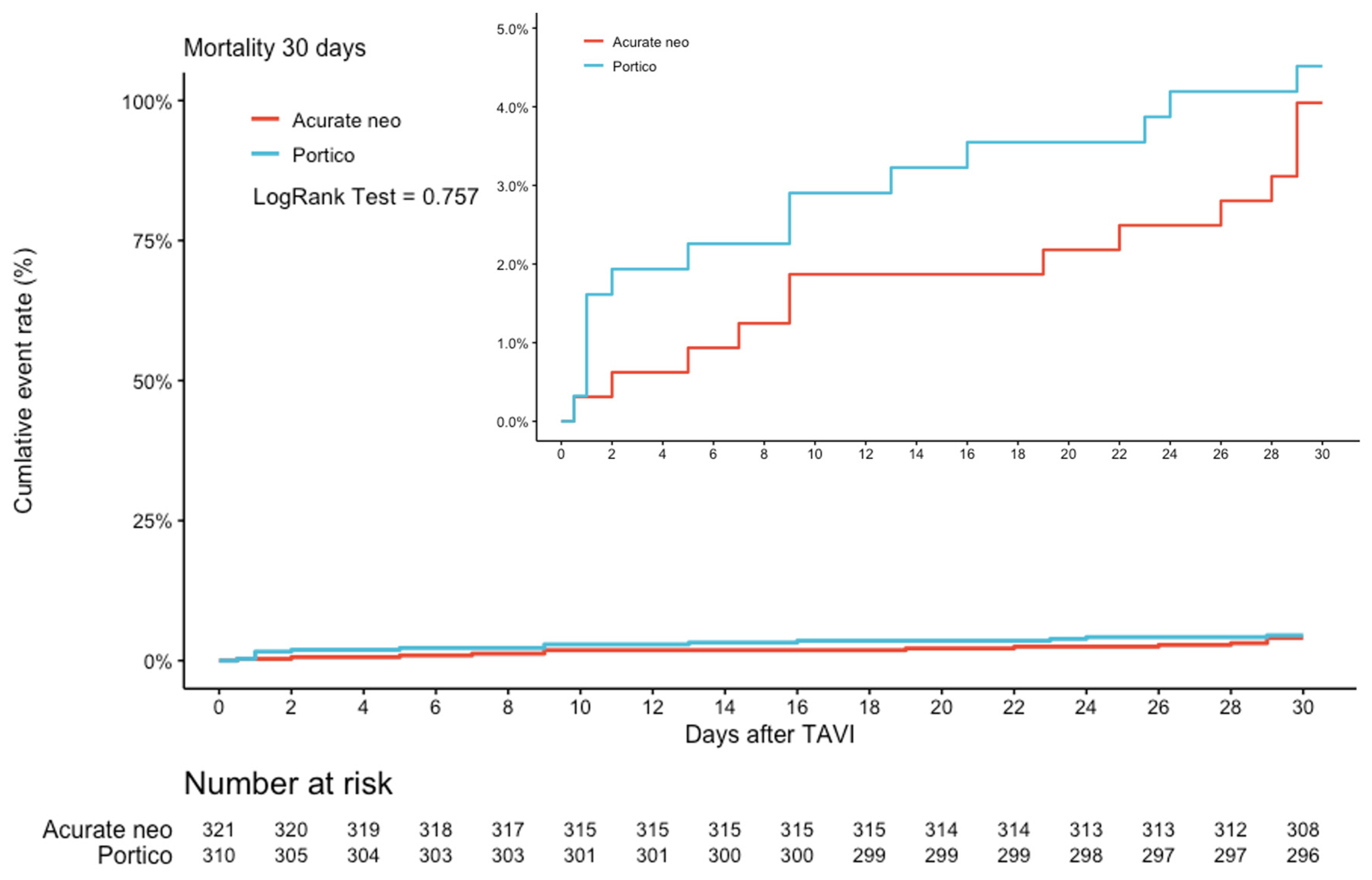
3.4. 30-Day Follow-Up
4. Discussion
4.1. Echocardiographic Data
4.2. Access-Related Complications and Major Bleeding Events
4.3. Permanent Pacemaker Implantation
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyregod, H.G.; Steinbruchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrom, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tolg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schafer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA 2014, 311, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.E.; Reardon, M.J.; Rajagopal, V.; Makkar, R.R.; Bajwa, T.K.; Kleiman, N.S.; Linke, A.; Kereiakes, D.J.; Waksman, R.; Thourani, V.H.; et al. Effect of Mechanically Expanded vs Self-Expanding Transcatheter Aortic Valve Replacement on Mortality and Major Adverse Clinical Events in High-Risk Patients With Aortic Stenosis: The REPRISE III Randomized Clinical Trial. JAMA 2018, 319, 27–37. [Google Scholar] [CrossRef]
- Kim, W.K.; Blumenstein, J.; Liebetrau, C.; Rolf, A.; Gaede, L.; Van Linden, A.; Arsalan, M.; Doss, M.; Tijssen, J.G.P.; Hamm, C.W.; et al. Comparison of outcomes using balloon-expandable versus self-expanding transcatheter prostheses according to the extent of aortic valve calcification. Clin. Res. Cardiol. 2017, 106, 995–1004. [Google Scholar] [CrossRef]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Mollmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J.; et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef]
- Pagnesi, M.; Kim, W.K.; Conradi, L.; Barbanti, M.; Stefanini, G.G.; Zeus, T.; Pilgrim, T.; Schofer, J.; Zweiker, D.; Testa, L.; et al. Transcatheter Aortic Valve Replacement With Next-Generation Self-Expanding Devices: A Multicenter, Retrospective, Propensity-Matched Comparison of Evolut PRO Versus Acurate neo Transcatheter Heart Valves. JACC Cardiovasc. Interv. 2019, 12, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Bleiziffer, S.; Thiele, H.; Scholtz, S.; Hildick-Smith, D.; Cunnington, M.; Wolf, A.; Barbanti, M.; Tchetche, D.; Garot, P.; et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients With Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial. Circulation 2020, 142, 2431–2442. [Google Scholar] [CrossRef]
- Mollmann, H.; Linke, A.; Holzhey, D.M.; Walther, T.; Manoharan, G.; Schafer, U.; Heinz-Kuck, K.; Van Boven, A.J.; Redwood, S.R.; Kovac, J.; et al. Implantation and 30-Day Follow-Up on All 4 Valve Sizes Within the Portico Transcatheter Aortic Bioprosthetic Family. JACC Cardiovasc. Interv. 2017, 10, 1538–1547. [Google Scholar] [CrossRef]
- Sondergaard, L.; Rodes-Cabau, J.; Hans-Peter Linke, A.; Fichtlscherer, S.; Schafer, U.; Kuck, K.H.; Kempfert, J.; Arzamendi, D.; Bedogni, F.; Asch, F.M.; et al. Transcatheter Aortic Valve Replacement With a Repositionable Self-Expanding Prosthesis: The PORTICO-I Trial 1-Year Outcomes. J. Am. Coll. Cardiol. 2018, 72, 2859–2867. [Google Scholar] [CrossRef]
- Willson, A.B.; Rodes-Cabau, J.; Wood, D.A.; Leipsic, J.; Cheung, A.; Toggweiler, S.; Binder, R.K.; Freeman, M.; DeLarochelliere, R.; Moss, R.; et al. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: First-in-human experience. J. Am. Coll. Cardiol. 2012, 60, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef]
- Mollmann, H.; Diemert, P.; Grube, E.; Baldus, S.; Kempfert, J.; Abizaid, A. Symetis ACURATE TF aortic bioprosthesis. EuroIntervention 2013, 9, S107–S110. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Kim, W.K.; Pellegrini, C.; Holzamer, A.; Walther, T.; Mayr, P.N.; Joner, M.; Kasel, A.M.; Trenkwalder, T.; Michel, J.; et al. Multicenter Comparison of Novel Self-Expanding Versus Balloon-Expandable Transcatheter Heart Valves. JACC Cardiovasc. Interv. 2017, 10, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Walther, T.; Burgdorf, C.; Mollmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; Thiele, H.; et al. One-Year Outcomes of a Randomized Trial Comparing a Self-Expanding With a Balloon-Expandable Transcatheter Aortic Valve. Circulation 2021, 143, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Pibarot, P.; Douglas, P.S.; Williams, M.; Xu, K.; Thourani, V.; Rihal, C.S.; Zajarias, A.; Doshi, D.; Davidson, M.; et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: Characterizing patients and impact on outcomes. Eur. Heart J. 2015, 36, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schafer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. The SAVI-TF Registry: 1-Year Outcomes of the European Post-Market Registry Using the ACURATE neo Transcatheter Heart Valve Under Real-World Conditions in 1,000 Patients. JACC Cardiovasc. Interv. 2018, 11, 1368–1374. [Google Scholar] [CrossRef]
- Mollmann, H.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schafer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention 2018, 13, e1764–e1770. [Google Scholar] [CrossRef] [Green Version]
- Dencker, D.; Taudorf, M.; Luk, N.H.; Nielsen, M.B.; Kofoed, K.F.; Schroeder, T.V.; Sondergaard, L.; Lonn, L.; De Backer, O. Frequency and Effect of Access-Related Vascular Injury and Subsequent Vascular Intervention After Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 118, 1244–1250. [Google Scholar] [CrossRef]
- Genereux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: A weighted meta-analysis of 3,519 patients from 16 studies. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef] [Green Version]
- Barbanti, M.; Buccheri, S.; Rodes-Cabau, J.; Gulino, S.; Genereux, P.; Pilato, G.; Dvir, D.; Picci, A.; Costa, G.; Tamburino, C.; et al. Transcatheter aortic valve replacement with new-generation devices: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 245, 83–89. [Google Scholar] [CrossRef]
- Husser, O.; Pellegrini, C.; Kim, W.K.; Holzamer, A.; Pilgrim, T.; Toggweiler, S.; Schafer, U.; Blumenstein, J.; Deuschl, F.; Rheude, T.; et al. Transcatheter Valve SELECTion in Patients With Right Bundle Branch Block and Impact on Pacemaker Implantations. JACC Cardiovasc. Interv. 2019, 12, 1781–1793. [Google Scholar] [CrossRef]
- Bruno, F.; D’Ascenzo, F.; Vaira, M.P.; Elia, E.; Omede, P.; Kodali, S.; Barbanti, M.; Rodes-Cabau, J.; Husser, O.; Sossalla, S.; et al. Predictors of pacemaker implantation after transcatheter aortic valve implantation according to kind of prosthesis and risk profile: A systematic review and contemporary meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Almendarez, M.; Avanzas, P.; Alvarez, R.; Arboine, L.A.; Del Valle, R.; Hernandez-Vaquero, D.; Alfonso, F.; Moris, C. Cusp-overlapping TAVI technique with a self-expanding device optimizes implantation depth and reduces permanent pacemaker requirement. Rev. Esp. Cardiol. 2022, 75, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Hernandez-Vaquero, D.; Alperi, A.; Almendarez, M.; Avanzas, P.; Kalavrouziotis, D.; Lorca, R.; Mesnier, J.; Arboine, L.; Mohammadi, S.; et al. Permanent Pacemaker Reduction Using Cusp-Overlapping Projection in TAVR: A Propensity Score Analysis. JACC Cardiovasc. Interv. 2022, 15, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Zappulla, P.; Barbanti, M.; Cirasa, A.; Todaro, D.; Rapisarda, G.; Picci, A.; Platania, F.; Tosto, A.; Di Grazia, A.; et al. Pacemaker dependency after transcatheter aortic valve implantation: Incidence, predictors and long-term outcomes. EuroIntervention 2019, 15, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Regueiro, A.; Abdul-Jawad Altisent, O.; Del Trigo, M.; Campelo-Parada, F.; Puri, R.; Urena, M.; Philippon, F.; Rodes-Cabau, J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003635. [Google Scholar] [CrossRef]
- Fadahunsi, O.O.; Olowoyeye, A.; Ukaigwe, A.; Li, Z.; Vora, A.N.; Vemulapalli, S.; Elgin, E.; Donato, A. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: Analysis From the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc. Interv. 2016, 9, 2189–2199. [Google Scholar] [CrossRef]
- Siontis, G.C.; Juni, P.; Pilgrim, T.; Stortecky, S.; Bullesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodes-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntane-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]

| Entire Population | Matched Population (1:1) | ||||
|---|---|---|---|---|---|
| PORTICO | ACURATE Neo | p-Value | ACURATE Neo | p-Value | |
| (n = 344) | (n = 1247) | (n = 344) | |||
| Clinical characteristics | |||||
| Age | 83.0 (79.7–86.0) | 82.0 (78.6–85.0) | 0.003 | 82.8 (79.6–86.0) | 0.863 |
| Gender (male): | 138 (40.1%) | 484 (38.8%) | 0.707 | 149 (43.3%) | 0.439 |
| BMI (kg/m2) | 26.7 (24.2–30.7) | 26.6 (23.9–30.4) | 0.533 | 26.9 (24.0–30.9) | 0.905 |
| Log Euroscore (%) | 17.4 (11.2–26.8) | 17.5 (11.2–26.4) | 0.841 | 17.4 (11.6–26.8) | 0.911 |
| Euroscore II (%) | 4.2 (2.7–7.0) | 4.1 (2.6–6.8) | 0.344 | 4.2 (2.6–7.2) | 0.731 |
| NYHA (III/IV) | 290 (84.3%) | 1008 (80.8%) | 0.164 | 298 (86.6%) | 0.449 |
| COPD | 70 (20.3%) | 241 (19.3%) | 0.729 | 72 (20.9%) | 0.925 |
| Hypertension | 308 (89.5%) | 1121 (89.9%) | 0.924 | 313 (91.0%) | 0.607 |
| Diabetes | 120 (34.9%) | 400 (32.1%) | 0.359 | 130 (37.8%) | 0.476 |
| eGFR (mL/min) | 54.0 (41.8–74.2) | 59.0 (43.0–78.0) | 0.059 | 59.0 (42.0–76.2) | 0.423 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 0.346 | 1.0 (0.8–1.3) | 0.971 |
| Dialysis | 6 (1.7%) | 32 (2.6%) | 0.494 | 4 (1.2%) | 0.750 |
| PAD | 48 (14.0%) | 173 (13.9%) | 1.000 | 45 (13.1%) | 0.824 |
| Previous Stroke | 31 (9.0%) | 168 (13.5%) | 0.034 | 27 (7.8%) | 0.681 |
| CAD | 230 (66.9%) | 821 (65.8%) | 0.772 | 227 (66.0%) | 0.872 |
| Previous MI | 37 (10.8%) | 142 (11.4%) | 0.817 | 33 (9.6%) | 0.705 |
| Previous PCI | 129 (37.5%) | 471 (37.8%) | 0.977 | 124 (36.0%) | 0.752 |
| Previous CABG | 31 (9.0%) | 125 (10.0%) | 0.648 | 31 (9.0%) | 1.000 |
| Echocardiographic characteristics | |||||
| LVEF (%) | 60.0 (52.0–65.0) | 62.0 (53.0–65.0) | 0.057 | 60.0 (50.0–65.0) | 0.474 |
| Pmean (mmHg) | 43.0 (35.0–50.0) | 41.0 (31.0–49.0) | 0.006 | 41.0 (32.0–51.0) | 0.405 |
| MR (≥Grade II) | 5 (1.5%) | 11 (0.9%) | 0.361 | 5 (1.5%) | 1.000 |
| TR (≥Grade II) | 5 (1.5%) | 17 (1.4%) | 0.800 | 4 (1.2%) | 1.000 |
| sPAP (≥60 mmHg) | 25 (8.9%) | 94 (9.0%) | 1.000 | 32 (9.3%) | 1.000 |
| Electrocardiographic characteristics | |||||
| Atrial fibrillation | 135 (39.2%) | 479 (38.4%) | 0.827 | 129 (37.5%) | 0.695 |
| LBBB | 34 (10.0%) | 109 (8.8%) | 0.568 | 36 (10.5%) | 0.900 |
| RBBB | 17 (5.0%) | 133 (10.7%) | 0.002 | 14 (4.1%) | 0.713 |
| Pacemaker | 66 (19.2%) | 147 (11.8%) | 0.001 | 49 (14.2%) | 0.102 |
| MSCT data | |||||
| Prosthesis area (cm2) | 4.3 (4.0–4.9) | 4.5 (4.0–4.9) | 0.435 | 4.6 (4.1–4.9) | 0.064 |
| Minimum diameter (mm) | 20.7 (19.2–22.0) | 20.3 (19.0–21.8) | 0.007 | 20.9 (19.6–22.0) | 0.197 |
| Maximum diameter (mm) | 26.9 (25.4–28.2) | 26.7 (25.1–28.0) | 0.131 | 27.0 (25.7–28.4) | 0.431 |
| Distance to LCA (mm) | 13.0 (11.6–15.0) | 13.1 (11.1–15.2) | 0.966 | 13.1 (11.9–15.4) | 0.321 |
| Distance to RCA (mm) | 17.0 (15.0–19.0) | 17.0 (15.0–19.0) | 0.422 | 17.1 (15.4–19.0) | 0.040 |
| Eccentricity | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.057 | 0.2 (0.2–0.3) | 0.630 |
| Calcification (AU) | 2366.0 (1661.0–3371.0) | 2197.5 (1400.5–3187.2) | 0.015 | 2512.0 (1641.0–3435.0) | 0.743 |
| Matched Population (1:1) | |||
|---|---|---|---|
| PORTICO | ACURATE Neo | p-Value | |
| (n = 344) | (n = 344) | ||
| Procedural data | |||
| Conscious sedation | 308 (89.5%) | 300 (87.2%) | 0.405 |
| THV Size | <0.001 | ||
| 23 | 6 (1.7%) | 49 (14.2%) | |
| 25 | 93 (27.0%) | 148 (43.0%) | |
| 27 | 123 (35.8%) | 147 (42.7%) | |
| 29 | 122 (35.5%) | 0 (0.0%) | |
| Pre-dilatation | 275 (79.9%) | 270 (78.5%) | 0.707 |
| Post-dilatation | 64 (18.6%) | 110 (32.0%) | <0.001 |
| Cerebral protection | 2 (0.6%) | 7 (2.0%) | 0.177 |
| Procedural time (min) | 52.0 (40.0–69.0) | 56.0 (38.0–72.2) | 0.853 |
| Contrast (mL) | 120.0 (100.0–160.0) | 100.0 (80.0–130.0) | <0.001 |
| Fluoroscopy dose (Gy) | 1466.5 (29.1–3463.8) | 1391.0 (27.1–3635.5) | 0.868 |
| Fluoroscopy time (s) | 12.5 (9.2–17.1) | 10.3 (8.0–14.6) | <0.001 |
| Echocardiographic characteristics | |||
| Postprocedural mean gradient (mmHg) | 8.0 (6.0–10.0) | 8.0 (6.0–10.0) | 0.982 |
| Postprocedural max gradient (mmHg) | 13.0 (10.0–18.0) | 14.0 [11.0;18.0] | 0.856 |
| Clinical events | |||
| Major stroke/minor stroke/TIA | 13 (3.8%) | 12 (3.5%) | 1.000 |
| Major vascular complications | 9 (4.5%) | 11 (5.4%) | 0.854 |
| Life-threatening bleeding (VARC) | 2 (1.0%) | 4 (2.0%) | 0.685 |
| Renal failure (AKIN 2/3) | 12 (3.5%) | 13 (3.8%) | 0.994 |
| Coronary artery obstruction with PCI | 0 (0.0%) | 3 (1.5%) | 0.248 |
| Myocardial infarction | 6 (3.0%) | 8 (3.9%) | 0.815 |
| Permanent pacemaker implantation 1 | 52 (18.7%) | 28 (9.5%) | 0.002 |
| Days in hospital | 7.0 (6.0–10.0) | 7.5 (6.0–11.0) | 0.965 |
| Days in the intensive care unit | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.825 |
| In-hospital mortality | 10 (2.9%) | 10 (2.9%) | 1.000 |
| PORTICO | ACURATE Neo | p-Value | |
|---|---|---|---|
| (n = 344) | (n = 344) | ||
| Device failure 1 | 26 (7.6%) | 25 (7.3%) | 1.000 |
| Procedural related death | 1 (0.5%) | 4 (2.0%) | 0.372 |
| Significant paravalvular leakage (>Grade II) | 12 (3.5%) | 16 (4.8%) | 0.546 |
| Elevated gradient (>20 mmHg) | 4 (1.2%) | 5 (1.5%) | 0.752 |
| Multiple valves | 10 (2.9%) | 7 (2.0%) | 0.623 |
| Conversion to sternotomy | 5 (1.5%) | 2 (0.6%) | 0.451 |
| PORTICO | ACURATE | |
|---|---|---|
| (n = 5) | (n = 7) | |
| Reasons for conversion | ||
| Coronary impairment | 0 (0.0%) | 1 (14.3%) |
| Embolization | 3 (60%) | 2 (28.6%) |
| Pericardial effusion | 1 (20%) | 4 (57.1%) |
| Severe mitral regurgitation (due to wire) | 1 (20%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumenstein, J.; Eckel, C.; Husser, O.; Kim, W.-K.; Renker, M.; Choi, Y.-H.; Hamm, C.W.; Al-Terki, H.; Sötemann, D.; Körbi, L.; et al. Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis. J. Clin. Med. 2022, 11, 4228. https://doi.org/10.3390/jcm11144228
Blumenstein J, Eckel C, Husser O, Kim W-K, Renker M, Choi Y-H, Hamm CW, Al-Terki H, Sötemann D, Körbi L, et al. Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis. Journal of Clinical Medicine. 2022; 11(14):4228. https://doi.org/10.3390/jcm11144228
Chicago/Turabian StyleBlumenstein, Johannes, Clemens Eckel, Oliver Husser, Won-Keun Kim, Matthias Renker, Yeong-Hoon Choi, Christian W. Hamm, Hani Al-Terki, Dagmar Sötemann, Leon Körbi, and et al. 2022. "Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis" Journal of Clinical Medicine 11, no. 14: 4228. https://doi.org/10.3390/jcm11144228
APA StyleBlumenstein, J., Eckel, C., Husser, O., Kim, W.-K., Renker, M., Choi, Y.-H., Hamm, C. W., Al-Terki, H., Sötemann, D., Körbi, L., Tiyerili, V., Grothusen, C., Gaede, L., Dohmen, G., & Möllmann, H. (2022). Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis. Journal of Clinical Medicine, 11(14), 4228. https://doi.org/10.3390/jcm11144228






