Comparison of PCWP and LVEDP Measurements in Patients with Severe Aortic Stenosis Undergoing TAVI—Same Same but Different?
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Procedure of RHC and LHC
2.3. Hemodynamic Definitions of PH
2.4. Transthoracic Echocardiography
2.5. TAVI Procedure
2.6. Clinical Follow Up and Study End Point
2.7. Statistical Analysis
3. Results
3.1. Patient Population
3.2. General Characteristics and Measurements
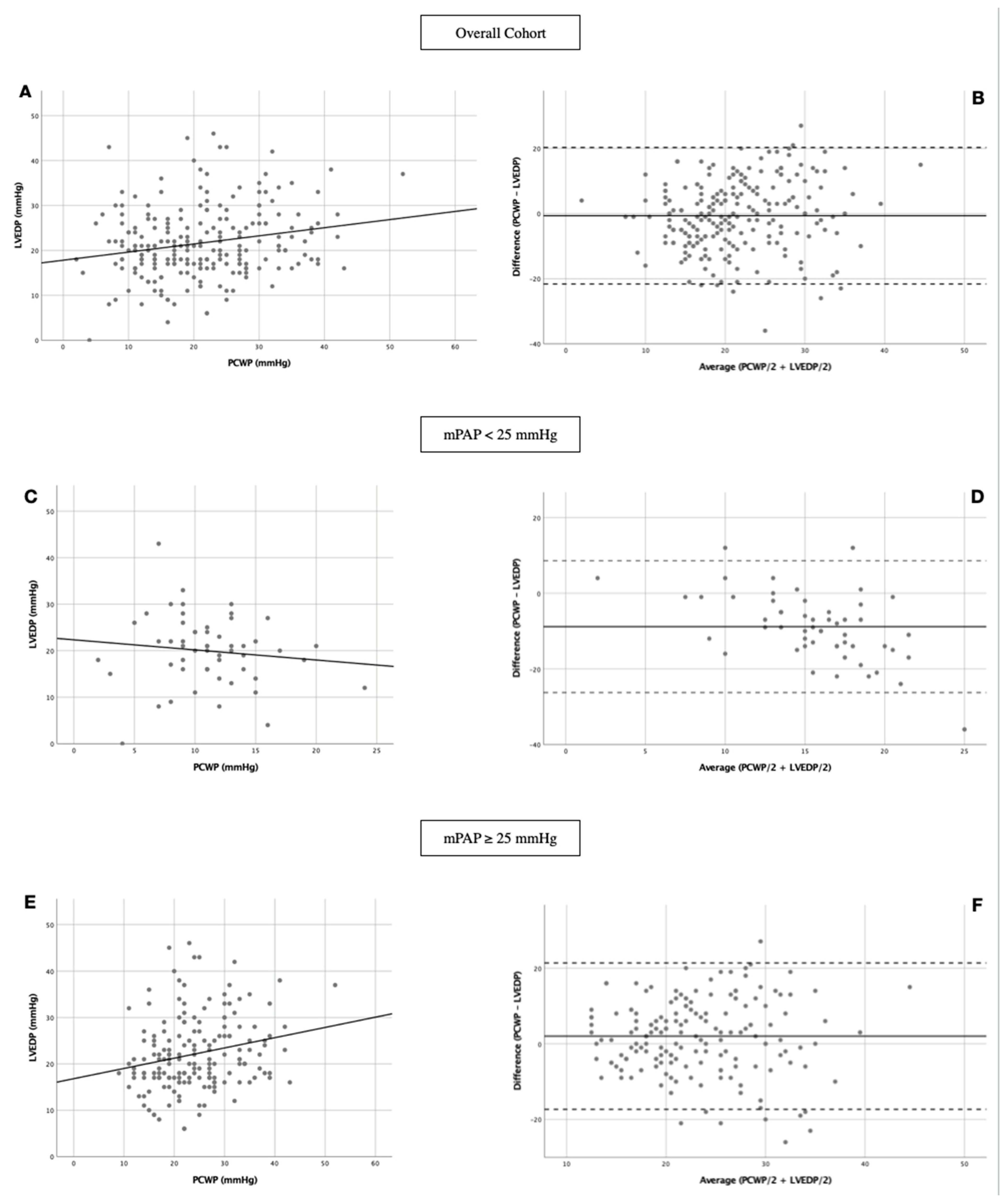
3.3. Linear Regression and Bland-Altman Analysis
3.4. Kaplan-Meier Curves
4. Discussion
4.1. Prevalances of PH and PH Subtypes Concerning PCWP and LVEDP Classification
4.2. Prec-PH via PCWP vs. LVEDP—Subgroup with Greatest Divergence in Terms of Hemodynamic Parameters and Mortality
4.3. Lack of Agreement between PCWP and LVEDP—Possible Reasons
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variances |
| AS | Aortic stenosis |
| borderlinepc-PH | Borderline post-capillary pulmonary hypertension |
| CO | Cardiac output |
| cpc-PH | Combined post-capillary pulmonary hypertension |
| dPAP | Diastolic pulmonary artery pressure |
| DPG | Diastolic pressure gradient |
| ESC | European Society of Cardiology |
| ipc-PH | Isolated post-capillary pulmonary hypertension |
| LHC | Left heart catheterization |
| LVEDP | Left ventricular end-diastolic pressure |
| LVEF | Left ventricular ejection fraction |
| mPAP | Mean pulmonary artery pressure |
| PCWP | Pulmonary capillary wedge pressure |
| PH | Pulmonary hypertension |
| prec-PH | Pre-capillary pulmonary hypertension |
| PVR | Pulmonary vascular resistance |
| RHC | Right hear catheterization |
| sPAP | Systolic pulmonary artery pressure |
| TAVI | Transcatheter aortic valve implantation |
| TPG | Transpulmonary pressure gradient |
| WHO | World Health Organization |
References
- Mehra, P.; Mehta, V.; Sukhija, R.; Sinha, A.K.; Gupta, M.; Girish, M.P.; Aronow, W.S. Pulmonary hypertension in left heart disease. Arch. Med. Sci. AMS 2019, 15, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Liu, X.; Lin, C.; He, Y.; Cai, X.; Xu, Q.; Hu, P.; Gao, F.; Jiang, J.; Lin, X.; et al. Meta-Analysis of Outcomes and Evolution of Pulmonary Hypertension Before and After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2017, 119, 91–99. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, Y.; McLaughlin, J.; Haag, E.; Rhee, P.; Passick, M.; Toole, R.; Cheng, J.; Berke, A.D.; Lachman, J.; et al. Left ventricular filling pressure assessment using left atrial transit time by cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging 2011, 4, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Haskell, R.J.; French, W.J. Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting left ventricular end-diastolic pressure. Am. J. Cardiol. 1988, 61, 136–141. [Google Scholar] [CrossRef]
- Varanasi, J.S.; Stouffer, G.A. Hypertrophic Cardiomyopathy. In Cardiovascular Hemodynamics for the Clinician; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 3. [Google Scholar] [CrossRef] [Green Version]
- Ruparelia, N.; Prendergast, B.D. TAVI in: Who, where and how? Heart 2015, 101, 1422–1431. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tuller, D.; et al. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation insights from the new proposed pulmonary hypertension classification. Circ. Cardiovasc. Interv. 2015, 8, 2358. [Google Scholar] [CrossRef] [Green Version]
- Weber, L.; Rickli, H.; Haager, P.K.; Joerg, L.; Weilenmann, D.; Brenner, R.; Taramasso, M.; Baier, P.; Maisano, F.; Maeder, M.T. Haemodynamic mechanisms and long-term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur. J. Heart Fail. 2019, 21, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Schewel, J.; Schmidt, T.; Kuck, K.H.; Frerker, C.; Schewel, D. Impact of Pulmonary Hypertension Hemodynamic Status on Long-Term Outcome After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2155–2168. [Google Scholar] [CrossRef]
- Sultan, I.; Fukui, M.; Bianco, V.; Brown, J.A.; Kliner, D.E.; Hickey, G.; Thoma, F.W.; Lee, J.S.; Schindler, J.T.; Kilic, A.; et al. Impact of Combined Pre and Postcapillary Pulmonary Hypertension on Survival after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 131, 60–66. [Google Scholar] [CrossRef]
- Kaple, R.; Sean, W.; Polydoros, K.; Amiran, B.; Micheas, Z.; Htoo, K.; Geoffrey, B.; Robert, M.; Richard, D.; Arash, S.; et al. Impact of etiology of pulmonary hypertension on post-procedural management and outcomes in patients undergoing transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2013, 61 (Suppl. S10), E1954. [Google Scholar] [CrossRef] [Green Version]
- Gerges, C.; Gerges, M.; Lang, M.B.; Zhang, Y.; Jakowitsch, J.; Probst, P.; Maurer, G.; Lang, I.M. Diastolic pulmonary vascular pressure gradient: A predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013, 143, 758–766. [Google Scholar] [CrossRef]
- Gerges, C.; Gerges, M.; Skoro-Sajer, N.; Zhou, Y.; Zhang, L.; Sadushi-Kolici, R.; Jakowitsch, J.; Lang, M.B.; Lang, I.M. Hemodynamic thresholds for precapillary pulmonary hypertension. Chest 2016, 149, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Naeije, R.; D’Alto, M. The Diagnostic Challenge of Group 2 Pulmonary Hypertension. Prog. Cardiovasc. Dis. 2016, 59, 22–29. [Google Scholar] [CrossRef]
- Dragu, R.; Hardak, E.; Ohanyan, A.; Adir, Y.; Aronson, D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int. J. Cardiol. 2019, 290, 138–143. [Google Scholar] [CrossRef]
- Mazimba, S.; Mejia-Lopez, E.; Black, G.; Kennedy, J.L.W.; Bergin, J.; Tallaj, J.A.; Abuannadi, M.; Mihalek, A.D.; Bilchick, K.C. Diastolic pulmonary gradient predicts outcomes in group 1 pulmonary hypertension (analysis of the NIH primary pulmonary hypertension registry). Respir. Med. 2016, 119, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [Green Version]
- Fontes Oliveira, M.; Oliveira, M.; Costa, R.; Dias Frias, A.; Silveira, I.; Cabral, S.; Santos, M.; Torres, S.; Reis, A. Predictors of survival in patients with precapillary pulmonary hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22 (Suppl. S1), 438. [Google Scholar] [CrossRef]
- Bitar, A.; Selej, M.; Bolad, I.; Lahm, T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PLoS ONE 2014, 9, 87304. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Opotowsky, A.R.; Assad, T.R.; Xu, M.; Doss, L.N.; Farber-Eger, E.; Wells, Q.S.; Brittain, E.L. Features Associated with Discordance Between Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure in Clinical Practice: Implications for Pulmonary Hypertension Classification. Chest 2018, 154, 1099–1107. [Google Scholar] [CrossRef]
- Halpern, S.D.; Taichman, D.B. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 2009, 136, 37–43. [Google Scholar] [CrossRef]
- De Oliveira, R.K.F.; Ferreira, E.V.M.; Ramos, R.P.; Messina, C.M.S.; Kapins, C.E.B.; Silva, C.M.C.; Ota-Arakaki, J.S. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J. Heart Lung Transplant. 2014, 33, 157–162. [Google Scholar] [CrossRef]
- Hildick-Smith, D.J.R.; Walsh, J.T.; Shapiro, L.M. Pulmonary capillary wedge pressure in mitral stenosis accurately reflects mean left atrial pressure but overestimates transmitral gradient. Am. J. Cardiol. 2000, 85, 512–515. [Google Scholar] [CrossRef]
- Slim, A.M.; Martinho, S.; Slim, J.; Davenport, E.; Castillo-Rojas, L.M.; Shry, E.A. The effect of airway pressure release ventilation on pulmonary catheter readings: Specifically pulmonary capillary wedge pressure in a swine model. Anesthesiol. Res. Pract. 2011, 2011, 371594. [Google Scholar] [CrossRef]
- Wrobel, J.P.; McLean, C.A.; Thompson, B.R.; Stuart-Andrews, C.R.; Paul, E.; Snell, G.I.; Williams, T.J. Pulmonary arterial remodeling in chronic obstructive pulmonary disease is lobe dependent. Pulm. Circ. 2013, 3, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Champion, H.C.; Michelakis, E.D.; Hassoun, P.M. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit state of the art and clinical and research implications. Circulation 2009, 120, 992–1007. [Google Scholar] [CrossRef] [Green Version]
- Her, C.; Cerabona, T.; Baek, S.H.; Shin, S.W. Increased pulmonary venous resistance in morbidly obese patients without daytime hypoxia: Clinical utility of the pulmonary artery catheter. Anesthesiology 2010, 113, 552–559. [Google Scholar] [CrossRef]
- Rahimtoola, S.H.; Loeb, H.S.; Ehsani, A.; Sinno, M.Z.; Chuquimia, R.; Lal, R.; Rosen, K.M.; Gunnar, R.M. Relationship of pulmonary artery to left ventricular diastolic pressures in acute myocardial infarction. Circulation 1972, 46, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Brienesse, S.C.; Davies, A.J.; Khan, A.; Boyle, A.J. Prognostic Value of LVEDP in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2018, 11, 33–35. [Google Scholar] [CrossRef]
- Weber, L.; Rickli, H.; Ammann, P.; Taramasso, M.; Brenner, R.; Ehl, N.F.; Buser, M.; Haager, P.K.; Maisano, F.; Maeder, M.T. Hemodynamic profile of patients with severe aortic valve stenosis and atrial fibrillation versus sinus rhythm. Int. J. Cardiol. 2020, 311, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Weber, L.; Seidl, S.; Weilenmann, D.; Hochholzer, D.; Joerg, L.; Chronis, J.; Rigger, J.; Haager, P.K.; Rickli, H. Wedge Pressure vs. Left Ventricular End-Diastolic Pressure for Pulmonary Hypertension Classification and Prognostication in Severe Aortic Stenosis. CJC Open 2021, 3, 1428–1437. [Google Scholar] [CrossRef] [PubMed]

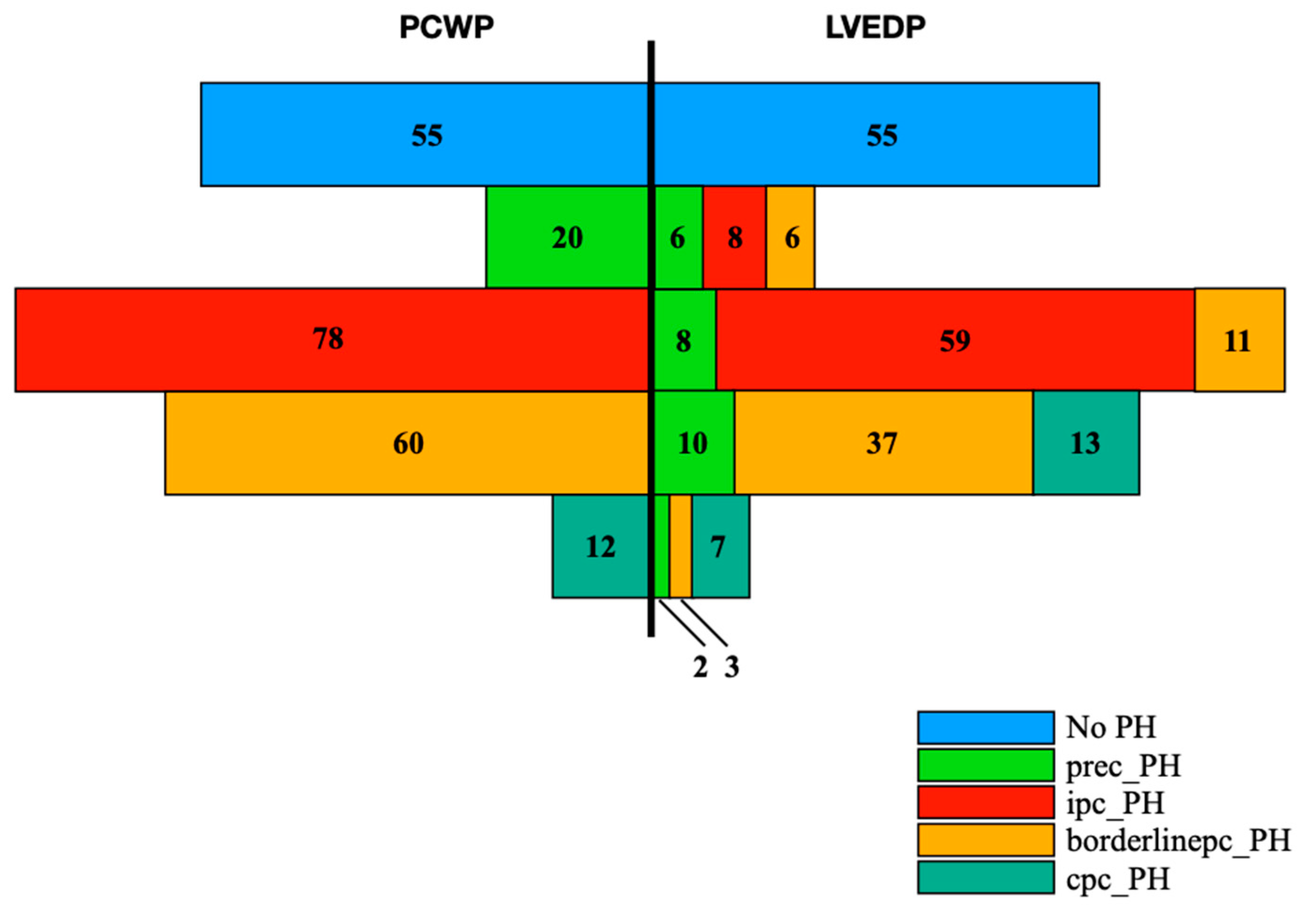


| Overall Cohort | No PH | Prec-PH | Ipc-PH | Borderlinepc-PH | Cpc-PH | ANOVA/Chi-Square | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCWP | LVEDP | PCWP | LVEDP | PCWP | LVEDP | PCWP | LVEDP | PCWP | LVEDP | |||||||||||||
| n = 225 | n = 55 | n = 20 | n = 26 | n = 78 | n = 67 | n = 60 | n = 57 | n = 12 | n = 20 | |||||||||||||
| Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | Mean | SD (±) | p-Value | p-Value | |
| Clinical data | ||||||||||||||||||||||
| Age (years) | 80.89 | 7.03 | 80.53 | 6.52 | 83.90 | 6.02 | 83.77 | 7.30 | 80.36 | 7.56 | 79.67 | 7.13 | 80.70 | 7.00 | 82.44 | 6.20 | 81.83 | 7.21 | 77.80 | 8.24 | 0.328 | 0.010 |
| Weight (kg) | 73.96 | 12.70 | 72.05 | 13.53 | 76.50 | 17.44 | 70.62 | 12.28 | 77.58 | 10.74 | 78.60 | 11.62 | 71.05 | 11.77 | 73.19 | 12.63 | 69.42 | 11.18 | 70.15 | 11.24 | 0.010 | 0.007 |
| Height (cm) | 164.42 | 8.55 | 162.65 | 8.37 | 163.55 | 8.64 | 162.81 | 6.88 | 166.42 | 8.08 | 167.27 | 8.31 | 163.37 | 8.62 | 162.82 | 7.75 | 166.25 | 10.29 | 166.40 | 11.44 | 0.080 | 0.007 |
| BMI (kg/m2) | 27.35 | 4.29 | 27.19 | 4.40 | 28.54 | 5.94 | 26.69 | 4.74 | 28.05 | 3.79 | 28.14 | 4.07 | 26.64 | 4.20 | 25.59 | 4.40 | 25.08 | 3.20 | 25.32 | 3.28 | 0.067 | 0.107 |
| STS-Score | 4.01 | 2.48 | 3.54 | 2.20 | 4.86 | 3.25 | 4.75 | 3.16 | 3.88 | 2.56 | 3.69 | 2.24 | 4.31 | 2.18 | 4.38 | 2.39 | 4.09 | 2.99 | 4.35 | 3.01 | 0.242 | 0.131 |
| EuroScore | 25.53 | 15.74 | 25.11 | 16.91 | 38.53 | 21.34 | 27.21 | 14.57 | 22.94 | 13.19 | 23.35 | 14.21 | 25.68 | 14.58 | 27.87 | 17.36 | 21.43 | 11.13 | 24.73 | 14.29 | 0.002 | 0.605 |
| Laboratory data | ||||||||||||||||||||||
| Creatinine (µmol/L) | 119.00 | 76.60 | 106.45 | 58.93 | 118.47 | 54.30 | 110.12 | 44.45 | 123.41 | 75.59 | 125.29 | 77.54 | 125.19 | 98.79 | 118.19 | 64.25 | 115.83 | 57.84 | 143.75 | 144.91 | 0.750 | 0.388 |
| C-reactive protein (mg/L) | 19.10 | 34.38 | 12.37 | 24.13 | 16.14 | 20.05 | 30.80 | 39.07 | 20.19 | 37.40 | 19.75 | 36.53 | 25.92 | 43.36 | 18.48 | 30.08 | 15.42 | 20.52 | 20.32 | 49.67 | 0.384 | 0.310 |
| Hemoglobin (mmol/L) | 7.54 | 1.07 | 7.55 | 0.99 | 7.68 | 0.80 | 7.46 | 1.16 | 7.39 | 1.00 | 7.34 | 1.05 | 7.64 | 1.25 | 7.78 | 1.02 | 7.69 | 1.39 | 7.59 | 1.32 | 0.704 | 0.347 |
| Concomitant diseases | ||||||||||||||||||||||
| Diabetes mellitus—no. (%) | 134 (59.60) | 31 (56.40) | 11 (55.00) | 15 (57.70) | 42 (53.80) | 39 (58.20) | 40 (66.66) | 33 (57.90) | 10 (83.33) | 16 (80.00) | 0.237 | 0.426 | ||||||||||
| Arterial hypertension (%) | 205 (91.10) | 51 (92.70) | 19 (95.00) | 25 (96.20) | 69 (88.50) | 59 (88.10) | 55 (91.66) | 52 (91.20) | 11 (91.66) | 18 (90.00) | 0.869 | 0.773 | ||||||||||
| Atrial fibrillation—no. (%) | 76 (33.80) | 18 (32.70) | 9 (45.00) | 8 (30.80) | 26 (33.33) | 25 (37.30) | 18 (30.00) | 18 (31.60) | 5 (41.70) | 7 (35.00) | 0.758 | 0.958 | ||||||||||
| Coronary heart disease ≥ 2 vessels—no. (%) | 58 (25.80) | 12 (21.80) | 5 (25.00) | 5 (19.20) | 21 (26.90) | 20 (29.90) | 18 (30.00) | 14 (24.60) | 2 (16.66) | 7 (35.00) | 0.811 | 0.636 | ||||||||||
| COPD—no. (%) | 50 (22.20) | 10 (18.20) | 5 (25.00) | 8 (30.80) | 18 (23.10) | 14 (20.90) | 13 (21.66) | 11 (19.30) | 4 (33.33) | 7 (35.00) | 0.825 | 0.426 | ||||||||||
| Myocardial infarction—no. (%) | 29 (12.90) | 9 (16.40) | 1 (5.00) | 3 (11.50) | 8 (10.30) | 4 (6.00) | 9 (15.00) | 9 (15.80) | 2 (16.66) | 4 (20.00) | 0.643 | 0.321 | ||||||||||
| Stroke—no. (%) | 32 (14.20) | 6 (10.90) | 5 (25.00) | 9 (34.60) | 14 (17.90) | 10 (14.90) | 6 (10.00) | 6 (10.50) | 1 (8.33) | 1 (5.00) | 0.342 | 0.022 | ||||||||||
| NYHA ≥ III—no. (%) | 186 (82.70) | 41 (74.50) | 16 (80.00) | 23 (88.50) | 66 (84.60) | 57 (85.10) | 54 (90.00) | 47 (82.50) | 9 (75.00) | 18 (90.00) | 0.183 | 0.238 | ||||||||||
| Echocardiographic data | ||||||||||||||||||||||
| EF (%) | 57.57 | 16.14 | 61.02 | 15.64 | 61.70 | 10.46 | 56.87 | 13.90 | 57.96 | 15.61 | 57.96 | 15.43 | 52.89 | 17.69 | 58.50 | 16.13 | 55.59 | 18.17 | 44.97 | 18.22 | 0.064 | 0.006 |
| LVEDD (mm) | 48.70 | 7.81 | 47.38 | 8.17 | 47.26 | 6.84 | 47.72 | 7.04 | 49.20 | 7.01 | 49.97 | 7.43 | 49.43 | 8.05 | 47.74 | 7.50 | 50.58 | 10.74 | 52.16 | 8.85 | 0.465 | 0.092 |
| LVESD (mm) | 32.14 | 9.27 | 30.00 | 9.51 | 30.69 | 7.47 | 31.69 | 8.99 | 32.04 | 8.34 | 33.09 | 8.91 | 34.03 | 9.87 | 30.50 | 8.18 | 36.38 | 12.34 | 40.46 | 9.32 | 0.224 | 0.005 |
| sPAP (mmHg) | 41.55 | 13.79 | 32.61 | 7.66 | 33.15 | 12.35 | 40.29 | 11.81 | 43.91 | 11.64 | 41.40 | 12.47 | 47.25 | 15.55 | 47.47 | 14.86 | 52.40 | 13.85 | 50.60 | 15.98 | < 0.001 | < 0.001 |
| AVA (cm2) | 0.65 | 0.18 | 0.67 | 0.21 | 0.64 | 0.19 | 0.65 | 0.18 | 0.67 | 0.17 | 0.68 | 0.18 | 0.61 | 0.15 | 0.61 | 0.15 | 0.66 | 0.22 | 0.60 | 0.14 | 0.351 | 0.295 |
| AV max (m/s) | 4.34 | 0.71 | 4.51 | 0.69 | 4.37 | 0.46 | 4.20 | 0.63 | 4.30 | 0.63 | 4.33 | 0.65 | 4.23 | 0.86 | 4.35 | 0.76 | 4.33 | 0.73 | 4.09 | 0.82 | 0.281 | 0.153 |
| AV dpmax (mmHg) | 78.96 | 26.42 | 83.52 | 24.59 | 77.69 | 15.71 | 70.16 | 18.14 | 77.12 | 23.04 | 76.91 | 23.00 | 76.87 | 33.63 | 83.44 | 32.01 | 81.08 | 30.09 | 71.96 | 30.86 | 0.654 | 0.101 |
| AV dpmean (mmHg) | 47.97 | 16.65 | 51.03 | 16.90 | 47.39 | 11.87 | 42.65 | 18.14 | 46.35 | 13.97 | 47.53 | 14.46 | 47.12 | 20.40 | 49.37 | 19.50 | 48.80 | 18.87 | 43.85 | 18.01 | 0.603 | 0.195 |
| Mitral regurgitation ≥ II°—no. (%) | 99 (44.00) | 20 (36.40) | 2 (10.00) | 11 (42.30) | 35 (44.90) | 26 (38.80) | 39 (65.00) | 32 (56.10) | 3 (25.00) | 10 (50.00) | < 0.001 | 0.275 | ||||||||||
| Tricuspid regurgitation ≥ II°—no. (%) | 95 (42.20) | 19 (34.50) | 6 (30.00) | 14 (53.80) | 34 (43.60) | 27 (40.30) | 29 (48.33) | 21 (36.80) | 7 (58.33) | 14 (70.00) | 0.439 | 0.044 | ||||||||||
| RHC & LHC data | ||||||||||||||||||||||
| RA (mmHg) | 10.41 | 5.53 | 5.44 | 3.24 | 7.89 | 2.79 | 10.63 | 3.83 | 12.47 | 4.70 | 11.18 | 3.94 | 12.66 | 5.51 | 12.16 | 5.95 | 13.33 | 6.11 | 16.83 | 5.36 | < 0.001 | < 0.001 |
| RV (mmHg) | 9.26 | 6.55 | 4.43 | 3.87 | 7.16 | 3.62 | 9.09 | 5.74 | 11.19 | 6.44 | 9.81 | 6.25 | 12.05 | 6.86 | 11.84 | 6.67 | 9.58 | 6.57 | 13.89 | 6.35 | < 0.001 | < 0.001 |
| sPAP (mmHg) | 52.77 | 17.93 | 33.91 | 6.53 | 43.95 | 7.10 | 53.92 | 14.42 | 55.30 | 14.25 | 51.81 | 12.45 | 65.83 | 14.44 | 64.63 | 25.47 | 72.17 | 20.03 | 72.55 | 16.74 | < 0.001 | < 0.001 |
| mPAP (mmHg) | 33.64 | 11.77 | 19.95 | 3.42 | 27.40 | 2.66 | 34.92 | 8.76 | 35.79 | 8.13 | 33.27 | 6.76 | 42.10 | 89.76 | 41.07 | 9.34 | 50.50 | 11.61 | 49.70 | 8.49 | < 0.001 | < 0.001 |
| dPAP (mmHg) | 19.87 | 8.83 | 10.16 | 4.24 | 16.80 | 2.63 | 21.31 | 6.69 | 21.62 | 6.62 | 19.76 | 5.20 | 24.53 | 6.72 | 24.05 | 7.62 | 34.75 | 8.32 | 33.10 | 5.72 | < 0.001 | < 0.001 |
| PCWP (mmHg) | 21.12 | 9.16 | 11.09 | 4.03 | 13.05 | 1.70 | 20.42 | 5.47 | 25.14 | 7.19 | 22.39 | 6.61 | 26.92 | 7.05 | 25.91 | 8.79 | 25.33 | 7.62 | 31.65 | 6.44 | < 0.001 | < 0.001 |
| DPGPCWP (mmHg) | −1.23 | 5.69 | −0.93 | 4.97 | 3.75 | 2.84 | 0.88 | 5.78 | −3.53 | 4.43 | −2.63 | 4.71 | −2.38 | 5.27 | −1.77 | 6.33 | 9.83 | 3.61 | 1.45 | 7.06 | < 0.001 | 0.011 |
| DPGLVEDP (mmHg) | −1.76 | 10.65 | −9.76 | 7.50 | −2.95 | 7.77 | 9.46 | 7.25 | −1.95 | 9.99 | −5.73 | 8.08 | 3.03 | 8.74 | 0.28 | 7.81 | 14.08 | 9.99 | 13.10 | 6.63 | < 0.001 | < 0.001 |
| TPGPCWP (mmHg) | 12.52 | 5.98 | 8.85 | 3.29 | 14.35 | 3.00 | 14.50 | 6.45 | 10.65 | 3.69 | 10.88 | 2.99 | 15.18 | 5.73 | 15.16 | 6.29 | 25.17 | 8.80 | 18.05 | 9.05 | < 0.001 | < 0.001 |
| TPGLVEDP (mmHg) | 12.01 | 12.97 | 0.02 | 8.78 | 7.65 | 7.77 | 23.07 | 9.22 | 12.23 | 11.02 | 7.78 | 8.94 | 20.60 | 9.09 | 17.30 | 8.79 | 29.83 | 12.19 | 29.70 | 9.34 | < 0.001 | < 0.001 |
| PVR (WU) | 3.25 | 2.15 | 2.64 | 1.58 | 3.11 | 1.08 | 3.55 | 2.89 | 1.83 | 0.63 | 1.91 | 0.64 | 5.02 | 2.28 | 4.34 | 2.13 | 6.00 | 2.50 | 5.77 | 1.99 | < 0.001 | < 0.001 |
| CO (L/min) | 4.19 | 1.18 | 4.18 | 1.32 | 4.22 | 1.06 | 3.95 | 9.54 | 4.62 | 1.14 | 4.65 | 1.01 | 3.68 | 1.00 | 3.85 | 1.18 | 3.91 | 0.83 | 3.91 | 1.20 | < 0.001 | 0.002 |
| LVEDP | 21.63 | 7.89 | 19.93 | 7.46 | 19.75 | 7.32 | 11.85 | 2.77 | 23.56 | 8.15 | 25.49 | 7.40 | 21.50 | 7.92 | 23.77 | 7.19 | 20.67 | 7.18 | 20.00 | 3.80 | 0.071 | < 0.001 |
| Procedurale data | ||||||||||||||||||||||
| Transfemoral approach—no. (%) | 193 (85.80) | 48 (87.30) | 18 (90.00) | 22 (84.60) | 65 (83.30) | 58 (86.60) | 52 (86.66) | 48 (84.20) | 10 (83.33) | 17 (85.00) | 0.928 | 0.991 | ||||||||||
| CoreValve—no. (%) | 57 (25.30) | 11 (20.00) | 7 (35.00) | 9 (34.60) | 22 (28.20) | 17 (25.40) | 14 (23.33) | 15 (26.30) | 3 (25.00) | 5 (25.00) | 0.684 | 0.728 | ||||||||||
| JenaValve—no. (%) | 28 (12.40) | 7 (12.70) | 1 (5.00) | 3 (11.50) | 10 (12.80) | 7 (10.40) | 8 (13.33) | 8 (14.00) | 2 (16.66) | 3 (15.00) | 0.866 | 0.971 | ||||||||||
| Edwards—no. (%) | 138 (61.30) | 37 (67.30) | 12 (60.00) | 14 (53.80) | 45 (57.70) | 43 (64.20) | 37 (61.66) | 32 (56.10) | 7 (58.33) | 12 (60.00) | 0.858 | 0.676 | ||||||||||
| Major vascular complications—no. (%) | 25 (11.10) | 8 (14.50) | 2 (10.00) | 2 (7.70) | 6 (7.7) | 4 (6.00) | 7 (11.66) | 8 (14.00) | 2 (16.66) | 3 (15.00) | 0.736 | 0.469 | ||||||||||
| R | R2 | F | B | B (SE) | Beta | p-Value | |
|---|---|---|---|---|---|---|---|
| Overall cohort | 0.210 | 0.044 | 10.292 | 0.244 | 0.076 | 0.210 | 0.002 |
| mPAP < 25 mmHg | 0.117 | 0.014 | 0.739 | −0.063 | 0.074 | −0.117 | 0.394 |
| mPAP ≥ 25 mmHg | 0.220 | 0.048 | 8.556 | 0.219 | 0.075 | 0.220 | 0.004 |
| Age < 80 years | 0.210 | 0.044 | 3.601 | 0.246 | 0.130 | 0.210 | 0.061 |
| Age ≥ 80 years | 0.195 | 0.038 | 5.678 | 0.226 | 0.095 | 0.195 | 0.018 |
| BMI < 30 kg/m2 | 0.253 | 0.064 | 11.189 | 0.288 | 0.086 | 0.253 | 0.001 |
| BMI ≥ 30 kg/m2 | 0.104 | 0.011 | 0.622 | 0.135 | 0.171 | 0.104 | 0.433 |
| Creatinine < 132 µmol/l | 0.238 | 0.057 | 9.320 | 0.266 | 0.087 | 0.238 | 0.003 |
| Creatinine ≥ 132 µmol/l | 0.357 | 0.127 | 7.012 | 0.540 | 0.204 | 0.357 | 0.011 |
| No Diabetes | 0.244 | 0.059 | 5.617 | 0.278 | 0.117 | 0.244 | 0.020 |
| Diabetes | 0.186 | 0.034 | 4.710 | 0.220 | 0.101 | 0.186 | 0.032 |
| No Hypertension | 0.090 | 0.008 | 0.148 | 0.151 | 0.392 | 0.090 | 0.705 |
| Hypertension | 0.224 | 0.050 | 10.697 | 0.251 | 0.077 | 0.224 | 0.001 |
| No Atrial Fibrillation | 0.207 | 0.043 | 6.613 | 0.233 | 0.090 | 0.207 | 0.011 |
| Atrial Fibrillation | 0.216 | 0.047 | 3.633 | 0.272 | 0.143 | 0.216 | 0.061 |
| Coronary Heart Disease < 2 vessels | 0.222 | 0.049 | 8.580 | 0.261 | 0.089 | 0.222 | 0.004 |
| Coronary Heart Disease ≥ 2 vessels | 0.163 | 0.027 | 1.532 | 0.182 | 0.147 | 0.163 | 0.221 |
| No COPD | 0.241 | 0.058 | 10.713 | 0.276 | 0.084 | 0.241 | 0.001 |
| COPD | 0.088 | 0.008 | 21.235 | 0.108 | 0.176 | 0.088 | 0.542 |
| No Myocardial Infarction | 0.221 | 0.049 | 9.923 | 0.252 | 0.080 | 0.221 | 0.002 |
| Myocardial Infarction | 0.095 | 0.009 | 0.248 | 0.126 | 0.252 | 0.095 | 0.622 |
| No Stroke | 0.202 | 0.041 | 8.119 | 0.237 | 0.083 | 0.202 | 0.005 |
| Stroke | 0.271 | 0.074 | 2.384 | 0.309 | 0.200 | 0.271 | 0.133 |
| NYHA < III | 0.027 | 0.001 | 0.019 | 0.036 | 0.265 | 0.027 | 0.893 |
| NYHA ≥ III | 0.267 | 0.071 | 14.126 | 0.308 | 0.082 | 0.267 | < 0.001 |
| EF < 50% | 0.261 | 0.068 | 4.526 | 0.323 | 0.152 | 0.261 | 0.037 |
| EF ≥ 50% | 0.110 | 0.012 | 1.834 | 0.122 | 0.090 | 0.110 | 0.178 |
| Mitral Regurgitation < II° | 0.107 | 0.012 | 1.356 | 0.116 | 0.099 | 0.107 | 0.247 |
| Mitral Regurgitation ≥ II° | 0.326 | 0.106 | 11.514 | 0.397 | 0.117 | 0.326 | 0.001 |
| Tricuspid Regurgitation < II° | 0.284 | 0.081 | 9.664 | 0.337 | 0.108 | 0.284 | 0.002 |
| Tricuspid Regurgitation ≥ II° | 0.200 | 0.040 | 3.857 | 0.240 | 0.122 | 0.200 | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boxhammer, E.; Mirna, M.; Bäz, L.; Alushi, B.; Franz, M.; Kretzschmar, D.; Hoppe, U.C.; Lauten, A.; Lichtenauer, M. Comparison of PCWP and LVEDP Measurements in Patients with Severe Aortic Stenosis Undergoing TAVI—Same Same but Different? J. Clin. Med. 2022, 11, 2978. https://doi.org/10.3390/jcm11112978
Boxhammer E, Mirna M, Bäz L, Alushi B, Franz M, Kretzschmar D, Hoppe UC, Lauten A, Lichtenauer M. Comparison of PCWP and LVEDP Measurements in Patients with Severe Aortic Stenosis Undergoing TAVI—Same Same but Different? Journal of Clinical Medicine. 2022; 11(11):2978. https://doi.org/10.3390/jcm11112978
Chicago/Turabian StyleBoxhammer, Elke, Moritz Mirna, Laura Bäz, Brunilda Alushi, Marcus Franz, Daniel Kretzschmar, Uta C. Hoppe, Alexander Lauten, and Michael Lichtenauer. 2022. "Comparison of PCWP and LVEDP Measurements in Patients with Severe Aortic Stenosis Undergoing TAVI—Same Same but Different?" Journal of Clinical Medicine 11, no. 11: 2978. https://doi.org/10.3390/jcm11112978
APA StyleBoxhammer, E., Mirna, M., Bäz, L., Alushi, B., Franz, M., Kretzschmar, D., Hoppe, U. C., Lauten, A., & Lichtenauer, M. (2022). Comparison of PCWP and LVEDP Measurements in Patients with Severe Aortic Stenosis Undergoing TAVI—Same Same but Different? Journal of Clinical Medicine, 11(11), 2978. https://doi.org/10.3390/jcm11112978








