Nurse-Led Counseling Intervention of Postoperative Home-Based Exercise Training Improves Shoulder Pain, Shoulder Disability, and Quality of Life in Newly Diagnosed Head and Neck Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consideration and Setting
2.2. Participants and Study Design
2.3. Routine Care
2.4. HBET
2.5. Nurse-Led Counseling Intervention
2.6. Measures
2.7. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
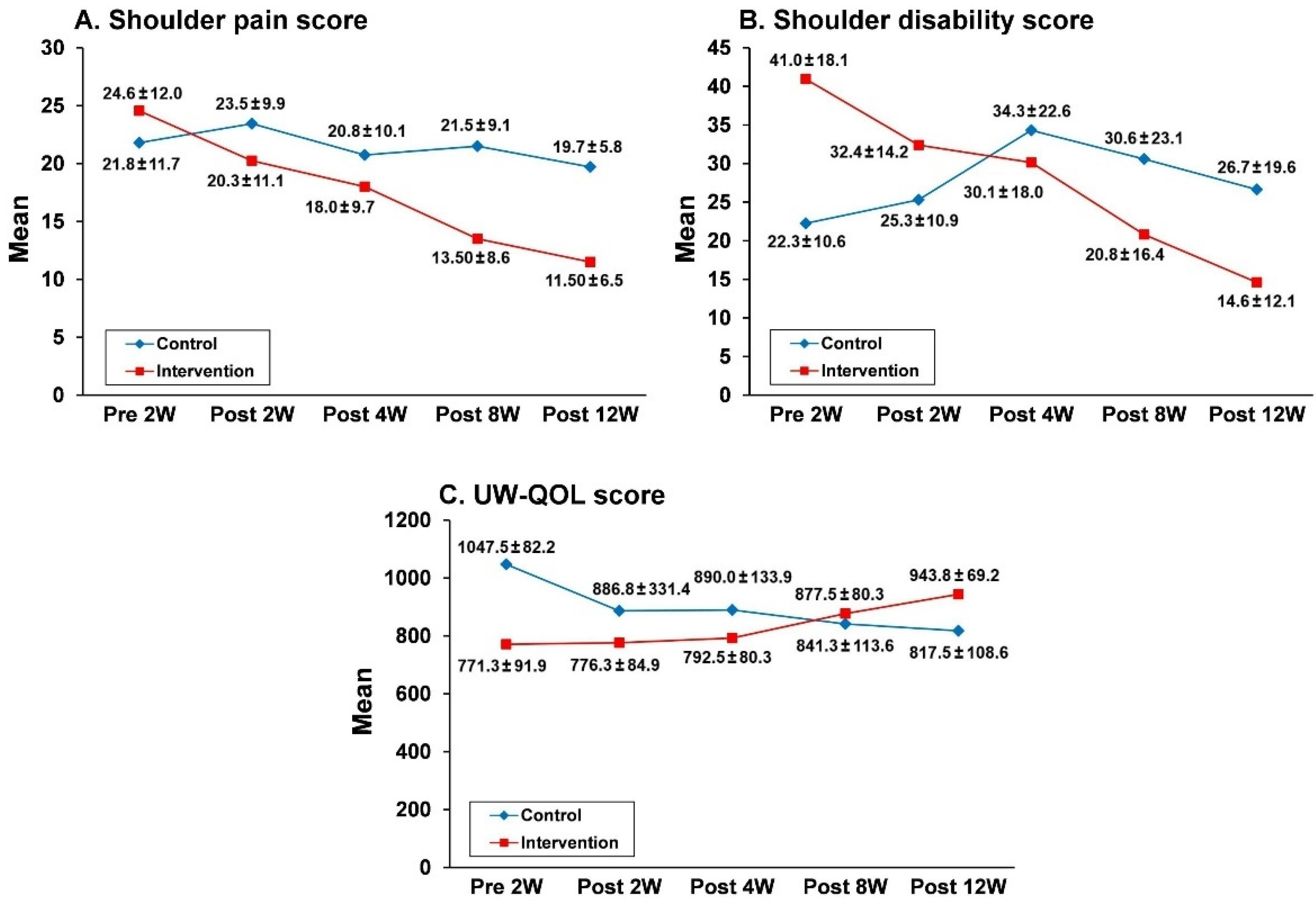
3.2. Effects of the Nurse-Led Counseling Intervention of HBET: GEE Analyses
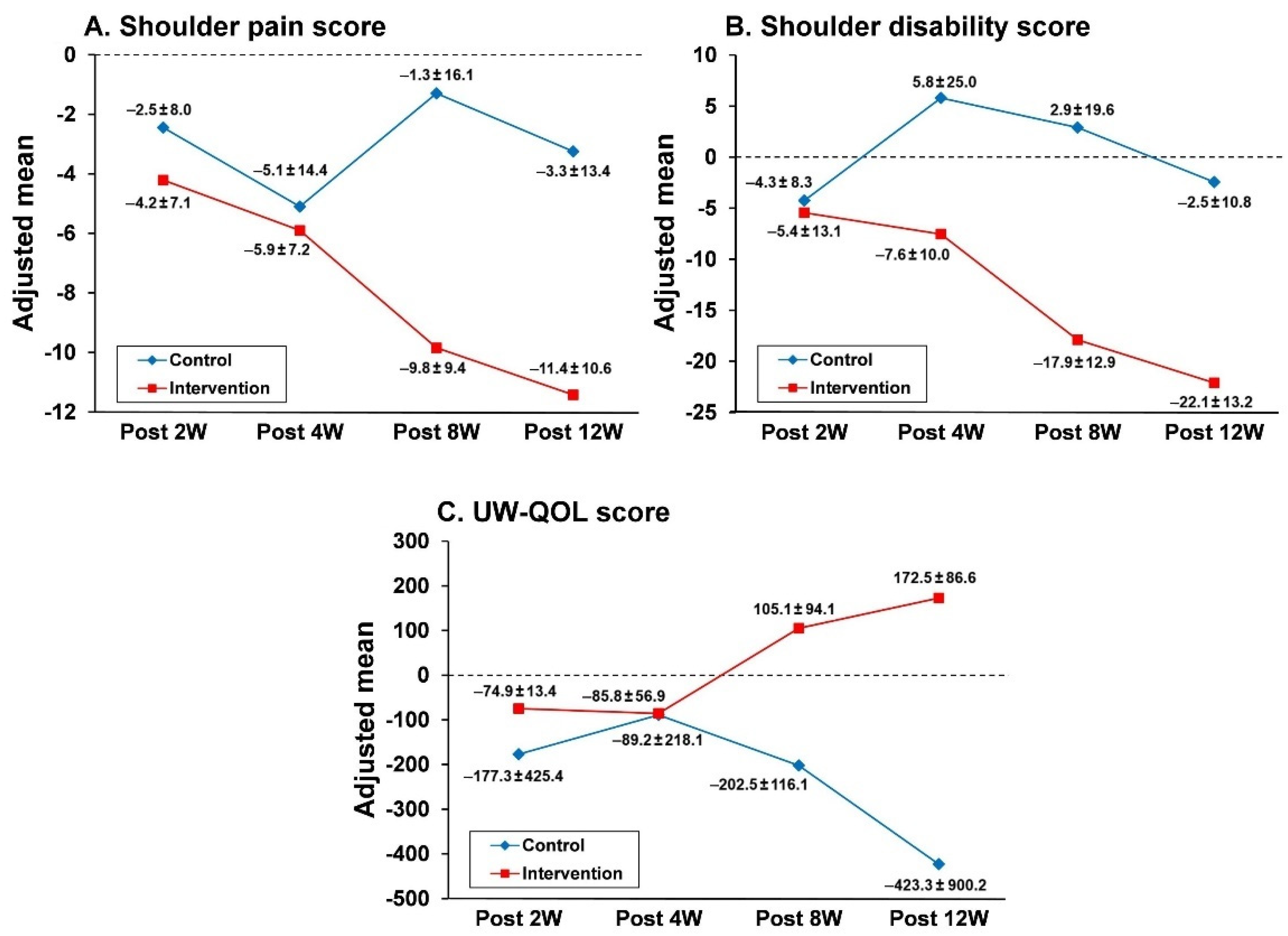
3.3. Effects of the Nurse-Led Counseling Intervention of HBET: ANCOVA Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferly, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hsu, W.L.; Yu, K.J.; Chiang, C.J.; Chen, T.C.; Wang, C.P. Head and neck cancer incidence trends in Taiwan, 1980–2014. Int. J. Head Neck Sci. 2017, 1, 180–189. [Google Scholar] [CrossRef]
- Spinelli, B.A. Shoulder pain and dysfunction after head and neck cancer and thyroid cancertreatment. Rehabil. Oncol. 2017, 35, 195–196. [Google Scholar] [CrossRef]
- Meccariello, G.; Maniaci, A.; Bianchi, G.; Cammaroto, G.; Iannella, G.; Catalano, A.; Sgarzani, R.; De Vito, A.; Capaccio, P.; Pelucchi, S.; et al. Neck dissection and trans oral robotic surgery for oropharyngeal squamous cell carcinoma. Auris Nasus Larynx 2022, 49, 117–125. [Google Scholar] [CrossRef]
- Wang, H.L.; Keck, J.F.; Weaver, M.T.; Mikesky, A.; Bunnell, K.; Buelow, J.M.; Rawl, S.M. Shoulder pain, functional status, and health-related quality of life after head and neck cancer surgery. Rehabil. Res. Pract. 2013, 2013, 601768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulougouris, A.; Doyle, P.C. Shoulder dysfunction and disability secondary to treatment for head and neck cancer. In Clinical Care and Rehabilitation in Head and Neck Cancer; Doyle, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 397–411. [Google Scholar] [CrossRef]
- Game, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. The relationship between physical impairments, quality of life and disability of the neck and upper limb in patients following neck dissection. J. Cancer Surviv. 2018, 12, 619–631. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Fengb, X.Q.; Fuc, M.R.; Yud, R.; Zhaod, H.L. Symptom patterns, physical function and quality of life among head and neck cancer patients prior to and after surgical treatment: A prospective study. Eur. J. Oncol. Nurs. 2020, 46, 101770. [Google Scholar] [CrossRef]
- Van Nieuwenhuizen, A.J.; Buffart, L.M.; van Uden-Kraan, C.F.; van der Velden, L.A.; Lacko, M.; Brug, J.; Leemans, C.R.; Verdonck-de Leeuw, I.M. Patient-reported physical activity and the association with health-related quality of life in head and neck cancer survivors. Support. Care Cancer 2018, 26, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.; Maiolino, L.; Di Mauro, P.; Licciardello, L.; Cocuzza, S. The senile functional evolution of the larynx after supracricoid reconstructive surgery. Eur. Arch. Otorhinolaryngol. 2016, 273, 4359–4368. [Google Scholar] [CrossRef]
- Möckelmann, N.; Lörincz, B.B.; Knecht, R. Robotic-assisted selective and modified radical neck dissection in head and neck cancer patients. Int. J. Surg. 2016, 25, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Guru, K.; Manor, U.K.; Supe, S.S. A comprehensive review of head and neck cancer rehabilitation: Physical therapy perspectives. Indian J. Palliat. Care 2012, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Komar, A.; Ringash, J.; Chan, C.; Davis, A.M.; Jones, J.; Martino, R.; McEwen, S. A scoping review of rehabilitation interventions for survivors of head and neck cancer. Disabil. Rehabil. 2019, 41, 2093–2107. [Google Scholar] [CrossRef]
- Midgley, A.W.; Levy, A.R.; Price, R.; Cunha, F.A.; Rogers, S.N. Should survivors of head and neck cancer be considered a distinct special population within the context of exercise prescription? Br. J. Oral Maxillofac. Surg. 2020, 58, 738–743. [Google Scholar] [CrossRef] [PubMed]
- McNeely, M.L.; Parliament, M.B.; Seakale, H.; Johan, N.; Magee, D.J.; Haykowsky, M.J.; Courtney, K.S. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: A randomized controlled trial. Cancer 2008, 113, 214–222. [Google Scholar] [CrossRef]
- McNeely, M.L.; Parliament, M.; Courneya, K.S.; Seikaly, H.; Jha, N.; Scrimger, R.; Hanson, J. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck 2004, 26, 518–530. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Anton, P.M.; Fogleman, A.; Hopkins-Price, P.; Verhulst, S.; Rao, K.; Malone, J.; Robbs, R.; Courneya, K.S.; Nanavati, P.; et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 2013, 35, 1178–1188. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.U.P.; Kurian, J.R.; Lin, P.J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef]
- Capozzi, L.C.; McNeely, M.L.; Lau, H.Y.; Reimer, R.A.; Giese-Davis, J.; Fung, T.S.; Culos-Reed, S.N. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer 2016, 122, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Samuel, S.R.; Maiya, G.; Babu, A.S.; Vidyasagar, M.S. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J. Med. Res. 2013, 137, 515–520. [Google Scholar]
- Su, T.L.; Chen, A.N.; Leong, C.P.; Huang, Y.C.; Chiang, C.W.; Chen, I.H.; Lee, Y.Y. The effect of home-based program and outpatient physical therapy in patients with head and neck cancer: A randomized, controlled trial. Oral Oncol. 2017, 74, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Do, J.H.; Yoon, I.J.; Cho, Y.K.; Ahn, J.S.; Kim, J.K.; Jeon, J.Y. Comparison of hospital based and home based exercise on quality of life, and neck and shoulder function in patients with spinal accessary nerve injury after head and neck cancer surgery. Oral Oncol. 2018, 86, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Lauchlan, D.T.; McCaul, J.A.; McCarron, T.; Patil, S.; McManners, J.; McGarva, J. An exploratory trial of preventative rehabilitation on shoulder disability and quality of life in patients following neck dissection surgery. Eur. J. Cancer Care 2011, 20, 113–121. [Google Scholar] [CrossRef]
- McGarvey, A.C.; Hoffman, G.R.; Osmotherly, P.G.; Chiarelli, P.E. Maximizing shoulder function after accessory nerve injury and neck dissection surgery: A multicenter randomized controlled trial. Head Neck 2015, 37, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Grote, M.; Maihöfer, C.; Weigl, M.; Davies-Knorr, P.; Belka, C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: A randomized controlled pilot feasibility trial. Radiat. Oncol. 2018, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Malone, J.; Rao, K.; Courneya, K.S.; Fogleman, A.; Tippey, A.; Markwell, S.J.; Robbins, K.T. Exercise preferences among patients with head and neck cancer: Prevalence and associations with quality of life, symptom severity, depression, and rural residence. Head Neck 2009, 31, 994–1005. [Google Scholar] [CrossRef]
- Falser, S.; Behrens, M.; Liese, J.; Strueder, D.F.; Rhode, K.; Junghanss, C.; Grosse-Thief, C. Feasibility and effects of a supervised exercise program suitable for independent training at home on physical function and quality of life in head and neck cancer patients: A pilot study. Integr. Cancer Ther. 2020, 19, 1534735420918935. [Google Scholar] [CrossRef]
- Senchak, J.J.; Fang, C.Y.; Bauman, J.R. Interventions to improve quality of life (QOL) and/or mood in patients with head and neck cancer (HNC): A review of the evidence. Cancers Head Neck 2019, 4, 2. [Google Scholar] [CrossRef]
- De Leeuw, J.; Prins, J.B.; Teerenstra, S.; Merkx, M.A.W.; Marres, H.A.M.; van Achtenberg, T. Nurse-led follow-up care for head and neck cancer patients: A quasi-experimental prospective trial. Support. Care Cancer 2013, 21, 537–547. [Google Scholar] [CrossRef]
- Turner, J.; Yates, P.; Kenny, L.; Gordon, L.G.; Burmeister, B.; Hughes Brett, G.M.; McCarthy, A.L.; Perry, C.; Chan, R.J.; Paviour, A.; et al. The ENHANCES study: A randomised controlled trial of a nurse-led survivorship intervention for patients treated for head and neck cancer. Support. Care Cancer 2019, 27, 4627–4637. [Google Scholar] [CrossRef]
- Xiao, W.; Chan, C.W.H.; Xiao, J.; Wong, C.L.; Chow, K.M. Development of a nurse-led educational intervention program in managing the nutrition impact symptom cluster in patients with nasopharyngeal carcinoma following the medical research council framework. Asia Pac. J. Oncol. Nurs. 2021, 8, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Khantwal, G.; Sharma, S.K.; Rani, R.; Agarwal, S.P. Effect of postsurgical nurse-led follow-ups on quality of life in head-and-neck cancer patients: A pilot randomized controlled trial. Asia Pac. J. Oncol. Nurs. 2021, 8, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H. Neck and Shoulder Exercise Program for Patients with Head and Neck Cancers; Wang, Y.C., Ed.; Taiwan Academy of Physical Medicine and Rehabilitation: Taipei, Taiwan, 2016. Available online: https://wd.vghtpe.gov.tw/pmr/files/N201711110934_06%E9%A0%AD%E9%A0%B8%E7%99%8C%E8%82%A9%E9%A0%B8%E7%96%BC%E7%97%9B%E5%85%A7%E6%96%87.pdf (accessed on 25 June 2022).
- Roach, K.E.; Budiman-Mak, E.; Songsiridej, N.; Lertratanakul, Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991, 4, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N. The University of Washington Quality of Life Scale. In Handbook of Disease Burdens and Quality of Life Measures; Preyed, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 101–128. [Google Scholar] [CrossRef]
- Cancer Council Western Australia. Available online: https://healthinfonet.ecu.edu.au/key-resources/resources/31523/?title=Guidelines+for+implementing+exercise+programs+for+cancer+patients&contentid=31523_1 (accessed on 1 May 2022).
- Idorn, M.; Thor Straten, P. Exercise and cancer: From “healthy” to “therapeutic”? Cancer Immunol. Immunother. 2017, 66, 667–671. [Google Scholar] [CrossRef] [Green Version]

| Total | Control | Intervention | p-Value | ||
|---|---|---|---|---|---|
| Variables | (n = 40) | (n = 20) | (n = 20) | ||
| Age (year) | 58.2 ± 10.1 | 58.1 ± 10.6 | 58.4 ± 9.8 | 0.58 | |
| Length of stay (day) | 11.1 ± 2.4 | 11.1 ± 2.4 | 11.2 ± 2.4 | 0.87 | |
| Sex | M | 30 (75) | 16 (80) | 14 (70) | 0.47 |
| F | 10 (25) | 4 (20) | 6 (30) | ||
| Cancer stage | I | 26 (65) | 15 (75) | 11 (55) | 0.40 |
| II | 8 (20) | 2 (10) | 6 (30) | ||
| III | 6 (15) | 3 (15) | 3 (15) | ||
| Elective neck dissection | 32 (80) | 17 (85) | 15 (75) | 0.69 | |
| Therapeutic neck dissection | 8 (20) | 3 (15) | 5 (25) | 0.69 | |
| Nodal positivity | 8 (20) | 3 (15) | 5 (25) | 0.69 | |
| Age | <40 years | 3 (7) | 1 (5) | 2 (10) | 0.86 |
| 41–50 years | 6 (15) | 5 (25) | 1 (5) | ||
| 51–60 years | 14 (35) | 4 (20) | 10 (50) | ||
| >61 years | 17 (43) | 10 (50) | 7 (35) | ||
| Work | No | 7 (17) | 2 (10) | 5 (25) | 0.76 |
| Yes | 33 (83) | 18 (90) | 15 (75) | ||
| Education | <9 years | 20 (50) | 7 (35) | 13 (65) | 0.06 |
| >12 years | 20 (50) | 13 (65) | 7 (35) | ||
| Marriage | Single | 12 (30) | 5 (25) | 7 (35) | 0.50 |
| Married | 28 (70) | 15 (75) | 13 (65) | ||
| Smoking | Never | 9 (23) | 3 (15) | 6 (30) | 0.41 |
| Abstained | 16 (40) | 12 (60) | 4 (20) | ||
| <1 pack/day | 3 (7) | 1 (5) | 2 (10) | ||
| >1 pack/day | 12 (30) | 4 (20) | 8 (40) | ||
| Alcohol | Never | 25 | 4 (20) | 6 (30) | 0.46 |
| Abstained | 10 (25) | 3 (15) | 7 (35) | ||
| <250 mL/day | 13 (33) | 11 (55) | 2 (10) | ||
| >250 mL/day | 7 (17) | 2 (10) | 5 (25) | ||
| Betel nut | Never | 7 (17) | 4 (20) | 3 (15) | 0.41 |
| Abstained | 24 (60) | 13 (65) | 11 (55) | ||
| <10/day | 2 (6) | 0 (0) | 2 (10) | ||
| >10/day | 7 (17) | 3 (15) | 4 (20) | ||
| Exercise activity | Yes | 14 (35) | 6 (30) | 8 (40) | 0.52 |
| No | 26 (65) | 14 (70) | 12 (60) | ||
| Chronic disease | No | 23 (58) | 10 (50) | 13 (65) | 0.35 |
| Yes | 17 (42) | 10 (50) | 7 (35) |
| SP | SD | UW-QOL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | β | SE | 95% CI | p | β | SE | 95% CI | p | β | SE | 95% CI | p |
| Intercept | 11.6 | 7.6 | −3.2 to 26.4 | <0.01 ** | 1.1 | 1.1 | −1.0 to 3.2 | <0.01 ** | 639.9 | 27.6 | 585.8 to 693.9 | <0.01 ** |
| Group | 22.1 | 5.1 | 11.9 to −32.2 | <0.01 ** | −2.3 | 1.9 | −6.1 to 1.5 | <0.01 ** | 422.9 | 35.44 | 353.5 to 492.5 | <0.01 ** |
| Time (Post 12W) | 2.2 | 1.3 | −9.8 to 4.0 | <0.01 ** | −0.2 | 0.2 | −0.6 to 0.2 | <0.01 ** | 76.8 | 7.6 | 61.8 to 91.8 | <0.01 * |
| Group × Time (Post 12W) | 6.9 | 1.5 | 1.5 to −9.8 | <0.01 ** | 0.1 | 0.4 | −0.6 to 0.8 | <0.05 * | -105.2 | 9.8 | −124.4 to −85.9 | <0.01 ** |
| Post 2W | Post 4W | Post 8W | Post 12W | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Adjusted Mean | β | SE | p | Adjusted Mean | β | SE | p | Adjusted Mean | β | SE | p | Adjusted Mean | β | SE | p |
| SPscore | ||||||||||||||||
| Intervention | −4.21 | −1.68 | 2.60 | 0.52 | −5.98 | −0.86 | 3.41 | 0.25 | −9.85 | −8.55 | 2.60 | <0.01 ** | −11.39 | −8.09 | 1.87 | <0.01 ** |
| Control | −2.53 | −5.11 | −1.29 | −3.30 | ||||||||||||
| SD score | ||||||||||||||||
| Intervention | −5.36 | −1.03 | 3.24 | 0.75 | −7.60 | −13.36 | 6.66 | <0.05 * | −17.96 | −20.91 | 6.11 | <0.01 ** | −22.07 | −19.49 | 5.26 | <0.01 ** |
| Control | −4.33 | 5.75 | 2.96 | −2.57 | ||||||||||||
| UW-QOL score | ||||||||||||||||
| Intervention | −74.90 | −102.44 | 178.8 | 0.32 | −85.78 | −3.43 | 119.89 | 0.97 | 105 | −90.65 | 73.80 | <0.01 ** | 172.5 | −143.48 | 269.0 | <0.01 ** |
| Control | −177.34 | −89.21 | −202.5 | −423.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-L.; Hsieh, T.-C.; Chen, P.-R.; Chang, S.-C. Nurse-Led Counseling Intervention of Postoperative Home-Based Exercise Training Improves Shoulder Pain, Shoulder Disability, and Quality of Life in Newly Diagnosed Head and Neck Cancer Patients. J. Clin. Med. 2022, 11, 4032. https://doi.org/10.3390/jcm11144032
Hong Y-L, Hsieh T-C, Chen P-R, Chang S-C. Nurse-Led Counseling Intervention of Postoperative Home-Based Exercise Training Improves Shoulder Pain, Shoulder Disability, and Quality of Life in Newly Diagnosed Head and Neck Cancer Patients. Journal of Clinical Medicine. 2022; 11(14):4032. https://doi.org/10.3390/jcm11144032
Chicago/Turabian StyleHong, Yu-Long, Tsung-Cheng Hsieh, Peir-Rong Chen, and Shu-Chuan Chang. 2022. "Nurse-Led Counseling Intervention of Postoperative Home-Based Exercise Training Improves Shoulder Pain, Shoulder Disability, and Quality of Life in Newly Diagnosed Head and Neck Cancer Patients" Journal of Clinical Medicine 11, no. 14: 4032. https://doi.org/10.3390/jcm11144032
APA StyleHong, Y.-L., Hsieh, T.-C., Chen, P.-R., & Chang, S.-C. (2022). Nurse-Led Counseling Intervention of Postoperative Home-Based Exercise Training Improves Shoulder Pain, Shoulder Disability, and Quality of Life in Newly Diagnosed Head and Neck Cancer Patients. Journal of Clinical Medicine, 11(14), 4032. https://doi.org/10.3390/jcm11144032






