Abstract
Background: Elderly patients are at high risk of both ischaemic and bleeding events, and the low body weight is considered a risk factor for major bleeding in atrial fibrillation (AF) patients on anticoagulation therapy. The aim of our study was to compare the safety and effectiveness of non-vitamin K antagonist oral anticoagulants (NOACs) versus well-controlled vitamin-K antagonists (VKA) therapy among AF patients aged >75 years and with a body weight <60 kg in a prospective registry setting. Methods: Data for this study were sourced from the Italian cohorts of PREFER in AF and PREFER in AF PROLONGATION registries. The occurrence of a composite of stroke, transient ischemic attack and systemic embolism (thromboembolic events) was the primary effectiveness endpoint. The occurrence of major bleeding was the primary safety endpoint. All-cause hospitalizations and all-cause death were the secondary endpoints. The net clinical benefit (NCB) was calculated in order to obtain an integrated assessment of the anti-thromboembolic and pro-haemorrhagic effects of NOACs vs. VKA. Results: Overall, 522 patients were included; 225 were on treatment with NOACs and 317 patients with VKA. The NOAC group more frequently featured a higher BMI and a higher prevalence of history of stroke/TIA and insulin-requiring diabetes; conversely, heart failure and chronic liver disease were less frequent in the NAOC group. In the unmatched study population, 18 patients (3.6% in the NOAC vs. 3.2% in the VKA group, p = 0.79) experienced thromboembolic events; 19 patients (1.78% in the NOAC vs. 4.73% in the VKA group, p = 0.06) experienced major bleeding events; and 68 patients were hospitalized during the follow-up (9.3% vs. 14.8%, p = 0.06). After balancing for potential confounders by using the 1:1 propensity score matching technique, 426 patients (213 on NOAC and 213 on VKA) were selected. We found no significant differences in terms of thromboembolic events (3.76% vs. 4.69%, p = 0.63), major bleeding events (n: 1.88% vs. 4.22%, p = 0.15) and hospitalizations (9.9% vs. 16.9%, p = 0.06) between NOAC vs. VKA matched population. Based on these incidences, we found a positive net clinical benefit (+1.6) of NOACs vs. VKAs. Conclusions: These real-world data suggest the safety and effectiveness of using NOACs in elderly patients with low body weight.
1. Introduction
Low body weight frequently occurs in elderly patients and is a risk factor for major bleeding in patients with atrial fibrillation (AF) on anticoagulation therapy—both with vitamin K oral anticoagulant (VKA) [1] and with the non-vitamin K antagonist oral anticoagulants (NOACs) [2]. Moreover, low body weight has been associated with a higher risk of thromboembolism compared with non-low body weight patients [3]. Patients with body weight <60 kg were clearly less represented in randomized clinical trials (RCT), occurring in <10% of the study populations [4]; however, the efficacy and safety of NOACs compared with warfarin in patients with low body weight seem to be consistent with the overall findings of the pivotal registration trials. There are still few clinical data on the safety and effectiveness of NOACs in elderly patients with AF and low body weight [5,6]. We therefore extracted data on AF patients aged ≥75 years and with body weight <60 kg from two real-world prospective registries, evaluating the clinical performance in terms of safety and effectiveness of NOACs vs. VKAs.
2. Methods
2.1. Study Population
Data from this study were pooled from two prospective, observational, European registries of anticoagulants in AF: the Prevention of thromboembolic events–European Registry in Atrial Fibrillation (PREFER in AF) [7] and the Prevention of thromboembolic events–European Registry in Atrial Fibrillation PROLONGATION (PREFER in AF PROLONGATION) [8], which enrolled AF patients between January 2012 and January 2013 and between June 2014 and May 2015, respectively, and followed for 1 year. The clinical endpoints were recorded in the registries as clinically judged by the investigators. All AF patients from Italy aged ≥75 years and with body weight <60 kg who received a treatment with VKAs or NOACs were included. Potentially eligible VKA and NOAC-treated patients were propensity score-matched to generate an analysis cohort with minimal differences in baseline characteristics. The occurrence of stroke, transient ischemic attack (TIA), systemic embolism (SE), major bleeding (MB) and hospitalizations were assessed. All-cause death was recorded. All patients provided written informed consent for the inclusion the Prefer AF registry and the present study was conducted in accordance with the Declaration of Helsinki and its amendments. Ethical approval was obtained according to the local regulations of each participating centre.
2.2. Definitions
MB included fatal bleeding or bleeding in a critical organ or clinically relevant bleeding with haemoglobin decrease ≥2 g/dL, consistent with the definition from the International Society on Thrombosis and Haemostasis [9]. Stroke was defined as the abrupt onset of a focal neurologic deficit lasting >24 h. TIA was defined as focal neurologic deficit lasting <24 h. Systemic embolic event was defined as abrupt arterial insufficiency with documentation of an arterial occlusion.
2.3. Outcomes
The occurrence of MB is the primary safety endpoint. The composite of stroke, TIA and SE is the primary effectiveness endpoint. Secondary endpoints were all-cause hospitalizations and all-cause death.
Statistical Analysis
Descriptive results for continuous variables were expressed as mean ± standard deviation (SD); those for categorical variables as frequency and percentage. The Student’s t-test and the χ2 test were used to compare the continuous and categorical variables, respectively. Propensity score matching (PSM), with ratio of matching was 1:1, was used to balance differences in baseline characteristics between patients receiving NOACs versus those receiving VKAs. The model included the following variables: age, sex, weight, arterial hypertension, diabetes mellitus, heart failure, coronary heart disease, peripheral artery disease and chronic kidney disease. The net clinical benefit (NBC) with NOACs vs. VKAs was calculated among matched cohorts with the following formula: NCB = (thromboembolic events incidence rate with VKAs − thromboembolic events incidence rate with NOACs) − weighting factor × (intracranial haemorrhage incidence (ICH) rate with NOACs − ICH incidence rate with VKAs), where we used a weighting factor of 1.5, as previously described [10,11]. The difference in rates of outcome events among matched groups during the follow-up period was assessed by the Kaplan–Meier method by means of the log-rank test. A two-sided p-value less than 0.05 was considered significant for all tests. All statistical analyses were performed using RStudio (RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, USA, available online: http://www.rstudio.com/ accessed on 15 February 2022.
3. Results
A total of 522 AF patients with age >75 years and weight <60 kg on treatment with a NOAC (n = 225) or a VKA (n = 317) were recorded in the two registries. Their baseline characteristics are summarized in Table 1. No data on international normalized ratio (INR) control in VKA-treated patients were available. However, INR control was assessed by collecting the last 3 INR measurements prior to enrolment. An adequate INR control (i.e., at least 2 of 3 INR values in the therapeutic range) was demonstrated in 72% of the VKA group.

Table 1.
Clinical characteristics of the study population.
The NOAC group was more likely to have a higher BMI and a higher prevalence of history of stroke/TIA and insulin-dependent diabetes; conversely, heart failure and chronic hepatic disease were less frequent. No significant difference in follow-up duration between the two groups were found (369 ± 31 vs. 369 ± 12 days; p = 0.3). Among the overall population, 19 patients (4 in NOAC vs. 15 in VKA group) experienced MB events; a trend of reduction of MB overall incidence was reported among NOAC group compared to VKA group (1.78% vs. 4.73%; p = 0.06). A total of 18 patients (8 in the NOAC vs. 10 in the VKA group) experienced TE events; no significant difference in the overall incidence of TE events was shown between the two groups (3.6% vs. 3.2%, p = 0.79). Sixty-eight patients were hospitalized during the follow-up (21 in the NOAC vs. 47 in the VKA group); a trend of lower rate of hospitalizations was present in the NOAC group compared to the VKA group (9.3% vs. 14.8%; p = 0.06). A total of 7 patients (6 in the NOAC vs. 1 in the VKA group) died; no significant difference in the overall incidence of death was shown between the two groups (2.8% vs. 0.4%, p = 0.12). Figure 1 shows the outcome events stratified according to age (75–80 yrs.; 81–90 yrs.; and >90 yrs.) and weight (<50 kg and 50–60 kg). No statistically significant differences were found across the subgroups, except for a statistically significant lower rate of all-cause hospitalizations among patients with weight 50–60 kg (8.6% vs. 26.1%; p = 0.0004) and those over 90 years (9.1% vs. 77.8%; p = 0.004) for NOACs vs. VKAs.
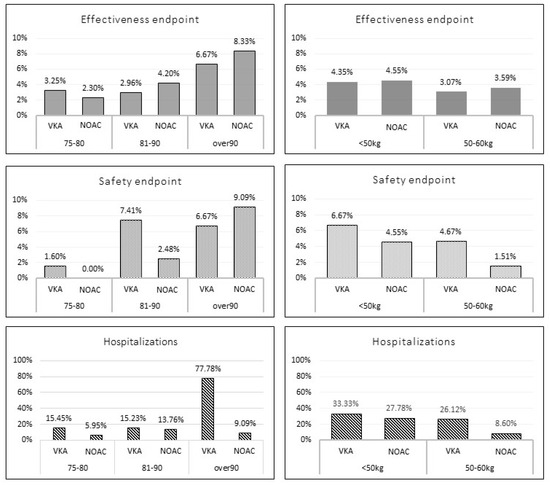
Figure 1.
Incidence of the outcome events according to age and weight stratification among unmatched study population. VKA: vitamin K antagonist; NOAC: direct oral anticoagulant.
Matched Population
After balancing for potential confounders, a population of 426 patients (213 on NOAC and 213 on VKA) were selected. Among the NOAC group, 48 patients (22.5%) were taking apixaban, 59 (27.7%) dabigatran and 106 (49.8%) rivaroxaban. Baseline characteristics of the study population are summarized in Table 2.

Table 2.
Clinical characteristics of matched study population.
No significant differences were found in terms of TE (3.8% vs. 4.7%, p = 0.63), MB events (1.9% vs. 4.2%, p = 0.15) and hospitalizations (9.9% vs. 15.9%; p = 0.06) overall incidences between NOAC vs. VKA matched populations. Figure 2 shows the incidence of outcome events stratified according to age (75–80 yrs.; 81–90 yrs.; and >90 yrs.) and weight (<50 kg and 50–60 kg). No statistically significant differences were found across the subgroups.
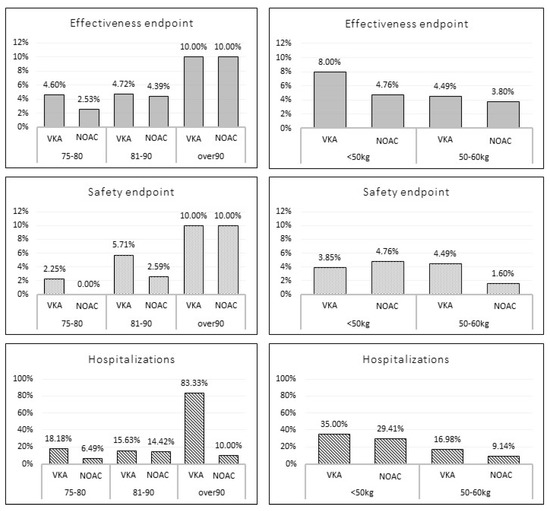
Figure 2.
Incidence of the outcome events according to age and weight stratification among matched study population. VKA: vitamin K antagonist; NOAC; non-vitamin K oral anticoagulant.
The Kaplan–Meier analysis showed no significant difference in 1 year survival from TE events (log rank: 0.08, p = 0.78), major bleeding (log rank: 1.05, p = 0.30) and all-cause death (log rank: 3.49, p = 0.06) in VKA versus NOAC group (Figure 3, Figure 4 and Figure 5).
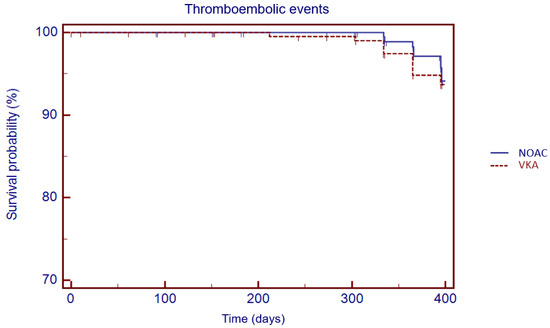
Figure 3.
Kaplan–Meier cumulative probability of thromboembolic event-free survival in NOAC and VKA treatment group.
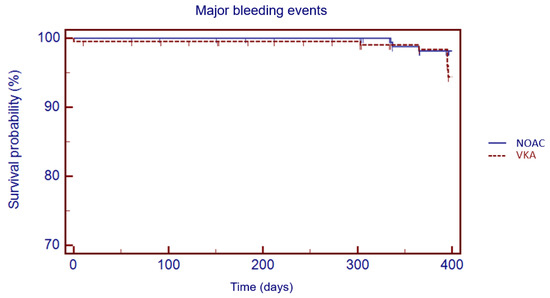
Figure 4.
Kaplan–Meier cumulative probability of major bleedings event-free survival in NOAC and VKA treatment group.
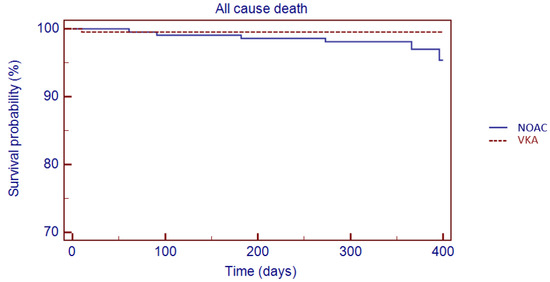
Figure 5.
Kaplan–Meier cumulative probability of all-cause death event-free survival in NOAC and VKA treatment group.
The NOAC group featured a positive net clinical benefit vs. VKAs (+1.6) between the two matched population (Figure 6).
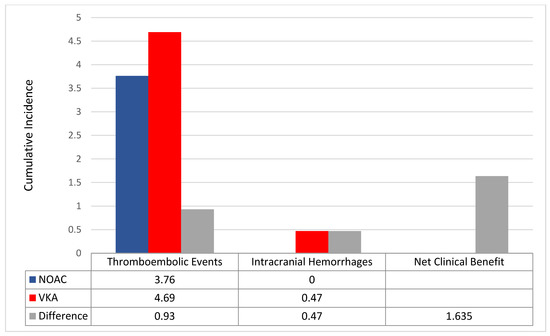
Figure 6.
Incidence rates of thromboembolic events and intracranial haemorrhages between the two matched population. Differences (Δ) between incidence rates were used to calculate the net clinical benefit (NCB). VKA: vitamin K antagonist; NOAC: non-vitamin K oral anticoagulant.
4. Discussion
The main results of the present analysis are that the overall incidence of both MB and TE events among elderly with atrial fibrillation and low body weight on oral anticoagulation therapy was high. NOACs showed a numerically lower incidence of both TE and MB events compared to VKAs across different weight and age, except for those aged >90 years. Among patients with body weight <50 kg, NOACs showed numerically higher MB events. NOACs also showed a better net clinical benefit than VKAs, mainly driven by the numerically lower rate of in TE events and intracranial haemorrhages; numerically lesser all-cause hospitalizations were shown among the NOAC vs. the VKA group.
The high overall incidence of MB events (3.64%) in our study population was expected, being both advanced age and the low body weight associated with a heightened risk for bleeding during anticoagulant treatment [1,12]. In contrast, the expected lower risk of TE events [13] seems to be attenuated by the concomitant low body weight, which has been associated with a paradoxical increase in thromboembolism risk among AF patients on oral anticoagulant treatment [3].
To date, few data on the safety or efficacy of NOACs among elderly patients with low body weight are available [4,5]. In a large Asian cohort including 21.679 AF patients with median age of 73 years and low body weight (≤60 kg) on oral anticoagulation therapy, NOACs, compared with VKAs, were associated with a lower risks of ischemic stroke, intracranial bleeding, hospitalization for major bleeding and all-cause death in patients with low body weight (≤60 kg); moreover, a consistent trend was observed in patients with extremely low body weight (<50 kg), except for hospitalization for gastrointestinal bleedings [4]. Recently, among an Italian cohort of octogenarians with atrial fibrillation and low body weight [5], Russo et al. showed a significantly lower incidence of all-cause death in patients on NOACs versus VKAs, confirming the evidence of a mortality advantage that emerged from randomized clinical trials (RCTs) on NOACs vs. VKAs in AF patients [13].
Among our study subgroup aged >90 years, no difference in thromboembolic and bleeding events was shown between NOACs and VKAs. Moreover, NOACs showed a numerically higher rate of major bleeding events among elderly AF patients with body weight <60 kg. This evidence suggests that caution should be adopted in considering NOACs “per se” a more effective and safer treatment than VKAs among very elderly patients with AF when a very low body weight and advanced age coexist [14,15,16].
Patients on NOACs showed a lower hospitalization rate compared with those on VKAs. The much lower rate of hospitalization among patient aged >90 years could not be justified by a lower incidence of MB or TE events alone, since it was similar across NOACs and VKAs matched groups. The conservative management of NOAC related complications [17] and the fear of intracranial haemorrhages in elderly patients on VKA therapy [18] might in part justify our results; however, further analyses of the Prefer in AF registry are needed to explore the reasons for hospitalizations [19].
Limitations
No outcome data on patients receiving edoxaban were provided. A direct comparison among NOACs was not performed, due to the small number of endpoint events during the observation period. Data about the appropriateness of NOACs dosage were not provided; however, we cannot exclude that octogenarians with AF may have received an inappropriate dose of NOAC [20]. No specific information about OAC adherence and persistence were available. Finally, our results were adjusted for possible confounding variables, but residual confounders cannot be excluded (i.e., previous stroke or TIA, history of bleeding and severely reduced clearance of creatinine).
5. Conclusions
Our study provides evidence of the safe and effective use of the NOACs among elderly patients with low body weight. This evidence was mainly accounted for by a numerical reduction in both TE events and intracranial haemorrhages. Moreover, the hospitalization rate was lower among patients on NOACs.
Author Contributions
Conceptualization, V.R. and R.D.C.; methodology, V.R. and R.T.; software, M.B. and M.C.M.; validation, P.K., E.A. and R.D.C.; formal analysis, M.B. and V.R.; investigation, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
PREFER in AF was sponsored by Daiichi Sankyo Europe GmbH, Munich, Germany.
Institutional Review Board Statement
The present study was conducted in accordance with the Declaration of Helsinki and its amendments. Ethical approval was obtained according to the local regulations of each participating centre.
Informed Consent Statement
All patients provided written informed consent for their inclusion on the Prefer AF registry.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no direct conflict of interest for this specific study. See other disclosures below.
References
- Park, C.S.; Choi, E.K.; Kim, H.M.; Lee, S.R.; Cha, M.J.; Oh, S. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm 2017, 14, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Inden, Y.; Yoshida, N.; Kato, H.; Miyoshi-Fujii, A.; Mizutani, Y.; Ito, T.; Kamikubo, Y.; Kanzaki, Y.; Hirai, M.; et al. Body mass index is associated with prognosis in Japanese elderly patients with atrial fibrillation: An observational study from the outpatient clinic. Heart Vessel. 2016, 31, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Boonyawat, K.; Caron, F.; Li, A.; Chai-Adisaksopha, C.; Lim, W.; Iorio, A.; Lopes, R.D.; Garcia, D.; Crowther, M.A. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: A systematic review and meta-analysis. J. Thromb. Haemost. 2017, 15, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caterina, R.; Lip, G.Y.H. The non-vitamin K antagonist oral anticoagulants(NOACs) and extremes of body weight—A systematicliterature review. Clin. Res. Cardiol. 2017, 106, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Attena, E.; Di Maio, M.; Carbone, A.; Parisi, V.; Rago, A.; Grieco, F.V.; Buonauro, A.; Golino, P.; Nigro, G. Non-vitamin K vs vitamin K oral anticoagulants in patients aged > 80 year with atrial fibrillation and low body weight. Eur. J. Clin. Investig. 2020, 50, e13335. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Park, C.S.; Han, K.-D.; Jung, J.-H.; Oh, S.; Lip, G.Y. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J. Am. Coll. Cardiol. 2019, 73, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Ammentorp, B.; Darius, H.; De Caterina, R.; Le Heuzey, J.-Y.; Schilling, R.J.; Schmitt, J.; Zamorano, J.L. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: Primary results of the Prevention of Thromboemolic events–European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014, 16, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Renda, G.; Pecen, L.; Patti, G.; Ricci, F.; Kotecha, D.; Siller-Matula, J.M.; Schnabel, R.B.; Wachter, R.; Sellal, J.M.; Rohla, M.; et al. Antithrombotic management and outcomes of patients with atrial fibrillation treated with NOACs early at the time of market introduction: Main results from the PREFER in AF Prolongation Registry. Intern. Emerg. Med. 2021, 16, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.E.; Chang, Y.; Fang, M.C.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Go, A.S. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann. Intern. Med. 2009, 151, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Attena, E.; Rago, A.; Melillo, E.; Di Micco, P.; Papa, A.A.; Napolitano, G.; D’Onofrio, A.; Golino, P.; Nigro, G. Clinical Outcome of Edoxaban vs. Vitamin K Antagonists in Patients with Atrial Fibrillation and Diabetes Mellitus: Results from a Multicenter, Propensity-Matched, Real-World Cohort Study. J. Clin. Med. 2020, 9, 1621. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, G.; Lucerna, M.; Pecen, L.; Siller-Matula, J.M.; Cavallari, I.; Kirchhof, P.; De Caterina, R. Thromboembolic risk, bleeding outcomes and effect of different antithrombotic strategies in very elderly patients with atrial fibrillation: A sub-analysis from the PREFER in AF (PREvention oF Thromboembolic Events- European Registry in Atrial Fibrillation). J. Am. Heart Assoc. 2017, 6, e005657. [Google Scholar] [CrossRef] [Green Version]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Russo, V.; Carbone, A.; Rago, A.; Golino, P.; Nigro, G. Direct Oral Anticoagulants in Octogenarians with Atrial Fibrillation: It Is Never Too Late. J. Cardiovasc. Pharmacol. 2019, 73, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Attena, E.; Di Maio, M.; Mazzone, C.; Carbone, A.; Parisi, V.; Rago, A.; D’Onofrio, A.; Golino, P.; Nigro, G. Clinical profile of direct oral anticoagulants versus vitamin K anticoagulants in octogenarians with atrial fibrillation: A multicentre propensity score matched real-world cohort study. J. Thromb. Thrombolysis 2020, 49, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hellenbart, E.L.; Faulkenberg, K.D.; Finks, S.W. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc. Health Risk Manag. 2017, 13, 325–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savioli, G.; Ceresa, I.F.; Luzzi, S.; Gragnaniello, C.; Giotta Lucifero, A.; Del Maestro, M.; Marasco, S.; Manzoni, F.; Ciceri, L.; Gelfi, E.; et al. Rates of Intracranial Hemorrhage in Mild Head Trauma Patients Presenting to Emergency Department and Their Management: A Comparison of Direct Oral Anticoagulant Drugs with Vitamin K Antagonists. Medicina 2020, 56, 308. [Google Scholar] [CrossRef] [PubMed]
- Brüggenjürgen, B.; Schliephacke, T.; Darius, H.; De Caterina, R.; Le Heuzey, J.Y.; Pittrow, D.; Reimitz, P.E.; Schilling, R.J.; Zamorano, J.L.; Kirchhof, P. Discontinuation and Hospitalisation Rates in Patients with Atrial Fibrillation: Follow-Up Results of the Prefer in Af Registry. Value Health 2014, 17, A473. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Santelli, F.; Bottino, R.; Attena, E.; Mazzone, C.; Parisi, V.; D’Andrea, A.; Golino, P.; Nigro, G.; Russo, V. Prevalence and clinical predictors of inappropriate direct oral anticoagulant dosage in octagenarians with atrial fibrillation. Eur. J. Clin. Pharmacol. 2022, 78, 879–886. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).