Prevalence and Risk of Infection in Patients with Diabetes following Primary Total Knee Arthroplasty: A Global Systematic Review and Meta-Analysis of 120,754 Knees
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Eligibility Criteria
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Analyses
3. Results
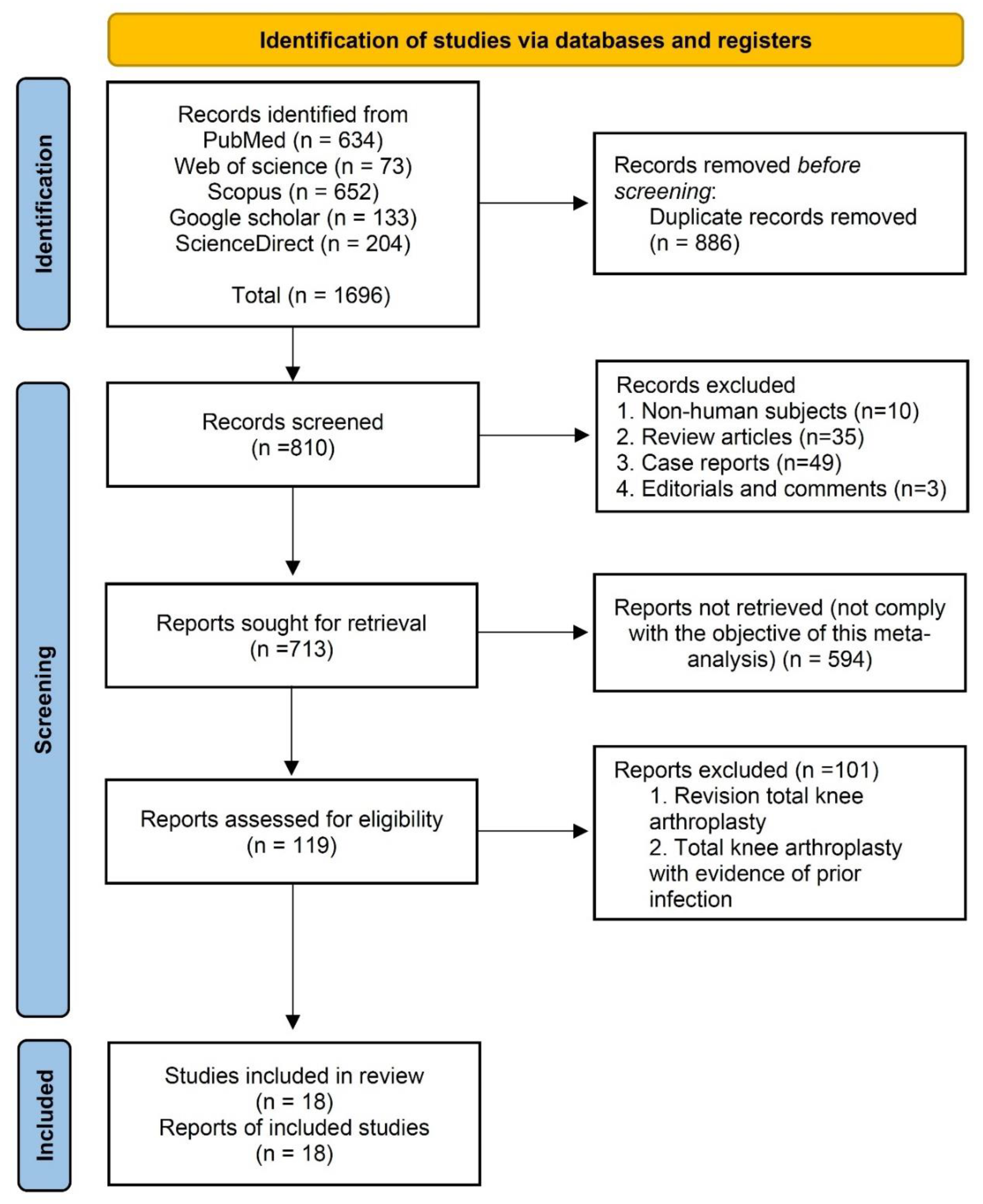
3.1. Study Selection
3.2. Characteristics of Included Studies
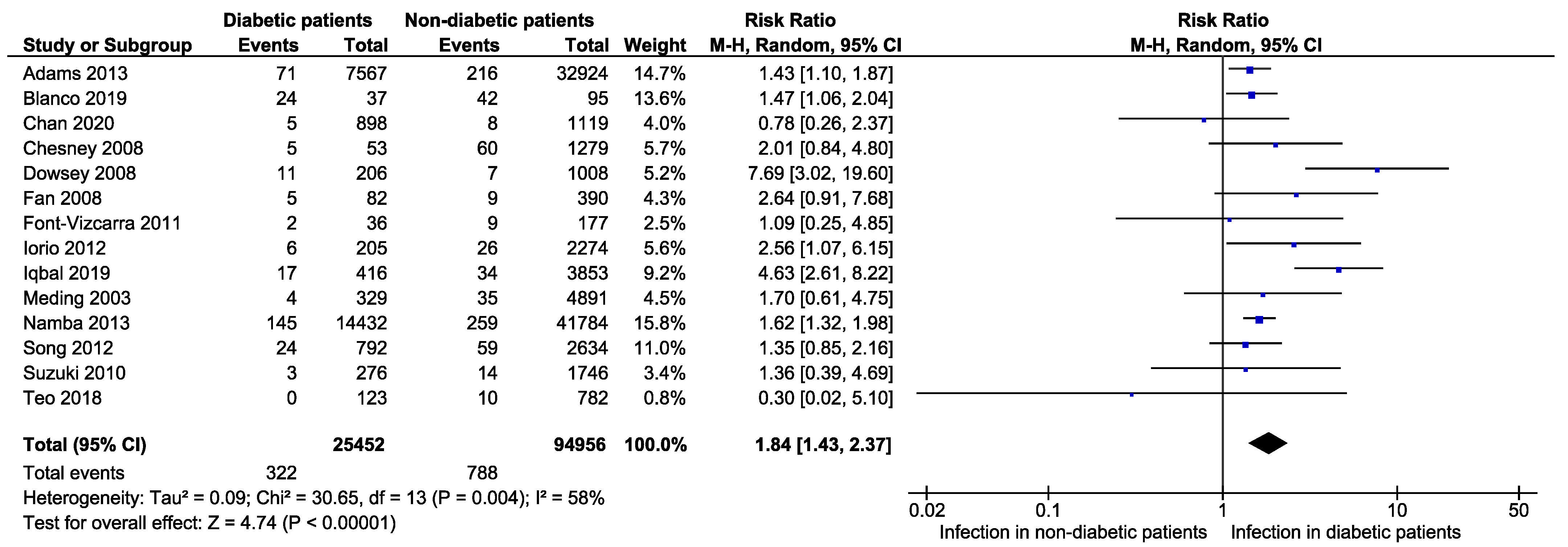
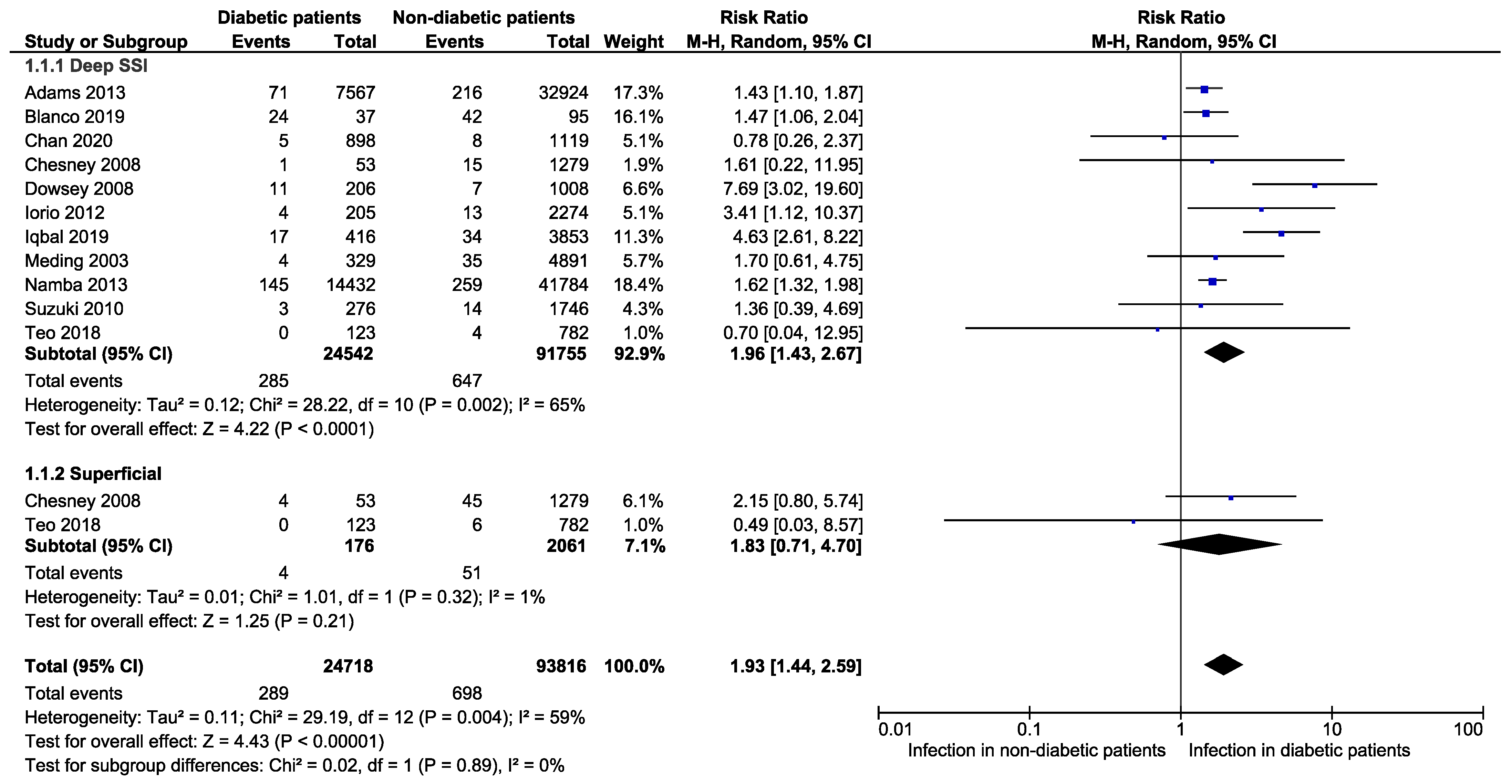
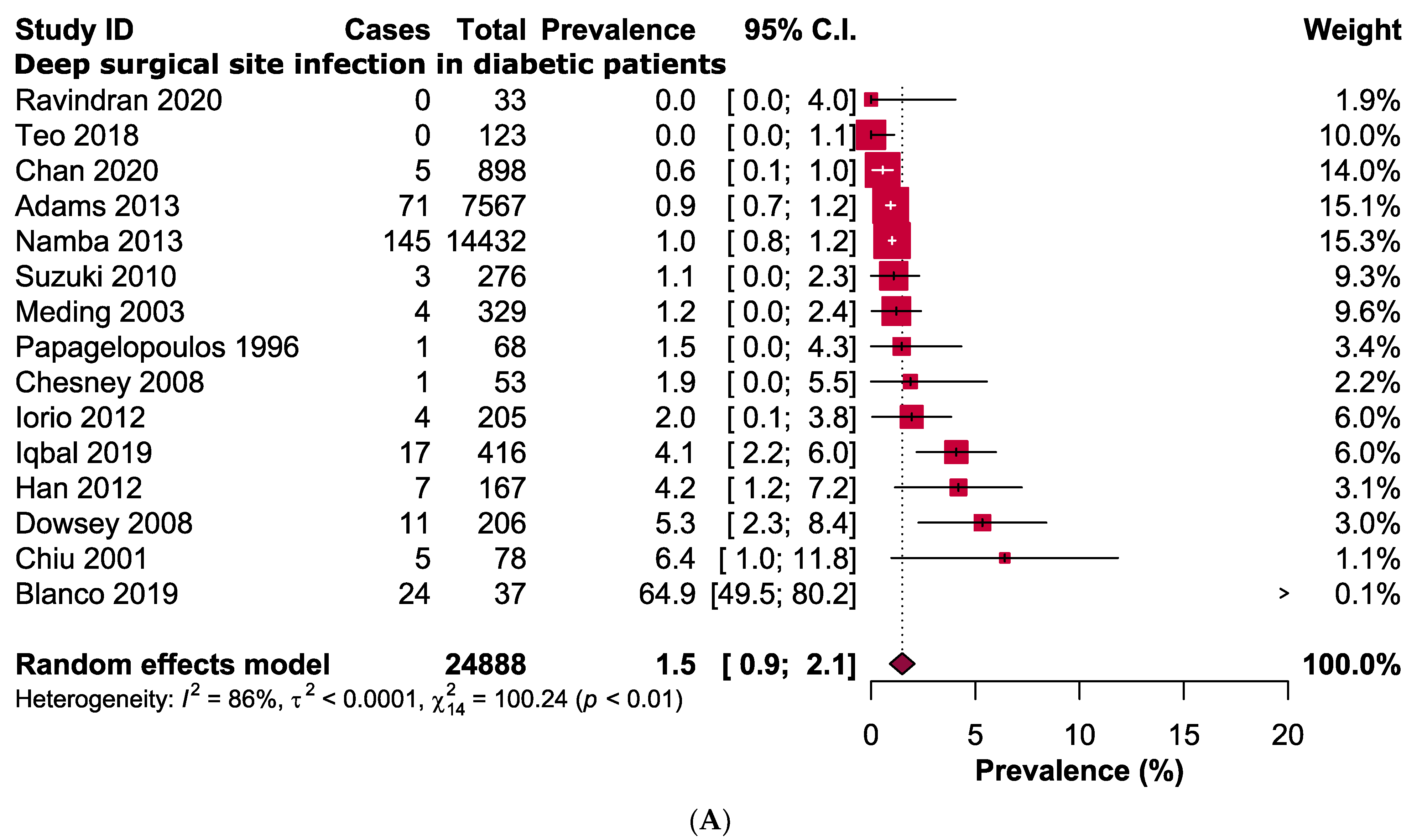
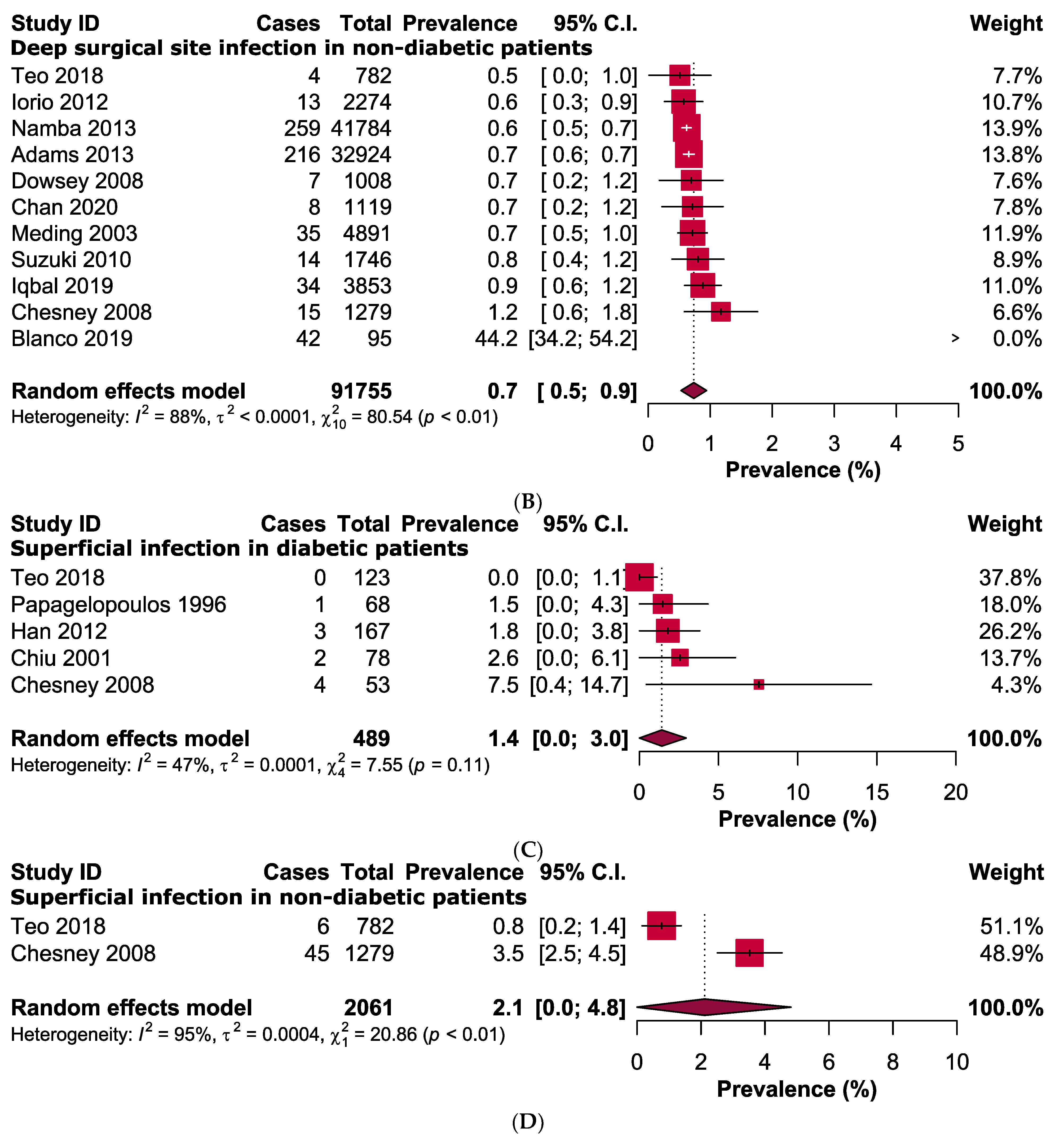
3.3. Primary Outcomes
3.4. Quality Assessment and Publication Bias
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, G.; Saito, S.; Ishii, T.; Motojima, S.; Tokuhashi, Y.; Ryu, J. Previous fracture surgery is a major risk factor of infection after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2010, 19, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.; Brown, J.; Taylor, A.; Pattison, G.; Whitehouse, S.; Bannister, G. Infection after total knee arthroplasty. J. Bone Joint. Surg. Br. 2004, 86, 688–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalore, N.V.; Gioe, T.J.; Singh, J.A. Diagnosis and management of infected total knee arthroplasty. Open J. Orthop. 2011, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Everhart, J.S.; Altneu, E.; Calhoun, J.H. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin. Orthop. 2013, 471, 3112–3119. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention: Morbidity and Mortality Weekly Report. 2014. Available online: http://www.cdc.gov/mmwr (accessed on 15 May 2022).
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Bolognesi, M.P.; Marchant, M.H., Jr.; Viens, N.A.; Cook, C.; Pietrobon, R.; Vail, T.P. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J. Arthroplast. 2008, 23, 92–98. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2017; Volume 5. [Google Scholar]
- Hajissa, K.; Islam, M.A.; Sanyang, A.M.; Mohamed, Z. Prevalence of intestinal protozoan parasites among school children in africa: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0009971. [Google Scholar] [CrossRef]
- Hajissa, K.; Marzan, M.; Idriss, M.I.; Islam, M.A. Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 932. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Cochrane Collaboration, Review Manager (RevMan) Version 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014; Available online: https://revman.cochrane.org/ (accessed on 31 May 2022).
- Adams, A.L.; Paxton, E.W.; Wang, J.Q.; Johnson, E.S.; Bayliss, E.A.; Ferrara, A.; Nakasato, C.; Bini, S.A.; Namba, R.S. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J. Bone Joint. Surg. Am. 2013, 95, 481. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.F.; Díaz, A.; Melchor, F.R.; da Casa, C.; Pescador, D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2019, 140, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.; Chan, P.; Woo, Y.; Fu, H.; Cheung, A.; Cheung, M.; Yan, C.; Chiu, K. Universal haemoglobin A1c screening reveals high prevalence of dysglycaemia in patients undergoing total knee arthroplasty. Hong Kong Med. J. 2020, 26, 304. [Google Scholar] [CrossRef]
- Chesney, D.; Sales, J.; Elton, R.; Brenkel, I.J. Infection after knee arthroplasty: A prospective study of 1509 cases. J. Arthroplast. 2008, 23, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-Y.; Lin, C.-F.; Chen, C.-M.; Lo, W.-H.; Chaung, T.-Y. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus: A prospective, randomised study. J. Bone Joint. Surg. Br. 2001, 83, 691–695. [Google Scholar] [CrossRef]
- Dowsey, M.M.; Choong, P.F. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin. Orthop. 2008, 467, 1577–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.C.; Hung, H.; Fung, K. Infection in primary total knee replacement. Hong Kong Med. J. 2008, 14, 40. [Google Scholar]
- Font-Vizcarra, L.; Lozano, L.; Ríos, J.; Forga, M.T.; Soriano, A. Preoperative nutritional status and post-operative infection in total knee replacements: A prospective study of 213 patients. Int. J. Artif. Organs 2011, 34, 876–881. [Google Scholar] [CrossRef]
- Han, H.-S.; Kang, S.-B. Relations between long-term glycemic control and postoperative wound and infectious complications after total knee arthroplasty in type 2 diabetics. Clin. Orthop. Surg. 2012, 5, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Iorio, R.; Williams, K.M.; Marcantonio, A.J.; Specht, L.M.; Tilzey, J.F.; Healy, W.L. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J. Arthroplast. 2012, 27, 726–729.e721. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Shafiq, B.; Zamir, M.; Noor, S.; Memon, N.; Memon, N.; Dina, T.K. Micro-organisms and risk factors associated with prosthetic joint infection following primary total knee replacement—Our experience in Pakistan. Int. Orthop. 2019, 44, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Meding, J.B.; Reddleman, K.; Keating, M.E.; Klay, A.; Ritter, M.A.; Faris, P.M.; Berend, M.E. Total knee replacement in patients with diabetes mellitus. Clin. Orthop. 2003, 416, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Namba, R.S.; Inacio, M.C.; Paxton, E.W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: An analysis of 56,216 knees. J. Bone Joint. Surg. Am. 2013, 95, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Papagelopoulos, P.J.; Idusuyi, O.B.; Wallrichs, S.L.; Morrey, B.F. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin. Orthop. 1996, 330, 124–132. [Google Scholar] [CrossRef]
- Ravindran, S.; Pratap, K.R. Clinical outcome of Primary Total Knee Arthroplasty in Diabetes Mellitus. J. Med. Sci. Clin. Res. 2020, 8, 249–260. [Google Scholar] [CrossRef]
- Song, K.-H.; Kim, E.S.; Kim, Y.K.; Jin, H.Y.; Jeong, S.Y.; Kwak, Y.G.; Cho, Y.K.; Sung, J.; Lee, Y.-S.; Oh, H.-B. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the Korean Nosocomial Infections Surveillance System (KONIS). Infect. Control Hosp. Epidemiol. 2012, 33, 1086–1093. [Google Scholar] [CrossRef]
- Teo, B.J.X.; Yeo, W.; Chong, H.-C.; Tan, A.H.C. Surgical site infection after primary total knee arthroplasty is associated with a longer duration of surgery. J. Orthop. Surg. 2018, 26, 2309499018785647. [Google Scholar] [CrossRef] [Green Version]
- Bozic, K.J.; Lau, E.; Kurtz, S.; Ong, K.; Berry, D.J. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin. Orthop. 2012, 470, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection burden for hip and knee arthroplasty in the United States. J. Arthroplast. 2008, 23, 984–991. [Google Scholar] [CrossRef]
- Goodson, W.H., III; Hunt, T.K. Studies of wound healing in experimental diabetes mellitus. J. Surg. Res. 1977, 22, 221–227. [Google Scholar] [CrossRef]
- Agos, F.; Shoda, C.; Bransford, D. Part II: Managing perioperative hyperglycemia in total hip and knee replacement surgeries. Nurs. Clin. 2014, 49, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.K.; Zeng, I.; Ravi, S.; Zhu, M.; Vince, K.G.; Young, S.W. Periprosthetic joint infection is the main cause of failure for modern knee arthroplasty: An analysis of 11,134 knees. Clin. Orthop. 2017, 475, 2194–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Huang, X.; Yang, H.; Shen, M. The influence of diabetes mellitus on patients undergoing primary total lower extremity arthroplasty: A systematic review and meta-analysis. Biomed. Res. Int. 2020, 2020, 6661691. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Pawasarat, I.M.; Azzam, K.A.; Joshi, A.; Hansen, E.N.; Bozic, K.J. Periprosthetic joint infection: The economic impact of methicillin-resistant infections. J. Arthroplast. 2010, 25, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e61. [Google Scholar] [CrossRef]




| Study ID | Country | Data Collection Period | Total Number of Knees (Diabetic/Non-Diabetics) | Total Number of Study Participants (Diabetic/Non-Diabetics) | Age of the Patients (Years) [Mean ± SD/Median (IQR)/Range] | Type of Infection | Study Design |
|---|---|---|---|---|---|---|---|
| Adams 2013 [15] | USA | 2001–2009 | 40,491 (7567/32,924) | 40,491 (7567/32,924) | 61–75 | Deep SSI | Case-control, retrospective |
| Blanco 2019 [16] | Spain | NR | 132 (37/95) | 132 (37/95) | Infected: 72.4 ± 7.0, Not infected: 70.1 ± 7.7 | Deep SSI | Case-control, retrospective |
| Chan 2020 [17] | Hong Kong | December 2014–May 2019 | 2017 (898/1119) | 1566 (704/862) | 23.0–93.0 | Deep SSI | Case-control, prospective |
| Chesney 2008 [18] | UK | October 1998–February 2005 | 1332 (53/1279) | 1332 (53/1279) | 64.0–75.0 | Superficial and deep SSI | Case-control, prospective |
| Chiu 2001 [19] | Taiwan | 1993–1998 | 78 (78/0) | 78 (78/0) | 69.0–72.0 | Superficial and deep SSI | Cohort, prospective |
| Dowsey 2008 [20] | USA | 1998–2005 | 1214 (206/1008) | 1214 (206/1008) | 72.0 (65.0–77.0) | Deep SSI | Case-control, retrospective |
| Fan 2008 [21] | Hong Kong | July 1997–June 2006 | 472 (82/390) | 348 (NR/NR) | 69.0 (40.0–88.0) | Superficial and deep SSI | Case-control, retrospective |
| Font-Vizcarra 2011 [22] | Spain | December 2007–May 2008 | 213 (36/177) | 213 (36/177) | Infected: 77.0 (74.0–80.0), Not infected: 72.0 (66.0–78.0) | Superficial and deep SSI | Case-control, prospective |
| Han 2012 [23] | South Korea | January 2001–March 2007 | 167 (167/0) | 115 (115/0) | 68.0 (49.0–82.0) | Superficial and deep SSI | Cohort, retrospective |
| Iorio 2012 [24] | USA | December 2004–December 2009 | 2479 (205/2274) | 2479 (205/2274) | NR | Superficial and deep SSI | Case-control, retrospective |
| Iqbal 2019 [25] | Pakistan | June 2008–December 2018 | 4269 (416/3853) | 4269 (416/3853) | 61.4 ± 10.2 | Deep SSI | Case-control, retrospective |
| Meding 2003 [26] | USA | June 1987–November 1999 | 5220 (329/4891) | 5220 (329/4891) | NR | Deep SSI | Case-control, prospective |
| Namba 2013 [27] | USA | April 2001–December 2009 | 56,216 (14,432/41,784) | 56,216 (14,432/41,784) | 67.4 ± 9.6 | Deep SSI | Case-control, prospective |
| Papagelopoulos 1996 [28] | USA | May 1978–May 1982 | 68 (68/0) | 51 (51/0) | NR | Superficial and deep SSI | Cohort, prospective |
| Ravindran 2020 [29] | India | July 2018–October 2019 | 33 (33/0) | 33 (33/0) | 48.0–71.0 | Deep SSI | Cohort, prospective |
| Song 2012 [30] | South Korea | 2006–2009 | 3426 (792/2634) | 3426 (792/2634) | SSI: 67.0 ± 8.8, No SSI: 68.6 ± 7.5 | Superficial and deep SSI | Case-control, retrospective |
| Suzuki 2010 [1] | Japan | 1995–2006 | 2022 (276/1746) | 1146 (NR/NR) | Infected: 69.5 ± 7.1, Not infected 70.7 ± 8.5 | Deep SSI | Case-control, retrospective |
| Teo 2018 [31] | Singapore | February 2004–July 2014 | 905 (123/782) | 905 (123/782) | 65.9 ± 7.7 | Superficial and deep SSI | Case-control, prospective |
| Risk Ratio Estimation | ||||||
|---|---|---|---|---|---|---|
| Strategies of Sensitivity Analyses | RR [95% CI] | Difference of Pooled RR Compared to the Main Result | Number of Studies Analysed | Total Number of Participants | Heterogeneity | |
| I2 | p-Value | |||||
| Excluding outlier studies | 1.5 [1.3–1.7] | 0.31 lower | 12 | 114,925 | 0% | 0.83 |
| Excluding small studies (n < 500) | 1.9 [1.4–2.6] | 0.09 higher | 11 | 119,591 | 66% | 0.001 |
| Excluding low- and moderate-quality studies | 1.8 [1.3–2.3] | 0.003 lower | 13 | 117,929 | 60% | 0.003 |
| Prevalence estimation (diabetic patients) | ||||||
| Strategies of sensitivity analyses | Prevalence [95% CI] % | Difference of pooled prevalence compared to the main result | Number of studies analysed | Total number of participants | Heterogeneity | |
| I2 | p-value | |||||
| Excluding outlier studies | 1.5 [1.1–2.0] | 0.4 lower | 17 | 25,761 | 74% | <0.0001 |
| Excluding small studies (n < 100) | 1.4 [0.9–1.8] | 0.5 lower | 11 | 25,411 | 78% | <0.0001 |
| Excluding low- and moderate-quality studies | 1.9 [1.3–2.6] | No difference | 16 | 25,560 | 88% | <0.0001 |
| Prevalence estimation (non-diabetic patients) | ||||||
| Excluding outlier studies | 1.1 [0.8–1.3] | 0.1 lower | 13 | 94,861 | 88% | <0.0001 |
| Excluding small studies (n < 100) | 1.2 [0.9–1.5] | No difference | 14 | 94,956 | 92% | <0.0001 |
| Excluding low- and moderate-quality studies | 1.2 [0.9–1.5] | No difference | 13 | 92,682 | 93% | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.; Ab Rahman, S.; Islam, M.A. Prevalence and Risk of Infection in Patients with Diabetes following Primary Total Knee Arthroplasty: A Global Systematic Review and Meta-Analysis of 120,754 Knees. J. Clin. Med. 2022, 11, 3752. https://doi.org/10.3390/jcm11133752
Ahmad MA, Ab Rahman S, Islam MA. Prevalence and Risk of Infection in Patients with Diabetes following Primary Total Knee Arthroplasty: A Global Systematic Review and Meta-Analysis of 120,754 Knees. Journal of Clinical Medicine. 2022; 11(13):3752. https://doi.org/10.3390/jcm11133752
Chicago/Turabian StyleAhmad, Mohd Aliff, Shaifuzain Ab Rahman, and Md Asiful Islam. 2022. "Prevalence and Risk of Infection in Patients with Diabetes following Primary Total Knee Arthroplasty: A Global Systematic Review and Meta-Analysis of 120,754 Knees" Journal of Clinical Medicine 11, no. 13: 3752. https://doi.org/10.3390/jcm11133752
APA StyleAhmad, M. A., Ab Rahman, S., & Islam, M. A. (2022). Prevalence and Risk of Infection in Patients with Diabetes following Primary Total Knee Arthroplasty: A Global Systematic Review and Meta-Analysis of 120,754 Knees. Journal of Clinical Medicine, 11(13), 3752. https://doi.org/10.3390/jcm11133752







